Evaluation of prolonged safety and efficacy of biodegradable polymer coated sirolimus-eluting coronary stent system: 1-year outcomes of the INDOLIMUS Registry
Introduction
The drug eluting stents (DES) are more efficient than bare metal stents (BMS), and it has been proven erstwhile in numerous studies (1-3). DES is associated with irrefutably lower risk of restenosis and revascularization as compared to BMS (4). The first generation DES constitutes of durable polymers, and reduces the risk of target lesion revascularization (TLR) (5). But, its usage suffers from detrimental inflammatory reaction, thereby prolonging healing process of vessel wall and restoration of endothelium (6,7). The chances of late thrombotic risk is also increased several folds (8-10). DES with biodegradable polymers can overcome the pros and cons associated with durable polymers. Biodegradable polymers can be absorbed after the drug has been eluted. Thus, only a metal stent is left over ensuring that there is no further damage of the arterial wall. The polymer material used in the study stent undergoes degradation within 9-12 months of stent implantation. While, the short-term safety and efficacy of the Indolimus SES is known (11), the prolonged safety and efficacy of the Indolimus SES at 1-year, after the degradation of polymer material, is unknown, although an important outcome to ponder.
Thus, our main aim is to evaluate prolonged safety and efficacy of the Indolimus coronary stent system in high risk patients with complex coronary lesions.
Methods
Study design and clinical end-points
The INDOLIMUS registry is a single center, non randomized, retrospective registry with the main aim of evaluating safety and efficacy of the Indolimus SES. The study was approved by the Institutional review board and was conducted in accordance with the principles of good clinical practice (GCP) and Declaration of Helsinki. The protocol and in-hospital, 30 days and 6-months results of the registry have been described elsewhere (11). The primary end-point of this follow-up is to determine 1-year incidence of major adverse cardiac events (MACEs). MACE includes cardiac death, TLR, target vessel revascularization (TVR), myocardial infarction (MI) and stent thrombosis (ST).
Stent description
The Indolimus stent consisted of L605 cobalt chromium alloy (Co-Cr) as its stent platform having strut thickness of 60 µm and drug load of 1.4 µg/mm2 coated with biodegradable polymer. The biodegradable polymer matrix consists of poly L-lactide, 50/50 poly DL lactide-co-glycolide and polyvinyl pyrrolidone to control the drug elution from stent coating. After unloading the drug within 48 days, the polymeric matrix undergoes hydrolysis. This process takes 9-12 months after which all the polymer gets degraded and gets excreted from the body in the form of metabolites.
Statistical analysis
Continuous variables are presented as mean ± standard deviation and categorical variables as counts and percentages. The event free survival curve was calculated according to the Kaplan-Meier method. All data were analysed using the Statistical Package for Social Sciences (SPSS; Chicago, IL, USA) program, version 15.
Results
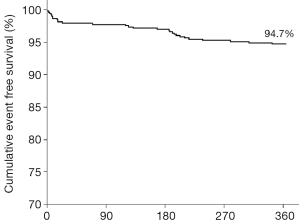
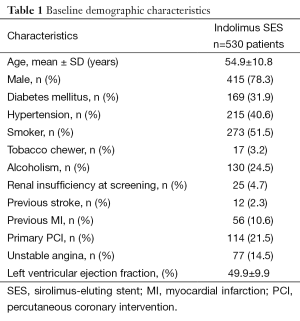
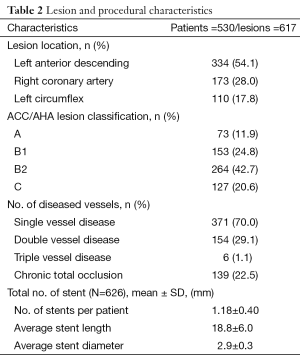
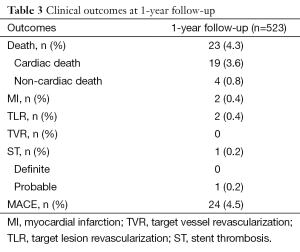
A total of 626 Indolimus stents were implanted in 617 lesions in 530 patients during the enrollment period. Out of 530 patients, 1-year follow-up was obtained for 523 (98.7%) patients. The patients retained the same baseline demographics (Table 1), and lesion and procedural characteristics (Table 2). At 1-year, cardiac death, TLR, and MI were reported in 19 (3.6%), 2 (0.4%), and 2 (0.4%) patients respectively, while ST was reported in 1 (0.2%) patients. The resultant MACE at 1-year was found to be 24 (4.5%) (Table 3). The event-free survival at 1-year follow-up by Kaplan-Meir method was found to be 94.7% (Figure 1).
Full table
Full table
Full table
Discussion
In the world of clinical trials, randomized controlled trial is considered as a “gold standard” for any intervention to establish its safety and efficacy. It enrolls clinically stable and straightforward lesions in contrast to real-life high-risk cohorts, involved in post-marketing surveillance registry. The INDOLIMUS registry was an “all-comers” registry, which means it also included high risk patients with complex lesions (21.5% primary PCI and 22.5% chronic total occlusions), and not ideal recruited lesions that gives an idea about the actual potential of the study stent in real-life setting. The in-hospital, 30-day and 6-month clinical outcomes have already established short-term safety and efficacy of the Indolimus stent (11).
Indolimus, a biodegradable polymer coated sirolimus-eluting stent (SES) has been envisioned to reduce the long term sequelae associated with durable polymer DES, due to its persistence beyond the period of controlled drug release. So, the perceptible potential of biodegradable polymer DES can be acclaimed only if prolonged safety and efficacy outcomes, after the degradation of polymer, are evaluated.
Giving a quick rundown, at 1-year, MACE was found to be 24 (4.5%), which consisted of 19 (3.6%) case of cardiac death, 2 (0.4%) cases of TLR and 2 (0.4%) cases of MI. There was only 1 (0.2%) case of ST, as defined by ARC. Such promising results of biodegradable polymer coated SES were observed in a study by Han et al. , in which, MACE at 1-year follow-up was found to be 4% (12).
The high prevalence of cardiac death after percutaneous coronary intervention, as observed in our clinical outcomes, can be attributed to higher burden of cardiac risk factors among the patients, especially in low-income-patients. Poor are intuitively less able to afford presciption drugs, leading to suboptimal compliance with discharge medications. Moreover, reduced access to medical care or delayed presentation to tertiary care hospital after primary consultation also possibly had an impact on the outcomes. The supporting evidence by Alter et al. suggests an inverse relationship between socioeconomic status and mortality from acute MI (13).
Many randomized trials and meta-analysis have demonstrated conflicting results regarding the advantage of biodegradable polymer coated DES in reducing restenosis. The ISAR-TEST 3 trial, which is a non-inferiority trial, demonstrates that biodegradable polymer DES is non-inferior to durable polymer in terms of anti-restenotic effects at 1-year (14). The results of ISAR-TEST 4 trial are in concomitant with ISAR-TEST 3 and retrieves no added advantage of biodegradable polymer DES over durable polymer DES (15). In contrast, pooled analysis of three large randomized trials (ISAR-TEST 3, ISAR-TEST 4 and LEADERS) has shown the advantage of biodegradable polymer DES over durable polymer DES and revealed clinically indicated TLR to be 7.6% at 1-year follow-up (16).
In our study, TLR was reported to be 0.4% and MI to be 0.4%. This gives an idea about the outstanding potential of the Indolimus in reducing restenosis and revascularization. Also, the lower strut thickness of Indolimus (60 µm) reduces the thrombogenic potential (17), which is evident from ST rate as low as 0.2%.
Thus, the prolonged clinical findings of the Indolimus registry satiate safety and efficacy needed for continued use of the Indolimus SES system.
Conclusions
At 1-year follow-up, there was substantially reduced rate of re-vascularization and only one case of ST. Thus, the lower incidence of MACE at 1-year follow-up clearly depicts the prolonged safety and efficacy of the Indolimus SES system. The major limitation of this registry is its observational design. It suffers the disadvantages of a nonrandomized/single-arm investigation.
Acknowledgements
None
Footnote
Conflicts of Interest: Dr. Ashok Thakkar and Shivani Kothari are employees of Sahajanand Medical Technologies Pvt. Ltd. and have provided detailed assistance in literature search and manuscript writing. Other authors declare that they have no conflict of interest.
Financial Disclosure: No grant was received from Sahajanand Medical Technologies Pvt. Ltd. for the study.
References
- Stone GW, Ellis SG, Cox DA, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation 2004;109:1942-7. [PubMed]
- Holmes DR Jr, Leon MB, Moses JW, et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation 2004;109:634-40. [PubMed]
- Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation 2003;108:788-94. [PubMed]
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315-23. [PubMed]
- Babapulle MN, Joseph L, Bélisle P, et al. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet 2004;364:583-91. [PubMed]
- Suzuki T, Kopia G, Hayashi S, et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 2001;104:1188-93. [PubMed]
- Drachman DE, Edelman ER, Seifert P, et al. Neointimal thickening after stent delivery of paclitaxel: change in composition and arrest of growth over six months. J Am Coll Cardiol 2000;36:2325-32. [PubMed]
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193-202. [PubMed]
- Ong AT, McFadden EP, Regar E, et al. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol 2005;45:2088-92. [PubMed]
- McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004;364:1519-21. [PubMed]
- Rajasekhar D, Vanajakshamma V, Shashank C, et al. The real world experience of the biodegradable polymer-coated sirolimus-eluting coronary stent system: Results From an "All-Comers" Clinical Experience. Catheter Cardiovasc Interv 2013. [Epub ahead of print].
- Han Y, Jing Q, Chen X, et al. Long-term clinical, angiographic, and intravascular ultrasound outcomes of biodegradable polymer-coated sirolimus-eluting stents. Catheter Cardiovasc Interv 2008;72:177-83. [PubMed]
- Alter DA, Naylor CD, Austin P, et al. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med 1999;341:1359-67. [PubMed]
- Mehilli J, Byrne RA, Wieczorek A, et al. Randomized trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis. Eur Heart J 2008;29:1975-82. [PubMed]
- Byrne RA, Kastrati A, Kufner S, et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur Heart J 2009;30:2441-9. [PubMed]
- Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J 2012;33:1214-22. [PubMed]
- Kastrati A, Mehilli J, Dirschinger J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 2001;103:2816-21. [PubMed]