
Effect of high-sensitivity C-reactive protein on the relationship between haemoglobin A1c and cardiovascular events in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a cohort study
Introduction
Haemoglobin A1c (HbA1c) is an effective and sensitive index reflecting glucose levels within 3 months. Several studies have demonstrated that HbA1c can indicate cardiovascular events in patients with or without diabetes (1-3). In acute coronary syndrome (ACS) patients treated with percutaneous coronary intervention (PCI), high HbA1c levels were related to an increased risk of long-term all-cause death [odds ratio (OR) 1.39, 95% CI: 1.16–1.68, P=0.001] (2). However, the results in other studies are not consistent. In a study of a small number of patients who received PCI, no correlation between HbA1c and prognosis was observed (3). A study of nondiabetic patients showed that there was no obvious correlation between HbA1c levels and cardiovascular events after PCI in patients with ST-segment elevated myocardial infarction (STEMI) (4). In addition, for patients with diabetes, the UK Prospective Diabetes Study (UKPDS) study showed that intensive blood glucose control reduced HbA1C by 11% compared to the conventional group over 10 years and reduced the risk of microvascular complications but not the risk of macrovascular complications (5). Another three large randomized trials also confirmed that the incidence of cardiovascular events did not decrease in patients with lower HbA1c (6-8). We think that one of the reasons for this inconsistency is that some factors may affect the ability of HbA1c as a predictor of cardiovascular events.
Studies have shown that inflammatory states are positively correlated with the level of HbA1c in patients with or without diabetes (9,10). Inflammation attaches importance to the formation and progression of coronary artery atherosclerosis. HsCRP is an acute-phase reactive and nonspecific inflammatory marker that is mainly produced in hepatocytes (11). Studies have shown that hsCRP can detect coronary heart disease and predict future cardiovascular events (12,13). Among patients undergoing PCI, higher CRP levels during the procedure can predict 10-year mortality and myocardial infarction (MI) (14). However, no one has studied the effect of inflammation on the correlation between HbA1c and cardiovascular events. Therefore, we aimed to test the hypothesis that hsCRP could modulate HbA1c-related cardiovascular risk in patients with ACS undergoing PCI. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-78/rc).
Methods
Study population
Patients who received PCI between January and December 2018 were continuously and retrospectively enrolled in this study. Demographic, medical history, PCI procedure information and medical treatment data at discharge were collected from the medical information recording system of Beijing Anzhen Hospital. The main inclusion criteria were: age ≥18 years old, diagnosed with ACS according to current guideline (15), indicated for new generation drug-eluting stents and procedurally successfully received PCI treatment. The main exclusion criteria included patients with infection, rheumatic immune diseases, haemoglobinopathies, such as β-thalassemia or sickle cell disease, taking glucocorticoids or other immunosuppressants, receiving renal replacement therapy, estimated glomerular filtration rate (eGFR) ≤30 mL/min/1.73 mm2, alanine aminotransferase or aspartate aminotransferase ≥5 normal upper limits, left ventricular ejection fraction <30%, and New York Heart Association (NYHA) or Killip cardiac function grade ≥ Grade III. Patients with incomplete key information, including hsCRP and HbA1c, and those who were lost to follow-up were also excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Beijing Anzhen Hospital of Capital Medical University (No. 2015031) and individual consent was waived for the retrospective analysis of the study.
Measurement
Body mass index (BMI) was calculated by weight (kg)/[height (m)]2. The criteria for diabetes included (I) diabetes previously treated with antidiabetic drugs (diet, oral drugs and/or insulin); (II) typical diabetes symptoms, random blood glucose ≥11.1 mmol/L, and/or fasting blood glucose (FBG) ≥7.0 mmol/L, and/or blood glucose ≥11.1 mmol/L 2 hours after the oral glucose tolerance test (16); and (III) HbA1c level ≥6.5% (17). Hypertension was defined as a definite diagnosis of hypertension or systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or the use of antihypertensive drugs (18). Unstable angina pectoris is defined as myocardial ischaemia at rest or during minimal exercise without myocardial cell necrosis (15). Acute myocardial infarction (AMI) is defined as an acute myocardial injury detected by abnormal cardiac biomarkers, particularly troponin, with evidence of acute myocardial ischaemia (19).
The patient fasted for at least 8 hours before the first fasting venous blood was drawn, and the samples were measured on the same day. Blood samples were collected immediately upon admission for STEMI patients. Blood samples were anticoagulated with sodium citrate, ethylenediaminetetraacetic acid (EDTA) or heparin, and the supernatant was collected after centrifugation at 3,000 rpm for 10 minutes and sent for detection. Liver and kidney function and blood lipid parameters were detected by standard laboratory methods in the central laboratory of Beijing Anzhen Hospital. High-performance liquid chromatography was used to measure HbA1c levels (Bio-Rad Variant II TURBO HbA1c analyser, USA). HsCRP was detected by the turbidimetric immunoassay method (Cias Latex CRP-H, Kanto Chemical Co. Inc., Tokyo, Japan).
Perioperative management
All patients were treated with aspirin, clopidogrel or ticagrelor before PCI, and unfractionated heparin 70–100 IU/kg was used during the operation. Patients with diabetes were treated with an appropriate dose of insulin or oral antidiabetic drugs according to their blood glucose levels. The detailed PCI strategy was determined by experienced cardiologists. Coronary angiogram data were analysed by at least two experienced cardiologists. The characteristics of the disease were defined as follows: (I) multivessel disease: more than two main epicardial coronary artery stenoses ≥50%; (II) chronic total occlusion (CTO): a coronary angiographically demonstrated lesion with thrombolysis in myocardial infarction (TIMI) grade 0 blood flow, no thrombus, an unstained proximal fibrous cap, and mature collateral circulation, with a duration of occlusion for more than 3 months (20); (III) diffuse lesions: a single stenotic lesion ≥20 mm in length. The severity of coronary artery disease was quantified by the Synergy Between PCI With Taxus and Cardiac Surgery (SYNTAX) score. All PCI procedures and medical treatments were performed in accordance with the current PCI guidelines of China (21).
Outcomes
Patients included in the study were routinely followed up by telephone conversation, rehospitalization record or outpatient service. All patients were followed up for 24 months unless lost to follow-up or they reached the endpoint of observation. The primary endpoint was the composite of MACCEs, which included all-cause death, nonfatal MI, unplanned revascularization and nonfatal ischaemic stroke. Nonfatal stroke was defined as acute cerebral infarction diagnosed by typical symptoms or imaging (22). Incident MI was defined according to the fourth general definition of MI (19). Unplanned repeat revascularization was defined as any accidental PCI or coronary artery bypass grafting (CABG) after indexing procedures on the target or nontarget vessels. If patients had multiple cardiovascular events, the most serious cardiovascular event (order of precedence: death, nonfatal stroke, nonfatal myocardial infarction, unplanned revascularization) was selected for final analysis.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation or median (interquartile range). One-way analysis of variance (ANOVA) was used for normally distributed data, and the Kruskal-Wallis test was used for nonnormally distributed data. Categorical variables are presented as the number of cases with percentages and were tested by the χ2 test (Fisher’s exact test). We evaluated the baseline characteristics based on the tertiles of HbA1c according to previous literature (1). Patients were divided into 3 groups: tertile 1: HbA1c ≤5.7%; tertile 2: 5.7%< HbA1c ≤6.2%, and tertile 3: HbA1c >6.2%. A Cox proportional hazard regression model was used to analyse the relationship between HbA1c and hsCRP and cardiovascular events. The stepwise regression method was used, and the fully adjusted model included sex, age, BMI, SBP, pulse, current smoker, hypertension, diabetes, dyslipidaemia, previous stroke, previous MI, previous CABG and PCI, triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), any antidiabetic agents, SYNTAX score, number of stents, mean stent diameters, and total length of stents. The statistical interaction between hsCRP and HbA1c was examined by incorporating multiplicative interaction terms. The Kaplan-Meier curve was used to show the cumulative incidence of cardiovascular events at different hsCRP and HbA1c levels over time, and the results were compared by the logarithmic rank test. Sensitivity analysis was performed according to whether the patient had diabetes. In the sensitivity analysis, we used different HbA1c tertiles and hsCRP median cut-off values according to different diabetes states. The receiver operating characteristic (ROC) curve was used to show the predictive ability of HbA1C to cardiovascular events in different hsCRP levels. We used SPSS 21.0 (IBM Corp., Armonk, NY, USA) for data analysis. P<0.05 was considered statistically significant (double tails).
Results
A total of 2,236 patients who met the inclusion criteria were included. Seventy-nine patients met the exclusion criteria, 45 patients were excluded due to incomplete key variables, and 89 patients were lost during follow-up. Finally, 2,023 patients were enrolled in the study. During the 24-month follow-up period, 152 (7.51%) MACCE events occurred and were included in this final analysis (Figure 1).
Baseline characteristics
The baseline characteristics are shown in Table 1. Of the 2,023 patients enrolled, 78.1% were men, aged 59.7±10.03 years, with an average BMI of 25.9±3.12 kg/m2, of whom 31.8% (n=644) had diabetes and 86.2% (n=1,744) had unstable angina. The median hsCRP was 1.21 mg/L, and the median SYNTAX score was 12. The average diameter of the implanted stent was 3.1 mm, and the median total length was 30 mm.
Table 1
| Variables | Total | HbA1c | P value | ||
|---|---|---|---|---|---|
| Tertile 1 (≤5.7%) | Tertile 2 (≤6.2%) | Tertile 3 (>6.2%) | |||
| N (%) | 2,023 | 793 (39.2) | 568 (28.1) | 662 (32.7) | – |
| Age, years | 59.7±10.03 | 57.3±10.21 | 61.4±9.77 | 61.2±9.47 | <0.001 |
| Male sex, n (%) | 1,579 (78.1) | 659 (83.1) | 443 (78.0) | 477 (72.1) | <0.001 |
| BMI, kg/m2 | 25.9±3.12 | 25.4±3.12 | 25.8±3.14 | 26.4±3.05 | <0.001 |
| Heart rate, bpm | 71.5±10.52 | 70.9±10.19 | 71±10.94 | 72.5±10.49 | 0.010 |
| SBP, mmHg | 129.6±17.05 | 129.6±16.67 | 128.7±17.16 | 130.3±17.39 | 0.254 |
| Current smoker, n (%) | 711 (35.1) | 287 (36.2) | 202 (35.6) | 222 (33.5) | 0.555 |
| Hypertension, n (%) | 1,313 (64.9) | 472 (59.5) | 366 (64.4) | 475 (71.8) | <0.001 |
| Diabetes, n (%) | 644 (31.8) | 47 (5.9) | 65 (11.4) | 532 (80.4) | <0.001 |
| Dyslipidemia, n (%) | 1,438 (71.1) | 571 (72.0) | 403 (71.0) | 464 (70.1) | 0.723 |
| Previous MI, n (%) | 248 (12.3) | 83 (10.5) | 72 (12.7) | 93 (14.0) | 0.109 |
| Previous stroke, n (%) | 110 (5.4) | 39 (4.9) | 20 (3.5) | 51 (7.7) | 0.004 |
| Previous PCI, n (%) | 505 (25.0) | 165 (20.8) | 149 (26.2) | 191 (28.9) | 0.001 |
| Previous CABG, n (%) | 47 (2.3) | 11 (1.4) | 20 (3.5) | 16 (2.4) | 0.035 |
| Laboratory tests | |||||
| FBG, mmol/L | 6.0±1.25 | 5.5±0.82 | 5.8±0.93 | 6.8±1.48 | <0.001 |
| HbA1c, % | 6.1±0.91 | 5.4±0.26 | 6.0±0.14 | 7.1±0.88 | <0.001 |
| TG, mmol/L | 1.29 [0.97, 1.74] | 1.26 [0.92, 1.69] | 1.245 [1.00, 1.66] | 1.37 [1.04, 1.95] | <0.001 |
| TC, mmol/L | 4.1±1.04 | 4.1±1.07 | 4.1±1.04 | 4±0.99 | 0.961 |
| HDL-C, mmol/L | 1.1±0.25 | 1.1±0.26 | 1.1±0.25 | 1.1±0.24 | 0.001 |
| LDL-C, mmol/L | 2.4±0.89 | 2.5±0.94 | 2.4±0.90 | 2.4±0.81 | 0.130 |
| hsCRP, mg/L | 1.21 [0.50, 3.15] | 1.17 [0.49, 3.37] | 1.30 [0.53, 3.02] | 1.24 [0.50, 2.93] | 0.645 |
| ACS status, n (%) | |||||
| Unstable angina | 1,744 (86.2) | 668 (84.2) | 488 (85.9) | 588 (88.8) | 0.040 |
| AMI | 279 (13.8) | 125 (15.8) | 80 (14.1) | 74 (11.2) | 0.040 |
| Medication at discharge, n (%) | |||||
| Aspirin | 1,993 (98.5) | 783 (98.7) | 557 (98.1) | 653 (98.6) | 0.567 |
| Clopidogrel | 1,468 (72.6) | 580 (73.1) | 412 (72.5) | 476 (71.9) | 0.870 |
| Ticagrelor | 555 (27.4) | 213 (26.9) | 156 (27.5) | 186 (28.1) | 0.870 |
| ACEI/ARB | 884 (43.7) | 307 (38.7) | 248 (43.7) | 329 (49.7) | <0.001 |
| β-Blocker | 1,233 (60.9) | 467 (58.9) | 347 (61.1) | 419 (63.3) | 0.229 |
| Statin | 1,999 (98.8) | 781 (98.5) | 562 (98.9) | 656 (99.1) | 0.536 |
| Antidiabetic agents | 471 (23.3) | 29 (3.7) | 38 (6.7) | 404 (61.0) | <0.001 |
| Angiographic coronary anatomy, n (%) | |||||
| Left main disease | 478 (23.6) | 182 (23.0) | 135 (23.8) | 161 (24.3) | 0.826 |
| Multivessel disease | 960 (47.5) | 345 (43.5) | 227 (40.0) | 388 (58.6) | <0.001 |
| CTO | 336 (16.6) | 139 (17.5) | 92 (16.2) | 105 (15.9) | 0.663 |
| Diffuse Lesions | 692 (34.2) | 271 (34.2) | 114 (20.1) | 307 (46.4) | <0.001 |
| SYNTAX score | 12 [9, 17] | 12 [12, 17] | 12 [9, 18] | 12 [9, 18] | 0.408 |
| Target vessel and intervention | |||||
| LM, n (%) | 276 (13.6) | 108 (13.6) | 75 (13.2) | 93 (14.0) | 0.911 |
| LAD, n (%) | 1,163 (57.5) | 454 (57.3) | 333 (58.6) | 376 (56.8) | 0.799 |
| LCX, n (%) | 498 (24.6) | 191 (24.1) | 140 (24.6) | 167 (25.2) | 0.881 |
| RCA, n (%) | 675 (33.4) | 258 (32.5) | 192 (33.8) | 225 (34.0) | 0.814 |
| Number of stents | 1 [1, 2] | 1 [1, 2] | 1 [1, 2] | 1 [1, 2] | 0.705 |
| Mean stent diameter, mm | 3.1±0.42 | 3.1±0.43 | 3.1±0.41 | 3±0.41 | <0.001 |
| Total stent length, mm | 30 [21, 51] | 30 [21, 51] | 30 [18, 51] | 33 [22, 54] | 0.205 |
Data are presented as the mean ± standard deviation or median [interquartile range] if not otherwise specified. ACS, acute coronary syndrome; ACEI, angiotensin-converting enzyme inhibitors; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; Cr, creatinine; CTO, chronic total occlusion; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; LAD, left anterior descending artery; LCX, left circumflex artery; LDL-C, low-density lipoprotein cholesterol; LM, left main; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SBP, systolic blood pressure; SYNTAX, Synergy Between PCI With Taxus and Cardiac Surgery; TC, total cholesterol; TG, triglyceride.
Patients with high HbA1c showed higher heart rate, BMI, TG, proportions of females, hypertension, history of stroke, previous histories of PCI, use of antidiabetic drugs, multivessel lesions and diffuse lesions. No significant difference was found in other angiographic parameters.
Relationship between HbA1c and cardiovascular events
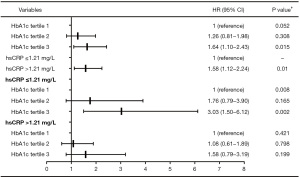
The relationship between HbA1c and cardiovascular events is described in Figure 2. The cardiovascular risk did not increase significantly with the increase in HbA1c [tertile 2 vs. tertile 1: hazard ratio (HR) 1.26, 95% confidence interval (CI): 0.81–1.98, P=0.308; tertile 3 vs. tertile 1: HR 1.64, 95% CI: 1.10–2.43, P=0.015; P=0.052 for trend]. We also found that compared with patients with hsCRP ≤1.21 mg/L, patients with hsCRP >1.21 mg/L had a significant 58% increase in cardiovascular risk (HR 1.58, 95% CI: 1.12–2.24, P=0.010]. Furthermore, taking hsCRP >1.21 or ≤1.21 mg/L as stratification criteria, the correlation between HbA1c and cardiovascular events was analysed again. The results showed that in patients with hsCRP ≤1.21 mg/L, taking the first HbA1c tertile as the reference, the risk of cardiovascular events increased by 76% in patients with the second HbA1c tertile and by approximately 3 times in patients with the third HbA1c tertile (tertile 2 vs. tertile 1: HR 1.76, 95% CI: 0.79–3.90, P=0.165, tertile 3 vs. tertile 1: HR 3.03, 95% CI: 1.50–6.12, P=0.002; P=0.008 for trend). However, there was no such correlation between HbA1c and cardiovascular events in patients with hsCRP >1.21 mg/L (tertile 2 vs. tertile 1: HR 1.08, 95% CI: 0.61–1.89, P=0.798; tertile 3 vs. tertile 1: HR 1.58, 95% CI: 0.79–3.19, P=0.199; P=0.421 for trend). We also found that there was a significant interaction for cardiovascular events between HbA1c tertiles and hsCRP (P=0.045).
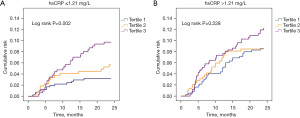
The Kaplan-Meier survival curves according to HbA1c tertiles and hsCRP binary during the 24-month follow-up are shown in Figure 3. Figure 3A shows that in patients with hsCRP ≤1.21 mg/L, the cumulative cardiovascular risk increased significantly with the increase in HbA1c quartile over time (P=0.002), but there was no similar correlation in patients with hsCRP >1.21 mg/L (P=0.228) (Figure 3B).
The ROC curve analysis of HbA1c to predict cardiovascular events in different hsCRP levels was shown in Figure S1. In the total population, the area under the curve (AUC) was 0.588 (95% CI: 0.541–0.635, P<0.001) (Figure S1A). When scarified the population into hsCRP ≤1.21 mg/L and hsCRP >1.21 mg/L, the AUC shows HbA1c had better predict value in patients hsCRP ≤1.21 mg/L (AUC: 0.553, 95% CI: 0.491–0.616, P=0.089 vs. AUC: 0.636, 95% CI: 0.566–0.706, P=0.001) (Figure S1B,S1C).
Table 2 presents the effect of different hsCRP levels on the relationship between HbA1c and cardiovascular risk in different diabetic states. Similarly, in patients without diabetes, cardiovascular risk was significantly associated with HbA1c tertiles when hsCRP ≤median (tertile 2 vs. tertile 1: HR 0.62, 95% CI: 0.21–1.81, P=0.379; tertile 3 vs. tertile 1: HR 2.42, 95% CI: 1.07–5.46, P=0.033; P=0.013 for trend) but not in patients with hsCRP above the median. However, no similar results were found in diabetic patients.
Table 2
| Variables | Diabetes | Non-diabetes | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value† | HR (95% CI) | P value† | ||
| hsCRP ≤ median# | |||||
| HbA1c* | |||||
| Tertile 1 | 1 (reference) | 0.962 | 1 (reference) | 0.013 | |
| Tertile 2 | 0.90 (0.28–2.92) | 0.858 | 0.62 (0.21–1.81) | 0.379 | |
| Tertile 3 | 1.08 (0.33–3.53) | 0.895 | 2.42 (1.07–5.46) | 0.033 | |
| hsCRP > median# | |||||
| HbA1c* | |||||
| Tertile 1 | 1 (reference) | 0.115 | 1 (reference) | 0.784 | |
| Tertile 2 | 1.29 (0.41–4.06) | 0.665 | 0.97 (0.49–1.92) | 0.938 | |
| Tertile 3 | 2.58 (0.93–7.17) | 0.069 | 1.21 (0.60–2.45) | 0.601 | |
†, adjusted for gender, age, BMI, SBP, pulse, current smoker, hypertension, dyslipidemia, previous MI, previous stroke, previous PCI, previous CABG, TC, TG, HDL-C, LDL-C, any antidiabetic agents, SYNTAX score, number of stents, mean stent diameters, total length of stents; #, median (hsCRP in diabetes) =1.185 mg/L, median (hsCRP in non-diabetes) =1.22 mg/L; *, HbA1c tertiles in diabetes: tertile 1, 6.3% (4.6%–6.6%); tertile 2, 6.9% (6.7%–7.2%); tertile 3, 7.9% (7.3%–13.0%). HbA1c tertiles in non–diabetes: tertile 1, 5.3% (4.2%–5.5%); tertile 2, 5.7% (5.6%–5.9%); tertile 3, 6.2% (6.0%–6.5%). BMI, body mass index; CABG, coronary artery bypass grafting; CI, confidence interval; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SYNTAX, Synergy Between PCI With Taxus and Cardiac Surgery; TC, total cholesterol; TG, triglyceride.
Discussion
To our knowledge, this is the first study to date that aims to determine the effect of hsCRP on the relationship between HbA1c and the risk of cardiovascular events in ACS patients after PCI. The results showed that in patients with low hsCRP levels, high HbA1c was closely related to the increased risk of cardiovascular events, but there was no such relationship in patients with high hsCRP levels. This finding suggests that there is a significant correlation between high HbA1c and residual cardiovascular risk in patients with low systematic inflammation but not in patients with high systematic inflammation.
These data provide new clues for hsCRP to precede HbA1c in predicting cardiovascular events after PCI. This study suggests that the risk of cardiovascular events may be underestimated when only HbA1c is used as a predictor of cardiovascular risk. Adding inflammatory markers such as hsCRP to the risk prediction model may improve the efficiency of cardiovascular risk prediction. In addition, this study indicates that hsCRP may be more helpful than HbA1C for clinicians to identify high-risk patients more accurately and provide more aggressive treatment.
We suspect that the possible reason for the result is that the effect of hyperglycaemia may be masked by other stronger risk factors. In other words, hsCRP may be better than HbA1c in predicting residual cardiovascular risk, masking the effects of HbA1c on cardiovascular events.
Indeed, studies have suggested that after controlling blood glucose to reduce cardiovascular risk, there are still great residual risk factors that lead to cardiovascular events. Many studies on type 2 diabetes mellitus (T2DM) patients have shown that blood glucose control does not always reduce cardiovascular events (23). In the ACCORD study, although the intensive treatment group reduced HbA1c by 11% (6.4% vs. 7.5%) compared with the standard treatment group, no difference in cardiovascular outcome was observed during the one-year follow-up (HR 0.90, 95% CI: 0.78–1.04, P=0.16) (6). The UKPDS study also observed at 1 year that low HbA1c levels after intensive hypoglycaemic therapy did not reduce the risk of macrovascular events (5). Although the 10-year follow-up of the UKPDS study showed that the risk of MI was reduced in the intensive treatment group, the group still had an absolute residual risk of 16.8% and the risk of stroke events did not improve (24). Except for the weakness of HbA1c as a blood glucose control marker in predicting cardiovascular events, its use in nondiabetic patients for prognosis is not quite clear (1,2,4). In addition to blood glucose control, the predictive ability of HbA1c is also affected by other factors.
Recently, the theory of residual inflammation has made great progress. According to current research, inflammation plays a vital role in the process of atherosclerosis, which may far exceed HbA1c. Various inflammatory factors play important roles in the early development of arteriosclerosis, in promoting plaque destabilization and inducing ACS, and in the response to myocardial cell death in MI (25). More and more evidence show that CRP is also a direct pathogenic and pro-inflammatory mediator of atherosclerosis and cardiovascular diseases. Two highly pro-inflammatory isoforms of pCRP* and monomer CRP (mCRP) have been shown to aggravate local tissue damage, MI and stroke under a wide range of pathological conditions, including atherosclerosis and thrombosis (26). Recent studies have also confirmed that controlling the inflammatory level can significantly reduce the residual risk. Canakinumab is a humanized monoclonal antibody against IL-1β that can reduce IL-6 and hsCRP (27). The CANTOS study showed that canakinumab could reduce the incidence of major cardiovascular events without affecting blood glucose and HbA1c (28,29).
In addition, a high level of hsCRP represents an abnormal pathological condition caused by many other factors, such as obesity, insulin resistance and metabolic syndrome, or some non-diabetes-related factors, such as smoking and acholic addiction, which may contribute to the occurrence of cardiovascular events (30-34). Thus, HbA1c is not sufficient to identify patients at risk in the presence of high levels of these risk factors reflected by hsCRP. HbA1c has better predictive value in the low levels of hsCRP level as a predictor of cardiovascular events. These pieces of evidence suggest that a series of pathological states represented by hsCRP may have a greater impact on prognosis than HbA1c.
There have been similar studies noting that there may be an interaction between hsCRP and HbA1c in predicting the progression of arteriosclerosis. In patients with advanced peripheral artery disease, both high hsCRP and HbA1c had a particularly high risk of cardiovascular events (35). In addition, hsCRP significantly interacts with HbA1c (P<0.001) and can be used together to predict the progression of carotid atherosclerosis. Compared with the patients with these two parameters in the first quartile, intimal-media thickness progressed most significantly in the patients with the fourth quartile, and the incidence of major adverse vascular events was significantly increased (36).
The definition of a high inflammatory state in this study is defined by the median hsCRP level. Indeed, our cut-off value for high inflammation was relatively low. Previous studies, especially those in Europe and America, mostly used 2.0 mg/L or even higher as the cut-off value of high inflammation (28,37,38). However, previous studies have shown that the inflammatory level of East Asian subjects is generally lower than that of European and American individuals (39,40). Therefore, we think it is reasonable to take 1.21 mg/L as the cut-off value to define the high inflammatory state in this study. However, because there are still no consistent opinions, large randomized controlled trials are needed to evaluate the appropriate cut-off value of hsCRP to identify high-risk patients.
In addition, we also found that hsCRP may affect the relationship between HbA1c and the risk of cardiovascular events for patients without diabetes but not patients with diabetes in the sensitivity analysis. We believe that this is due to the decrease in test efficiency caused by insufficient sample size, and this needs to be confirmed by further large sample clinical studies. Another possible reason is that diabetic patients generally have higher levels of inflammation, which may be the reason why there is no interaction between hsCRP and HbA1c in predicting cardiovascular events after PCI in diabetic patients.
Limitations
This study has several limitations. First, as a single-centre observational cohort study, this study may have introduced selection bias. The statistical capacity may be limited because of the short follow-up period. Thus, a randomized controlled trial with a larger sample or a large prospective cohort study is needed to confirm the results of this study. Second, the study has strict inclusion and exclusion criteria, so the results may not be extrapolated to other populations. Third, this study cannot show the effects of dynamic changes in hsCRP on cardiovascular events. Fourth, although we have adjusted the confounding factors that are most likely to affect the results, we cannot rule out the unknown confounding factors that may have a great effect on the result. Finally, the definition of high inflammation needs to be confirmed by larger clinical studies.
Conclusions
This study showed that hsCRP may affect the relationship between HbA1c and the risk of cardiovascular events in patients with ACS after PCI. This finding suggests that the risk of cardiovascular events may be underestimated when only HbA1c is used as a predictor of cardiovascular risk. HbA1c has better predictive value in the absence or low levels of inflammation states represented by hsCRP as a predictor of cardiovascular events.
Acknowledgments
The manuscript was polished by AJE language editing service (Durham, North Carolina, USA).
Funding: This work was supported by the National Natural Science Foundation of China (No. 81970291 and No. 82170344), the Major State Basic Research Development Program of China (No. 2015CB554404).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-78/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-78/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-78/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are responsible for all aspects of the work to ensure that issues related to the accuracy or completeness of any part of the work are properly investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Beijing Anzhen Hospital of Capital Medical University (No. 2015031) and individual consent was waived for the retrospective analysis of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sinning C, Makarova N, Völzke H, et al. Association of glycated hemoglobin A1c levels with cardiovascular outcomes in the general population: results from the BiomarCaRE (Biomarker for Cardiovascular Risk Assessment in Europe) consortium. Cardiovasc Diabetol 2021;20:223. [Crossref] [PubMed]
- Li Y, Li XW, Zhang YH, et al. Prognostic significance of the hemoglobin A1c level in non-diabetic patients undergoing percutaneous coronary intervention: a meta-analysis. Chin Med J (Engl) 2020;133:2229-35. [Crossref] [PubMed]
- Lemesle G, Bonello L, de Labriolle A, et al. Prognostic value of hemoglobin A1C levels in patients with diabetes mellitus undergoing percutaneous coronary intervention with stent implantation. Am J Cardiol 2009;104:41-5. [Crossref] [PubMed]
- Shin D, Ahn J, Cha KS, et al. Impact of initial glycosylated hemoglobin level on cardiovascular outcomes in prediabetic patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis 2016;27:40-6. [Crossref] [PubMed]
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837-53. Erratum in: Lancet 1999 Aug 14;354(9178):602. [Crossref] [PubMed]
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-59. [Crossref] [PubMed]
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560-72. [Crossref] [PubMed]
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129-39. [Crossref] [PubMed]
- Seo JW, Park SB. The Association of Hemoglobin A1c and Fasting Glucose Levels with hs-CRP in Adults Not Diagnosed with Diabetes from the KNHANES, 2017. J Diabetes Res 2021;2021:5585938. [Crossref] [PubMed]
- Seo YH, Shin HY. Relationship between hs-CRP and HbA1c in Diabetes Mellitus Patients: 2015-2017 Korean National Health and Nutrition Examination Survey. Chonnam Med J 2021;57:62-7. [Crossref] [PubMed]
- Fu Y, Wu Y, Liu E. C-reactive protein and cardiovascular disease: From animal studies to the clinic Exp Ther Med 2020;20:1211-9. (Review). [Crossref] [PubMed]
- Sharif S, Van der Graaf Y, Cramer MJ, et al. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc Diabetol 2021;20:220. [Crossref] [PubMed]
- Denegri A, Boriani G. High Sensitivity C-reactive Protein (hsCRP) and its Implications in Cardiovascular Outcomes. Curr Pharm Des 2021;27:263-75. [Crossref] [PubMed]
- Oemrawsingh RM, Cheng JM, Akkerhuis KM, et al. High-sensitivity C-reactive protein predicts 10-year cardiovascular outcome after percutaneous coronary intervention. EuroIntervention 2016;12:345-51. [Crossref] [PubMed]
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289-367. [Crossref] [PubMed]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53. [Crossref] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43:S14-31. [Crossref] [PubMed]
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021-104. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018;138:e618-51. [Crossref] [PubMed]
- Ybarra LF, Rinfret S, Brilakis ES, et al. Definitions and Clinical Trial Design Principles for Coronary Artery Chronic Total Occlusion Therapies: CTO-ARC Consensus Recommendations. Circulation 2021;143:479-500. [Crossref] [PubMed]
- Section of Interventional Cardiology of Chinese Society of Cardiology of Chinese Medical Association. Editorial Board of Chinese Journal of Cardiology. Chinese guideline for percutaneous coronary intervention(2016). Zhonghua Xin Xue Guan Bing Za Zhi 2016;44:382-400.
- Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344-51. [Crossref] [PubMed]
- Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med 1991;90:450-9. [Crossref] [PubMed]
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89. [Crossref] [PubMed]
- Lawler PR, Bhatt DL, Godoy LC, et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J 2021;42:113-31. [PubMed]
- Zeller J, Bogner B, McFadyen JD, et al. Transitional changes in the structure of C-reactive protein create highly pro-inflammatory molecules: Therapeutic implications for cardiovascular diseases. Pharmacol Ther 2022;235:108165. [Crossref] [PubMed]
- Ridker PM. Clinician's Guide to Reducing Inflammation to Reduce Atherothrombotic Risk: JACC Review Topic of the Week. J Am Coll Cardiol 2018;72:3320-31. [Crossref] [PubMed]
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017;377:1119-31. [Crossref] [PubMed]
- Everett BM, Donath MY, Pradhan AD, et al. Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J Am Coll Cardiol 2018;71:2392-401. [Crossref] [PubMed]
- Ebrahimi M, Heidari-Bakavoli AR, Shoeibi S, et al. Association of Serum hs-CRP Levels With the Presence of Obesity, Diabetes Mellitus, and Other Cardiovascular Risk Factors. J Clin Lab Anal 2016;30:672-6. [Crossref] [PubMed]
- Fizelova M, Jauhiainen R, Kangas AJ, et al. Differential Associations of Inflammatory Markers With Insulin Sensitivity and Secretion: The Prospective METSIM Study. J Clin Endocrinol Metab 2017;102:3600-9. [Crossref] [PubMed]
- Chen J, Wildman RP, Hamm LL, et al. Association between inflammation and insulin resistance in U.S. nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2960-5. [Crossref] [PubMed]
- Kianoush S, Yakoob MY, Al-Rifai M, et al. Associations of Cigarette Smoking With Subclinical Inflammation and Atherosclerosis: ELSA-Brasil (The Brazilian Longitudinal Study of Adult Health). J Am Heart Assoc 2017;6:005088. [Crossref] [PubMed]
- Iakunchykova O, Averina M, Kudryavtsev AV, et al. Evidence for a Direct Harmful Effect of Alcohol on Myocardial Health: A Large Cross-Sectional Study of Consumption Patterns and Cardiovascular Disease Risk Biomarkers From Northwest Russia, 2015 to 2017. J Am Heart Assoc 2020;9:e014491. [Crossref] [PubMed]
- Schillinger M, Exner M, Amighi J, et al. Joint effects of C-reactive protein and glycated hemoglobin in predicting future cardiovascular events of patients with advanced atherosclerosis. Circulation 2003;108:2323-8. [Crossref] [PubMed]
- Sander D, Schulze-Horn C, Bickel H, et al. Combined effects of hemoglobin A1c and C-reactive protein on the progression of subclinical carotid atherosclerosis: the INVADE study. Stroke 2006;37:351-7. [Crossref] [PubMed]
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195-207. [Crossref] [PubMed]
- Arroyo-Espliguero R, Avanzas P, Quiles J, et al. Predictive value of coronary artery stenoses and C-reactive protein levels in patients with stable coronary artery disease. Atherosclerosis 2009;204:239-43. [Crossref] [PubMed]
- Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem 2008;54:1027-37. [Crossref] [PubMed]
- Lear SA, Chen MM, Birmingham CL, et al. The relationship between simple anthropometric indices and C-reactive protein: ethnic and gender differences. Metabolism 2003;52:1542-6. [Crossref] [PubMed]


