
Long-term course of pulmonary arterial hypertension in adults with congenital heart disease under targeted therapy: a retrospective analysis of a single tertiary center
Introduction
Congenital heart disease (CHD) is the most common single organ malformation, with a prevalence of approximately 9 per 1,000 live births (1,2). Nowadays, in the industrialized world, up to 97% of the affected patients reach adulthood (3), resulting in a large number of adults with congenital heart disease (ACHD). The number of ACHD, in fact, exceeds that of children with CHD (4,5). These patients present with several unique challenges for the attending physician or even specialist (6). Independent of their repair status, all ACHD are considered to have chronic heart disease and face a wide range of cardiac and extra-cardiac comorbidities (7-9) requiring lifelong follow-up, preferably by an ACHD specialist (10-12).
Even decades after successful primary repair of the underlying heart defect, pulmonary arterial hypertension (PAH) can persist, recur, or develop (13). With an estimated prevalence of PAH in ACHD ranging from 3.2% to 28%, PAH, along with pulmonary vascular disease (PVD) in general, is one of the most serious complications in ACHD, causing significant morbidity and mortality (14-17).
Invasive measurement is essential for PAH diagnosis and therapeutic management, and the European Society of Cardiology (ESC), in their ‘2020 ESC Guidelines for the management of adult congenital heart disease’, expressly recommends cardiac catheterization in PAH-CHD for “major decisions, such as start and follow-up of vasodilator therapy, pregnancy, or surgery” (12). The definition of pulmonary hypertension (PH) has been updated in 2018, and now, PAH or precapillary-type PH, which is most common in PAH-CHD, is defined as an elevated mean pulmonary artery pressure (mPAP) of ≥20 mmHg at rest in combination with a pulmonary vascular resistance (PVR) of ≥3 Wood units and a pulmonary artery wedge pressure ≤15 mmHg (18).
The clinical classification of PH comprises four groups: Eisenmenger syndrome (ES), PAH associated with a predominant systemic-to-pulmonary shunt, PAH associated with a small defect, and PAH associated with a repaired defect (19). Of these, ES is the most extreme form of PAH in CHD. It is defined by a reversal of the primary left-right shunt to a right-left shunt caused by an increase in PVR and a pulmonary artery pressure exceeding or equivalent to systemic arterial pressure. Affected patients clinically present with progredient cyanosis and often with cardiac or extra-cardiac complications (20). Until recently, the prognosis for these patients was disastrous, and even today, life expectation remains greatly reduced (21,22).
Meanwhile, targeted medical therapy for PAH has been available for more than 20 years. Nevertheless, in the special subgroup of PAH-CHD, there are only limited data, from clinical trials and international registries such as COMPERA (23), on the course of disease under targeted therapy. In particular, comprehensive data on the choice of the appropriate targeted PAH medication and on indications for treatment modification or therapy escalation are lacking.
The aim of the present study was to retrospectively analyze the medium-term outcome of patients with PAH-CHD receiving targeted PAH therapy. In addition to clinical parameters and outcome data, the focus was on the analysis of targeted PAH therapy regimens. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-266/rc).
Methods
Study design
The current study is a retrospective, cross-sectional analysis of ACHD with PAH cared for at the German Heart Center Munich between 2004 and 2020. A focused search of our institution’s database at the German Heart Center Munich was undertaken to identify patients with a diagnosis related to the following keywords: congenital heart disease, pulmonary hypertension/pulmonary arterial hypertension, pulmonary vascular disease, Eisenmenger syndrome (ES), Fontan/total cavopulmonary connection (TCPC); and receiving any of the following targeted PAH therapies: endothelin receptor antagonists (ERA), phosphodiesterase type 5 inhibitors (PDE5 inhibitors), soluble guanylyl cyclase stimulators (sGC-S), tyrosine kinase inhibitors (TKI), or prostacyclin analogs (PCA). Inclusion criteria were diagnosis of CHD independent of repair status, a minimum age of 18 years at last observation, and targeted treatment with PAH medication at any timepoint during the study interval.
For the study, PAH was defined as mPAP ≥25 mmHg at rest, as this was the valid definition at the time of observation onset (24). Also included were patients with any type of Fontan circulation, independent of their mPAP or transpulmonary gradient (TPG), if they received targeted PAH therapy for “failing Fontan” at any time during the study period. Patients who did not meet the above-mentioned inclusion criteria were excluded from the study.
For structured analysis, patients’ cardiac defects were classified anatomically-pathophysiologically as pre-tricuspid, post-tricuspid, or having complex anatomy, depending on the location of the shunt.
In addition, based on the 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension (19) and according to hemodynamic and clinical considerations of their underlying CHD, patients were assigned to one of the following subgroups:
- ES;
- Prevalent systemic-to-pulmonary shunt; comprising patients possibly developing ES, but as of yet without shunt reversal;
- Patients with an anatomically or functionally repaired heart defect;
- Severe PVD in the context of complex CHD;
- Patients with Fontan circulation.
To note, we added the subgroups of ‘severe PVD in complex CHD’ and ‘Fontan patients’ to better allocate our study population. This is justified by the fact that anatomical-pathophysiological classification comprises a nonhomogeneous group of heart defects under ‘complex anatomy’. We modified the clinical ESC classification somewhat, because by definition ES implies a reversal of a primary left-right shunting; therefore, some complex defects with uncorrected univentricular physiology or collateralized lung perfusion can formally not develop ES. Such patients are classified as ‘severe PVD in complex CHD’ in the present study.
Study parameters
Patient visits were included in the analysis if one or more of the following events occurred within the study period: initial or final patient visit; change in targeted PAH therapy; clinical worsening/event (e.g., signs and symptoms of heart failure, edema, progressive dyspnea, progredient cyanosis, syncope, hemoptysis, decrease in exercise capacity); hospitalization due to or related to PAH; cardiac catheterization; intervention/surgery targeting the heart defect; death.
The following were obtained from patient records: demographic data, underlying heart defect, status of cardiac repair, cardiac catheterization data, functional class, exercise tests (6-minute-walk-distance, spiroergometry), laboratory values, medical therapy, and clinical symptoms/complications.
All changes in targeted PAH therapy that occurred during the study period were recorded, and the reason for therapy modification was evaluated.
Consent to participate
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice guidelines. The study was in accordance to the local data privacy regulation (BayKrG-27) and was approved by the institutional ethics board (2489/09 S). All participants gave written informed consent and agreed to an anonymous publication of their data. Guidelines on good pharmacoepidemiological practice and data protection guidelines were followed.
Statistical analysis
Metric parameters are given as median with interquartile range (IQR). Categorical parameters are given as relative or absolute frequency. Survival rates were visualized by Kaplan-Meier curves and compared by log-rank test. Central tendencies within subgroups were analyzed using Wilcoxon test. Statistical significance was assumed for P<0.05, although due to the retrospective, explorative character of this study, statistical findings must be put into perspective. Statistical analysis was performed using IBM SPSS Statistics Version 28 (IBM Corp., Armonk, NY, USA).
Results
Study population
A total of 103 PAH-ACHD patients undergoing targeted medical PAH therapy were included. The observational period was up to 14 years (median 6.2, IQR 3.3; 10.1 years). The study population was predominantly female (n=68, 66%), and the median age at study entrance was 36.0 years (IQR 25.0; 46.0 years), and age at last follow-up was 44.5 years (IQR 32.0; 51.0 years) (Tables 1-4).
Table 1
| Demographics | Overall | ES | Prevalent systemic-to-pulmonary shunt | Repaired defect (anatomically/functionally) | Severe PVD in complex CHD | Fontan circulation |
|---|---|---|---|---|---|---|
| Overall (%) | 103 (100.0) | 47 (45.6) | 4 (3.9) | 20 (19.4) | 21 (20.4) | 11 (10.7) |
| Female (%) | 68 (66.0) | 33 (70.2) | 3 (75.0) | 14 (70.0) | 12 (57.1) | 6 (54.5) |
| BMI (kg/m²) at start | 23.4 (20.3–26.5) | 23.6 (21.0–27.0) | 25.6 (21.4–26.6) | 23.9 (20.5–28.2) | 22.6 (20.1–26.4) | 21.6 (18.9–23.0) |
| Down syndrome (%) | 18 (17.5) | 17 (36.2) | 0 | 1 (5.0) | 0 | 0 |
| Age (years): start; end | 36.0 (25.0–46.0) | 35.0 (23.0–46.0) | 30.0 (25.8–49.3) | 40.0 (29.3–52.8) | 37.0 (30.5–42.0) | 33.0 (20.0–43.0) |
| 44.5 (32.0–51.0) | 45.0 (29.8–50.5) | 36.0 (30.5–55.0) | 48.5 (31.8–55.3) | 44.0 (33.0–52.0) | 34.0 (30.0–48.0) |
Values are presented as absolute numbers and relative frequency or as median with interquartile range. ES, Eisenmenger syndrome; PVD, pulmonary vascular disease; CHD, congenital heart disease; BMI, body mass index.
Table 2
| Clinical parameters | Overall | ES | Prevalent systemic-to-pulmonary shunt | Repaired defect (anatomically/functionally) | Severe PVD in complex CHD | Fontan circulation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial visit | Final follow-up | Initial visit | Final follow-up | Initial visit | Final follow-up | Initial visit | Final follow-up | Initial visit | Final follow-up | Initial visit | Final follow-up | ||||||
| Functional class | |||||||||||||||||
| I/II | 38 | 41 | 11 | 17 | 2 | 2 | 8 | 12 | 9 | 5 | 8 | 5 | |||||
| III | 53 | 44 | 29 | 24 | 2 | 2 | 9 | 5 | 10 | 10 | 3 | 3 | |||||
| IV | 8 | 9 | 5 | 3 | 0 | 0 | 2 | 0 | 1 | 4 | 0 | 2 | |||||
| Ø | 4 | 6 | 2 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 1 | |||||
| SpO2 (%) | 84.0 (78.3–92.0) | 83.0 (78.0–93.0) | 80.0 (74.0–84.0) | 80.0 (75.8–84.0) | 92.0 (80.0 to –) | 94.5 (83.5–98.8) | 94.5 (92.0–98.0) | 94.5 (93.0–96.8) | 80.5 (76.3–86.5) | 79.0 (71.5–82.5) | 91.5 (87.0–96.0) | 94 (80.0–95.0) | |||||
| PeakVO2 (mL/kg/min) | 13.00 (11.40–16.80) | 13.65 (12.23–17.55) | 12.40 (10.00–15.40) | 15.65 (13.40–18.05) | 11.30 (11.30–11.30) | 13.20 (12.50 to –) | 14.95 (12.18–18.60) | 17.20 (11.40 to –) | 12.70 (11.50–14.50) | 8.00 (8.00–8.00) | 16.35 (13.43–26.03) | 13.40 (13.40–13.40) | |||||
| 6 MWD (m) | 371.5 (300.0–444.8) | 390.0 (315.0–415.5) | 305.3 (234.8–420.3) | 397.0 (277.5–464.8) | 360.0 (360.0–360.0) | 420.0 (390.0 to –) | 378.0 (371.5–470.0) | 300.0 (300.0–300.0) | 420.0 (360.0–472.5) | 345.0 (320.0 to –) | 524.0 (524.0–524.0) | – | |||||
| NT-proBNP (pg/mL) | 427.0 (213.0–1,430.0) | 787.0 (295.5–2,040.0) | 409.0 (224.0–1,245.0) | 740.0 (349.0–1,565.0) | 705.0 (502.0 to –) | 359.0 (95.0 to –) | 292.0 (162.0–1,485.0) | 680.5 (143.8–2,755.0) | 681.0 (231.5–2,400.0) | 2650.0 (666.0–7,010.0) | 404.0 (157.0–1,640.0) | 259.5 (113.5–1,841.0) | |||||
Values are presented as absolute numbers or as median with interquartile range. ES, Eisenmenger syndrome; PVD, pulmonary vascular disease; CHD, congenital heart disease; SpO2, peripheral oxygen saturation; peakVO2, peak oxygen uptake; 6MWD, 6-minute walk distance; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Table 3
| Cardiac catheterization at therapy start | Overall | Overall excl. Fontan | ES | Prevalent systemic-to-pulmonary shunt | Repaired defect (anatomically/functionally) | Severe PVD in complex CHD | Fontan circulation |
|---|---|---|---|---|---|---|---|
| mPAP (mmHg) | 57.0 (46.0–77.0) | 63.0 (48.5–79.0) | 81.0 (59.0–85.0) | 61.5 (46.0 to –) | 48.0 (39.3–56.5) | 66.0 (53.0–75.0) | 13.5 (12.0–16.3) |
| PVR (indexed Wood units) | 21.3 (11.8–29.9) | 21.7 (15.9–31.6) | 30.0 (21.7–44.6) | 28.6 (28.6–28.6) | 15.0 (7.5–16.5) | 25.4 (17.6–29.9) | 2.2 (1.9–3.1) |
Values are presented as median with interquartile range. ES, Eisenmenger syndrome; PVD, pulmonary vascular disease; CHD, congenital heart disease; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance.
Table 4
| Frequency of clinical complications and death | Overall | ES | Prevalent systemic-to-pulmonary shunt | Repaired defect (anatomically/functionally) | Severe PVD in complex CHD | Fontan circulation |
|---|---|---|---|---|---|---|
| Decompensation (%) | 29 (28.2) | 10 (21.3) | 0 (0) | 3 (15.0) | 10 (47.6) | 6 (54.5) |
| Progredient edema (%) | 42 (40.8) | 16 (34.0) | 1 (25.0) | 7 (35.0) | 12 (57.1) | 6 (54.5) |
| Reduced exercise capacity (%) | 50 (48.5) | 23 (48.9) | 1 (25.0) | 5 (25.0) | 14 (66.7) | 7 (63.6) |
| Progredient cyanosis (%) | 18 (17.5) | 8 (17.0) | 1 (25.0) | 0 (0) | 5 (23.8) | 4 (36.4) |
| Progredient dyspnea (%) | 40 (38.8) | 20 (42.6) | 1 (25.0) | 3 (15.0) | 10 (47.6) | 6 (54.5) |
| Syncope (%) | 12 (11.7) | 8 (17.0) | 1 (25.0) | 1 (5.0) | 1 (4.8) | 1 (9.1) |
| Hemoptysis (%) | 5 (4.9) | 3 (6.4) | 0 (0) | 1 (5.0) | 1 (4.8) | 0 (0) |
| Death (%) | 25 (24.3) | 9 (19.1) | 0 (0) | 2 (10.0) | 9 (42.9) | 5 (45.5) |
| Female (% of deaths in subgroup) | 16 (64.0) | 7 (77.8) | 0 (0) | 1 (50.0) | 6 (66.7) | 2 (40.0) |
| Down syndrome (% of deaths in subgroup) | 6 (24.0) | 6 (66.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Clinical complications are presented as number of patients with percentages given within subgroups. Relative frequency of death is presented as a percentage value representing the total percentage of death within each subgroup, with additional values provided for the percentages of deaths in each subgroup occurring in female patients and down syndrome patients. ES, Eisenmenger syndrome; PVD, pulmonary vascular disease; CHD, congenital heart disease.
Of a total of 1055 consultations, 496 visits of interest, as defined above, were identified and included in the analysis. The number of patients with an initial visit was n=103, the number of patients with at least one follow-up examination was n=100, and 25 patients (24.3%) died during the study period. Detailed information on the cause of death was available for 8 of the patients, a brief external message for 7, and no information at all for 10. To avoid a bias from unintended exclusion of the 17 patients who died at the end of the study period due to missing data, we implemented patients’ data from the time point the patient was last seen alive.
All included patients (n=103) had severe disease complexity according to the 2020 ESC Guidelines for the management of ACHD (12). Three patients were able to be downgraded to moderate complexity during follow-up after repair and a consecutive decrease of mPAP, even allowing termination of PH-targeted therapy.
Underlying congenital heart defects
The leading cardiac anomaly was classified according to the anatomical-pathophysiological classification of each patient’s case of CHD (25). Most patients were assigned to the group of complex anomalies (52.4%, n=54). The most common CHD was VSD with n=27 (26.2%), followed by ASD and AVSD with n=15 (14.6%) each (Table 5). Many patients had several coexisting heart defects.
Table 5
| Variables | n | % |
|---|---|---|
| Pre-tricuspid shunts | 16 | 15.5 |
| ASD | 15 | 14.6 |
| APVR | 1 | 1.0 |
| Post-tricuspid shunts | 33 | 32.0 |
| VSD | 27 | 26.2 |
| PDA | 5 | 4.9 |
| AP-window | 1 | 1.0 |
| Complex anomalies | 54 | 52.4 |
| AVSD | 15 | 14.6 |
| Tetralogy of Fallot | 1 | 1.0 |
| Pulmonary atresia with VSD | 10 | 9.7 |
| Tricuspid atresia with VSD | 7 | 6.8 |
| Univentricular heart | 1 | 1.0 |
| DORV | 4 | 3.9 |
| DILV | 6 | 5.8 |
| ccTGA with VSD | 3 | 2.9 |
| TGA with VSD | 5 | 4.9 |
| TGA with intact ventricular septum | 2 | 1.9 |
| Overall | 103 | 100 |
Leading congenital cardiac anomaly according to the anatomical-pathophysiological classification of the ESC/ERS Guidelines from 2009 (25). ASD, atrial septal defect; APVR, anomalous pulmonary venous return; VSD, ventricular septal defect; PDA, patent ductus arteriosus; AP-window, aortopulmonary window; AVSD, atrioventricular septal defect; DORV, double outlet right ventricle; DILV, double inlet left ventricle; ccTGA, congenitally corrected transposition of the great arteries; TGA, transposition of the great arteries.
The distribution of PAH-CHD patients with regard to hemodynamic considerations according to the modified clinical ESC classification was as follows:
- ES (n=47, 45.6%);
- Prevalent systemic-to-pulmonary shunt (n=4, 3.9%);
- Coincidental/small defects (n=0);
- Post-defect correction (n=20, 19.4%);
- Severe PVD in complex CHD (n=21, 20.4%);
- Fontan circulation (n=11, 10.7%).
Eight patients (median age at surgery 55.2 years, IQR 31.6; 69.3 years) underwent operative or interventional repair during the study period; another two patients had completion of TCPC (Table 6).
Table 6
| Type of operation/intervention | n | Age in years |
|---|---|---|
| ASD closure | 5 | 51, 53, 61, 72, 79 |
| VSD closure | 2 | 25, 57 |
| AVSD repair | 1 | 22 |
| TCPC completion | 2 | 14, 43 |
Number, age, and type of operation/intervention of patients who underwent surgery during the study period. ASD, atrial septal defect; VSD, ventricular septal defect; AVSD, atrioventricular septal defect; TCPC, total cavopulmonary connection.
Clinical parameters, events, and survival
Important clinical parameters obtained during the follow-up examinations included functional class, oxygen saturation (SpO2), exercise test results, and n-terminal pro brain natriuretic peptide (NT-proBNP) level (Tables 1-4). At baseline, most patients with ES or with severe PVD (n=45; 66.2%) were in functional class III or IV.
Details on clinical events are shown in Table 4. Cardiopulmonary decompensation may comprise several of the below-listed symptoms. The highest complication rates were seen in the subgroups of patients with severe PVD in complex CHD and failing Fontan patients. Notably, syncope was overrepresented in ES patients, while patients with repaired CHD had the lowest frequency of symptoms, in contrast to their proportion in the total study cohort.
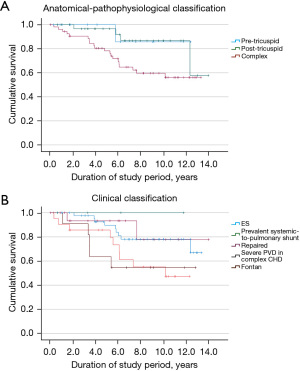
A total of 25 patients (24.3%) died during the study period, with a median age at death of 44.0 (IQR 36.0; 51.0) years. Survival was lowest in patients with severe PVD associated with complex CHD, followed by Fontan patients under PAH-targeted therapy. The highest survival rates were seen in patients with prevalent systemic-to-pulmonary shunt, followed by patients with repaired CHD.
Survival curves as a function of anatomical-pathophysiological classification are shown in Figure 1A. Survival curves as a function of modified clinical ESC classification are shown in Figure 1B.
Eight (32%) of the deaths were attributable to heart failure, six (24%) were directly related to the underlying condition (e.g., lung bleeding, intracerebral abscess), one (4%) was due to extra-cardiac reason (traffic accident), and in ten (40%), the cause of death was unknown. Reasons for death due to heart failure were cardiac decompensation (n=4), cardiac arrest (n=2), myocardial infarction (n=1), and death after heart transplantation (n=1).
There was no relevant difference in survival according to patient sex (P=0.924).
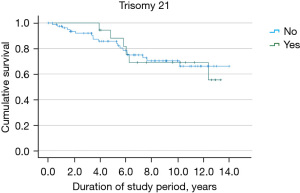
Trisomy 21
Six (24%) of the deceased PAH-CHD patients had trisomy 21 (Down syndrome). There was no relevant difference between survival of the trisomy 21 patients and patients without trisomy 21 (P=0.996) (Figure 2). The median age at death in the trisomy 21 subgroup was 44.5 (IQR 24.3; 50.0) years, compared with a median of 44.0 (IQR 38.0; 51.0) years in patients without trisomy 21.
PAH-targeted medical therapy
During the study period, targeted PAH therapy was initiated in 73 cases. Of these, 52 (71.2%) underwent cardiac catheterization before targeted therapy was initiated. Catheterization data (mPAP and PVR) are listed in Tables 1-4.
A total of 14 patients (13.6%) were pre-treated with calcium channel blockers (CCB); however, CCB therapy was suspended in 12 patients when modern targeted PAH therapy became available. In one case, it was stopped during the study period, and in one case it is still ongoing.
At baseline, most patients (n=86, 83.5%) received monotherapy and only 14 patients (13.6%) were on combination therapy. At the end of the study period, monotherapy was still the most common strategy, but combination therapy (double or triple) was applied in 41 (41.0%) patients (Figure 3).
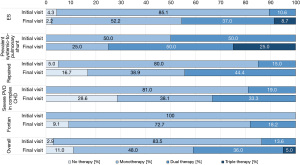
At study entry, the most common targeted PAH medication was PDE5 inhibitors, taken by 62 patients (60.2%), and the second most common was ERA, taken by 50 patients (48.5%). Combination therapy with PDE5 inhibitor + ERA at baseline was used for 12 patients (11.7%).
At the final visit, PDE5 inhibitors were still the most frequently used (n=67, 67.0%), followed by ERA (n=58, 58.0%). It should, however, be noted that more patients at this time had combination therapy with PDE5 inhibitors and ERA (n=37, 37.0%) (Figure 4).
During the study period, a total of 249 changes in targeted PAH therapy were recorded. In 73 of these cases, targeted PAH medication was initiated. In 45 cases, targeted PAH therapy was escalated from monotherapy to dual therapy, or from dual therapy to triple therapy. In 49 cases, dose scaling to maximum dose was implemented. In 19 cases, a therapy adaptation was required due to intolerance or side effects. In 18 cases, no further information was provided, and in 7 cases, medication adjustment took place at an external facility without further information being reported (Table 7).
Table 7
| Variables | n | % |
|---|---|---|
| Initiation of targeted therapy | 73 | 100 |
| After cardiac catheterization | 50 | 68.5 |
| After cardiac catheterization with simultaneous escalation (from CCB pretreatment) | 2 | 2.7 |
| Clinical decision | 14 | 19.2 |
| Not further specified | 7 | 9.6 |
| Therapy escalation | 45 | 100 |
| Clinical decision | 22 | 48.9 |
| After cardiac catheterization | 11 | 24.4 |
| Not further specified | 8 | 17.8 |
| For optimization of situation | 4 | 8.9 |
| Therapy change | 61 | 100 |
| Insufficient effect/clinical worsening under therapy | 15 | 24.6 |
| Side effect | 13 | 21.3 |
| Other | 9 | 14.8 |
| Not further specified | 6 | 9.8 |
| Another drug more suitable | 6 | 9.8 |
| Intolerance | 4 | 6.6 |
| Not further necessary | 4 | 6.6 |
| Patient’s wish | 2 | 3.3 |
| Changed at external institution, not further specified | 2 | 3.3 |
| Dose adjustments | 70 | 100 |
| Adjustment to targeted dose | 49 | 70.0 |
| Not further specified | 12 | 17.1 |
| Changed at external institution, not further specified | 5 | 7.1 |
| Intolerance | 2 | 2.9 |
| Patient’s wish | 2 | 2.9 |
| Overall | 249 |
Reasons for change in the targeted PAH treatment during the study period. Percentages are given for each subgroup. PAH, pulmonary arterial hypertension; CCB, calcium channel blocker.
Considering all treatment modifications, including therapy initiation and targeted dose adjustments, the median number of therapy adaptations during the study period was 2.0 (IQR 1.0; 3.0), with a median interval between therapy adjustments of 0.9 (IQR 0.3; 3.0) years.
Excluding targeted dose adjustments, which are a logical consequence after therapy initiation, patients had a median of 2.0 (IQR 1.0; 3.0) changes in targeted PAH-medication therapy with a median interval of 1.3 (IQR 0.5; 4.2) years.
In 47 cases, a rapid change of therapy was noted, meaning that therapy had to be readjusted within one year. We reported these rapid therapy changes under exclusion of adjustments to the targeted dose. The median interval of these rapid changes in therapy was 0.39 (IQR 0.22; 0.74) years.
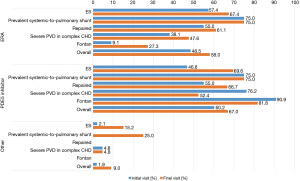
The intensity of targeted PAH therapy differed between deceased patients and patients still alive at the end of the study. In the entire study cohort, there was a clear trend towards targeted PAH combination therapy. In contrast, deceased patients were disproportionately often on monotherapy or received no targeted therapy (Figure 5).
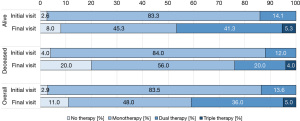
The Wilcoxon test indicated a significant difference with respect to the therapeutic regimen between the first and the last observation in patients alive at the end of the study (P<0.001), while the corresponding analysis of deceased patients showed no significant difference (P=1.000).
Discussion
To our knowledge, this is the first study to describe in detail the contemporary treatment of adults with CHD using targeted PAH medication over more than a decade. This is of paramount importance because of the lack of data about the optimal timing and type of targeted treatment, treatment escalation, optimal combination therapy, and objective parameters for therapeutic decisions.
Demographics, clinical course, and mortality
ACHD who received targeted PAH therapy were included in the study. This cohort comprises ACHD with very severe disease patterns, a status that was reflected by the relevant morbidity and mortality observed during the study.
The highest complication rates occurred in the subgroup of patients on targeted therapy for severe PVD associated with complex cardiac malformations and in the subgroup of failing Fontan patients.
For decades, PAH-CHD was poorly treatable, and outcomes were unfavorable, particularly in cyanotic patients with ES (22). In Paul Wood’s original landmark publication on ES (in 1958), the mean age at death was 33 years for shunts at the ventricular or aortopulmonary level and 36 years for atrial septal defects (26). Studies from the 1950s to as recently as the 1990s have reported a mean age at death between 30 and 37 years in ES patients (27,28). It was not until the introduction of targeted PAH therapy that morbidity was significantly reduced and survival was significantly increased in ES patients (29). Accordingly, in the current study, patients with a median age of 35.0 (IQR 23.0; 46.0) years were available for inclusion and it was possible to stabilize most of the patients who were susceptible to complications and comorbidities (28,30,31). This is particularly evident in our observation of stabilized functional class or, in ES patients, even an improved functional class. Also observed were unchanged SpO2 values over time, which we interpret positively, considering historic data on spontaneous disease progression. In the present cohort, 10-year survival under targeted PAH treatment in ES was 78.0%.
The highest morbidity and mortality rates were seen in patients with severe PVD in complex CHD, paralleling the results of a study by Diller et al. (21) and findings from the COMPERA registry (23). Compared with the ES group, baseline mPAP and PVR at therapy start was lower in these patients, whereas SpO2 was similar. Not only was survival remarkably lower in the group with severe PVD and complex CHD but also deterioration in functional class and NT-proBNP values was observed despite similar therapy strategy at baseline. SpO2 remained almost unchanged during the course of the study.
One special group of patients was managed during the study period according to the ‘treat and repair’ strategy. In this group, very favorable outcomes were seen. In three patients, targeted PAH therapy was even able to be stopped after repair. There is reasonable assumption that this is a consequence of careful patient selection and successful pre-treatment with targeted PAH therapy. A similar outcome has already been observed in other rare cases (32-34). It should be noted, however, that these patients have an even greater need for continuous, experienced medical follow-up than PAH patients in general.
The included group of patients with failing Fontan circulation was small. They showed, unfortunately, a high mortality, reflecting their fragility.
Regarding mortality across groups in the present study, it is noteworthy that deceased patients received less aggressive targeted PAH therapy (P=1.000) than patients who were alive at the end of the observation period (P<0.001). At final follow-up, they were less likely to receive combination therapy (46.7% vs. 24.0%), although the targeted drug regimen and clinical parameters were similar at enrollment.
There was no difference in survival between female and male patients. Moreover, the survival of patients with trisomy 21 in this study was comparable to that of the remaining patients. In the literature, the rate of patients with trisomy 21 and ES receiving targeted PAH-treatment is considerably lower than that of non-syndromic Eisenmenger patients (35), although it has been shown that trisomy 21 patients respond similarly to targeted PAH therapy. Randomized data on this are even sparser than in the overall PAH-CHD cohort (36). In the present study, the frequency of changes in PAH targeted therapy (17.7%) in the trisomy 21 group was equivalent to the corresponding share of trisomy 21 patients in the study group (17.5%). This result serves to highlight the importance of looking closely at the treatment received by trisomy 21 patients before drawing any conclusions from future differences in mortality associated with trisomy 21.
Targeted PAH therapy management
Data on optimal targeted treatment strategy in CHD-PAH patients are limited, especially regarding the outcome in patients with complex CHD. Several small studies have shown that targeted PAH treatment improves clinical course in ES (29,37,38). Although only limited data were available, the use of targeted PAH medication has become widespread in PAH-CHD. The therapeutic approach has even shifted in the last decade towards more aggressive use of targeted PAH medication and combination therapy, along with recognition of the effectiveness of combination therapy (39,40). In one small study, initial combination therapy in PAH-CHD patients was found to improve clinical course compared with monotherapy (41). In another study, combination therapy using bosentan and sildenafil resulted in clinical improvement in CHD patients after failure of bosentan monotherapy (42). Another recent study showed an improvement in functional class and mPAP in an upfront combination therapy of sildenafil and ambrisentan (43). In our study population, we also found a shift towards more aggressive targeted PAH treatment over time. At baseline, most patients at our center received PDE5-inhibitor monotherapy. At study end, patients with a combination therapy of PDE5 inhibitors and ERA exceeded the number of patients taking either of these drugs in monotherapy.
The most extensive data on the use of PAH-specific drugs in ES patients are available for ERA, which have demonstrated a clear reduction of morbidity and mortality (44). Accordingly, in larger PAH registry studies such as COMPERA, more patients were on ERA than on PDE5 inhibitors at latest observation (39). The slight divergence in our study, in which PDE5 inhibitors remained the most common medication, may be due to the fact that some of our patients were minors and already on medication before study inclusion. They were cared for by pediatric cardiologists at our institution, for whom PDE5 inhibitors, such as sildenafil, have long been the drug of choice for children and adolescents. Besides, some patients were started on a PDE5 inhibitors before the BREATHE-5 study (45) was published, and remained on this medication with no changes and with good tolerability.
Although PAH-targeted therapy is beneficial for many PAH-CHD patients, questions remain about the optimal timing and type of therapy escalation, introduction of combination therapy, and, in particular, the best objective parameters for therapeutic decision making.
An early general treatment algorithm for PAH-CHD patients was proposed in 2008 by Galie et al. (46). However, even in the 2015 ESC PAH guidelines, recommendations are vague except for recommendations for CHD patients in functional class III/IV. Criteria for therapy initiation, as well as the use of combination therapy, are solely considered for ES patients (19). In the 2020 ESC Guidelines for the management of ACHD, recommendations were extended only by adding a treatment strategy for patients with repaired simple lesions and pre-capillary PAH, recommending that high-risk subgroups should be treated with initial combination therapy (12). A less aggressive approach is recommended for uncorrected ES patients with reduced exercise capacity: these patients should initially receive monotherapy with an ERA, followed by combination therapy if they fail to improve. For important therapeutic decisions, cardiac catheterization is recommended, but the threshold for invasive assessment is higher in patients with ES as it can be hazardous, and because catheterization data are often not required for guiding therapeutic interventions over time (12).
PAH-targeted therapy for PAH-CHD patients with complex CHD (e.g., patients with unrepaired single ventricle physiology, pulmonary perfusion by aortopulmonary collaterals, or segmental PAH in CHD), is beyond the scope of all current guidelines. Regarding such complicated situations, there are only very few reports on mostly small case series (47-50).
At our institution, current therapy strategies for ES patients were expanded to selected patients with complex CHD, with the choice of which PAH-targeted medication to implement based on clinical or invasive considerations. However, attention must be paid if there is no improvement with targeted PAH therapy or especially if signs of clinical deterioration occur, as this may be due to volume loading from increased pulmonary blood flow secondary to decreasing PVR.
During the study period, the vast majority of ES patients stayed on targeted PAH medication. In contrast, for patients with complex CHD with severe PVD, the treatment regimen split during the course of the study. At the time of study inclusion, 0%/81%/19% received no/mono/combined therapy, compared with 28.6%/38.1%/33.3% at the final follow-up visit, reflecting a much more variable response to targeted PAH therapy compared to the remaining CHD-PAH patients.
In the present study, we analyzed 249 patient visits with modifications of PAH-targeted therapy. In 71.2% of the therapy starts, the decision to initiate targeted PAH therapy was made after invasive diagnostics, while in 19.2%, it was solely a clinical decision. Some of the latter patients had received invasive measurements in the past but targeted therapy was not initiated until later in the course for clinical reasons; 8.2% received dual therapy from the beginning of their targeted treatment.
Therapy changes, including change in drug class, deescalation, discontinuation of single drugs or all PAH therapy and combination of these changes as part of combination therapy, were made in 61 cases. These changes were mostly attributable to side effects/intolerance (n=17, 27.9%) or inadequate efficacy/clinical worsening with therapy (n=15, 24.6%).
Therapy escalation by adding another PAH-specific drug was documented in 45 cases during follow-up. The decisions were mainly driven by clinical considerations (n=26, 57.8%); only 11 (24.4%) patients received cardiac catheterization in this situation (in consideration of the high periprocedural risk).
Limitations
The present study recruited a remarkably large sample of patients with PAH-CHD and focused on specific details regarding targeted PAH treatment in ACHD not described before in detail. However, some limitations must be considered when interpreting the current results.
The retrospective character of the study and the small number of cases, especially when performing subgroup analysis, are obvious limitations. Data could only be analyzed as documented at the time of the visit. As there was no defined follow-up interval, patients with severe disease were seen more often. Lack of documented data was greater at the end of the study than at the beginning, most likely due to the fact that the study period began in most cases with an event of interest such as a change in therapy or clinical worsening, which necessitates a full investigation. In contrast, the last obtained visit often was a routine follow-up, sometimes even at an external institution.
The sample of patients seen at a tertiary care center does not represent the typical population of patients with CHD seen by a general practitioner, internist, or by a general cardiologist. The prevalence of more severe forms of PAH-CHD in these institutions is likely to be higher than in community-based hospitals and even in departments of cardiology.
Lastly, the presented data derive solely from patients living in Germany. The extent of generalizability of the conclusions and transmission to patients living in other countries or different culture groups is debatable.
Conclusions and future implication
Optimal decision-making for targeted therapy in PAH-CHD remains challenging, and many questions remain unexplored.
The present retrospective analysis examines clinical course, outcome, and treatment in a comparatively large group of patients with PAH-CHD receiving targeted PAH therapy. Considering the recommendations from the literature and our experience with CHD-PAH patients, targeted PAH combination therapy is usually well tolerated and contributes to the stabilization of clinical course in this high-risk cohort. Most treatment changes can and should be guided by clinical decision-making, given the significant periprocedural risks. Invasive measurements seem to be crucial in cases of otherwise unclear hemodynamic conditions and in the absence of improvement under initiated therapy. Especially in patients with complex CHD with severe PVD, therapy must be monitored very closely to allow early detection of individuals whose condition worsens with targeted PAH therapy.
Acknowledgments
The authors thank the German Heart Foundation (“Deutsche Herzstiftung e.V.”), the German patient organization “Herzkind e. V.”, and also the German health care insurance AOK-Bayern for the promotion of ACHD research. We explicitly thank Dr. Claudia S. Copeland, New Orleans, USA for the professional editing of the final draft of the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-266/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-266/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-266/coif). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” was commissioned by the editorial office without any funding or sponsorship. HK served as the unpaid Guest Editor of the series, and reports sponsorship/honoraria received from Actelion/Janssen, Bristol-Myers Squibb. HK is on steering board for COMPERA International Steering Board, received research grant/support from patient organizations: Deutsche Herzstiftung and Herzkind e.V, and received consulting fees from Janssen Pharmaceuticals. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice guidelines. The study was in accordance to the local data privacy regulation (BayKrG-27) and was approved by the institutional ethics board (2489/09 S). All participants gave written informed consent and agreed to an anonymous publication of their data. Guidelines on good pharmacoepidemiological practice and data protection guidelines were followed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241-7. [Crossref] [PubMed]
- Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol 2019;48:455-63. [Crossref] [PubMed]
- Mandalenakis Z, Giang KW, Eriksson P, et al. Survival in Children With Congenital Heart Disease: Have We Reached a Peak at 97%? J Am Heart Assoc 2020;9:e017704. [Crossref] [PubMed]
- Marelli AJ, Ionescu-Ittu R, Mackie AS, et al. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014;130:749-56. [Crossref] [PubMed]
- Kompetenznetz Angeborene Herzfehler e.V. 2021. Available online: https://www.kompetenznetz-ahf.de/wir/presse/zahlen-und-fakten/. Accessed 12.10.2021 2021.
- Warnes CA. Adult congenital heart disease: the challenges of a lifetime. Eur Heart J 2017;38:2041-7. [PubMed]
- Neidenbach R, Niwa K, Oto O, et al. Improving medical care and prevention in adults with congenital heart disease-reflections on a global problem-part I: development of congenital cardiology, epidemiology, clinical aspects, heart failure, cardiac arrhythmia. Cardiovasc Diagn Ther 2018;8:705-15. [Crossref] [PubMed]
- Neidenbach RC, Lummert E, Vigl M, et al. Non-cardiac comorbidities in adults with inherited and congenital heart disease: report from a single center experience of more than 800 consecutive patients. Cardiovasc Diagn Ther 2018;8:423-31. [Crossref] [PubMed]
- Singh S, Desai R, Fong HK, et al. Extra-cardiac comorbidities or complications in adults with congenital heart disease: a nationwide inpatient experience in the United States. Cardiovasc Diagn Ther 2018;8:814-9. [Crossref] [PubMed]
- Diller GP, Orwat S, Lammers AE, et al. Lack of specialist care is associated with increased morbidity and mortality in adult congenital heart disease: a population-based study. Eur Heart J 2021;42:4241-8. [Crossref] [PubMed]
- Seidel L, Nebel K, Achenbach S, et al. Facts about the General Medical Care of Adults with Congenital Heart Defects: Experience of a Tertiary Care Center. J Clin Med 2020;9:1943. [Crossref] [PubMed]
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 2021;42:563-645. [Crossref] [PubMed]
- van Riel AC, Blok IM, Zwinderman AH, et al. Lifetime Risk of Pulmonary Hypertension for All Patients After Shunt Closure. J Am Coll Cardiol 2015;66:1084-6. [Crossref] [PubMed]
- van Riel AC, Schuuring MJ, van Hessen ID, et al. Contemporary prevalence of pulmonary arterial hypertension in adult congenital heart disease following the updated clinical classification. Int J Cardiol 2014;174:299-305. [Crossref] [PubMed]
- Engelfriet PM, Duffels MG, Möller T, et al. Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart 2007;93:682-7. [Crossref] [PubMed]
- Maurer SJ, Stöckemann K, Pujol C, et al. Pulmonary Arterial Hypertension Associated with Congenital Heart Disease in Adults over the Age of 40 Years. J Clin Med 2020;9:4071. [Crossref] [PubMed]
- Lowe BS, Therrien J, Ionescu-Ittu R, et al. Diagnosis of pulmonary hypertension in the congenital heart disease adult population impact on outcomes. J Am Coll Cardiol 2011;58:538-46. [Crossref] [PubMed]
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Nashat H, Kempny A, McCabe C, et al. Eisenmenger syndrome: current perspectives. Research Reports in Clinical Cardiology 2017;8:1-12. [Crossref]
- Diller GP, Dimopoulos K, Broberg CS, et al. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J 2006;27:1737-42. [Crossref] [PubMed]
- Diller GP, Kempny A, Inuzuka R, et al. Survival prospects of treatment naive patients with Eisenmenger: a systematic review of the literature and report of own experience. Heart 2014;100:1366-72. [Crossref] [PubMed]
- Kaemmerer H, Gorenflo M, Huscher D, et al. Pulmonary Hypertension in Adults with Congenital Heart Disease: Real-World Data from the International COMPERA-CHD Registry. J Clin Med 2020;9:1456. [Crossref] [PubMed]
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013;62:D42-50. [Crossref] [PubMed]
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493-537. [Crossref] [PubMed]
- Wood P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. Br Med J 1958;2:755-62. [Crossref] [PubMed]
- Saha A, Balakrishnan KG, Jaiswal PK, et al. Prognosis for patients with Eisenmenger syndrome of various aetiology. Int J Cardiol 1994;45:199-207. [Crossref] [PubMed]
- Cantor WJ, Harrison DA, Moussadji JS, et al. Determinants of survival and length of survival in adults with Eisenmenger syndrome. Am J Cardiol 1999;84:677-81. [Crossref] [PubMed]
- Dimopoulos K, Inuzuka R, Goletto S, et al. Improved survival among patients with Eisenmenger syndrome receiving advanced therapy for pulmonary arterial hypertension. Circulation 2010;121:20-5. [Crossref] [PubMed]
- Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J 2010;31:1220-9. [Crossref] [PubMed]
- Maurer SJ, Bauer UMM, Baumgartner H, et al. Acquired Comorbidities in Adults with Congenital Heart Disease: An Analysis of the German National Register for Congenital Heart Defects. J Clin Med 2021;10:314. [Crossref] [PubMed]
- Akagi S, Kasahara S, Sarashina T, et al. Treat-and-repair strategy is a feasible therapeutic choice in adult patients with severe pulmonary arterial hypertension associated with a ventricular septal defect: case series. Eur Heart J Case Rep 2018;2:yty033. [Crossref] [PubMed]
- Hu Z, Xie B, Zhai X, et al. Midterm results of "treat and repair" for adults with non-restrictive ventricular septal defect and severe pulmonary hypertension. J Thorac Dis 2015;7:1165-73. [PubMed]
- Kijima Y, Akagi T, Takaya Y, et al. Treat and Repair Strategy in Patients With Atrial Septal Defect and Significant Pulmonary Arterial Hypertension. Circ J 2016;80:227-34. [Crossref] [PubMed]
- Kempny A, Hjortshøj CS, Gu H, et al. Predictors of Death in Contemporary Adult Patients With Eisenmenger Syndrome: A Multicenter Study. Circulation 2017;135:1432-40. [Crossref] [PubMed]
- Crepaz R, Romeo C, Montanaro D, et al. Long-term results of treatment with bosentan in adult Eisenmenger's syndrome patients with Down's syndrome related to congenital heart disease. BMC Cardiovasc Disord 2013;13:74. [Crossref] [PubMed]
- Arnott C, Strange G, Bullock A, et al. Pulmonary vasodilator therapy is associated with greater survival in Eisenmenger syndrome. Heart 2017. [Epub ahead of print]. pii: heartjnl-2017-311876. doi:
10.1136/heartjnl-2017-311876 .10.1136/heartjnl-2017-311876 - Berger RM, Beghetti M, Galiè N, et al. Atrial septal defects versus ventricular septal defects in BREATHE-5, a placebo-controlled study of pulmonary arterial hypertension related to Eisenmenger's syndrome: a subgroup analysis. Int J Cardiol 2010;144:373-8. [Crossref] [PubMed]
- Kaemmerer AS, Gorenflo M, Huscher D, et al. Medical treatment of pulmonary hypertension in adults with congenital heart disease: updated and extended results from the International COMPERA-CHD Registry. Cardiovasc Diagn Ther 2021;11:1255-68. [Crossref] [PubMed]
- D'Alto M, Constantine A, Balint OH, et al. The effects of parenteral prostacyclin therapy as add-on treatment to oral compounds in Eisenmenger syndrome. Eur Respir J 2019;54:1901401. [Crossref] [PubMed]
- Galiè N, Barberà JA, Frost AE, et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med 2015;373:834-44. [Crossref] [PubMed]
- D'Alto M, Romeo E, Argiento P, et al. Bosentan-sildenafil association in patients with congenital heart disease-related pulmonary arterial hypertension and Eisenmenger physiology. Int J Cardiol 2012;155:378-82. [Crossref] [PubMed]
- Mohammed S, Vijayvergiya R, Malhotra S, et al. A randomized, double-blind, placebo-controlled study to evaluate sildenafil, ambrisentan combination therapy in pulmonary hypertension, particularly of Eisenmenger syndrome. Indian Heart J 2021;73:633-6. [Crossref] [PubMed]
- Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369:809-18. [Crossref] [PubMed]
- Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006;114:48-54. [Crossref] [PubMed]
- Galie N, Manes A, Palazzini M, et al. Management of pulmonary arterial hypertension associated with congenital systemic-to-pulmonary shunts and Eisenmenger's syndrome. Drugs 2008;68:1049-66. [Crossref] [PubMed]
- Condliffe R, Clift P, Dimopoulos K, et al. Management dilemmas in pulmonary arterial hypertension associated with congenital heart disease. Pulm Circ 2018;8:2045894018792501. [Crossref] [PubMed]
- Schuuring MJ, Bouma BJ, Cordina R, et al. Treatment of segmental pulmonary artery hypertension in adults with congenital heart disease. Int J Cardiol 2013;164:106-10. [Crossref] [PubMed]
- Apostolopoulou SC, Vagenakis G, Rammos S. Pulmonary vasodilator therapy in tetralogy of Fallot with pulmonary atresia and major aortopulmonary collaterals: case series and review of literature. Cardiol Young 2017;27:1861-4. [Crossref] [PubMed]
- Lim ZS, Vettukattill JJ, Salmon AP, et al. Sildenafil therapy in complex pulmonary atresia with pulmonary arterial hypertension. Int J Cardiol 2008;129:339-43. [Crossref] [PubMed]


