
The frozen elephant trunk procedure: indications, outcomes and future directions
Introduction
The aortic arch may be implicated in a myriad of complex disease states, ranging from aneurysms and penetrating ulcers, to acute and chronic dissections. The surgical management for such extensive pathologies concomitantly involving the arch and descending aortic segments continues to represent a significant technical challenge, even in the contemporary era. Until the 1990s, reconstruction of the transverse aortic arch and proximal descending aorta incorporated initial replacement of the aortic arch, followed by the remaining descending thoracic aorta either in the same operation employing median sternotomy with a thoracoabdominal incision, or clamshell incision, or 4–12 weeks later as a two-stage procedure (1,2). Although offering a definitive treatment for multi-segment aortic disease and yielding acceptable outcomes, the surgical complexity, protracted operative durations and particularly the extended hypothermic circulatory arrest times necessary imparted significant morbidity and mortality risks to the patient undergoing such an invasive procedure (3). Furthermore, access via the posterolateral left thoracotomy usually required for the second-stage procedure might be complicated by dense adhesions surrounding the aortic arch graft, and risk inadvertent injury during clamping to the adjacent pulmonary artery, left recurrent laryngeal or vagus nerves, oesophagus or trachea (3). The advent of two-staged procedures for extensive aortic replacement emerged in 1983 with the so-called elephant trunk (ET) procedure, when Borst and colleagues suspended the distal aortic arch graft freely within the proximal descending aorta, facilitating its second-stage anastomosis via lateral thoracotomy with a descending aortic graft (4). The introduction of novel ET prostheses in the 1990s combining a covered stent sutured distally to a conventional tube-graft to simultaneously address the aortic arch and proximal descending aorta via a median sternotomy, led to the development of the frozen elephant trunk (FET) procedure by Kato and colleagues (5). This review aims to discuss the indications, technique, advantages and potential complications, contemporary results and future developments regarding the FET technique.
The conventional ET (CET) procedure
The treatment of thoracic aortic aneurysms in the era preceding the emergence of extra-corporeal circulation and deep hypothermic circulatory arrest (6) consisted of techniques such as aortic ligation and wrapping with cellophane, which achieved unpredictable results. Since Borst and colleagues pioneered the revolutionary CET procedure (Figure 1) (7) in 1983 in the treatment of mega-aorta syndrome (4), the technique has since been performed worldwide as a standard approach for the staged repair of multi-segment thoracic aortic aneurysmal disease (8). There are essentially two components to the CET procedure which are performed in a staged fashion. Firstly, a tube-graft prosthesis is invaginated and its reflected end sutured end-to-end into the transected aortic arch distal to the left subclavian artery. The invaginated portion of the prosthesis is then retrieved and anastomosed proximally, with the creation of orifices for implantation of the arch branches individually or together as an “island”. This completes the reconstruction of the aortic arch as described in the modifications of the technique by Crawford (3) and Svensson (9), resulting in a distal graft segment which floats freely within the descending aorta with an ET appearance. In the second stage of the CET procedure, descending thoracic or thoraco-abdominal aortic repair is accomplished via a left postero-lateral thoracotomy and anastomosis of the distal end of the previously implanted CET graft to a descending thoracic graft.
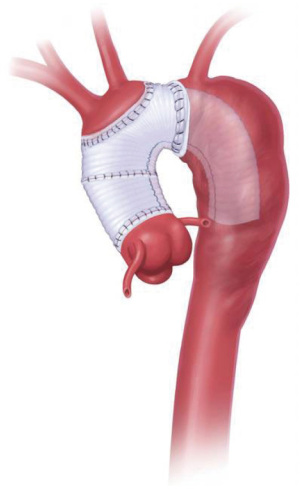
The CET technique affords safe proximal control and thus simplifies the second stage of aortic repair by obviating the need for clamping or excessive manipulation of the fragile descending aorta, since the ET graft implanted during the first stage is itself directly clamped after retrieval from the aortic lumen. Stroke and paraplegia rates are also reduced because clamping of the proximal left subclavian artery is not necessary (10). Furthermore, avoidance of re-dissection amongst dense adhesions in the proximity of the aortic arch reduces the risk of pulmonary artery or aortic injury that was commonplace during the early experiences of thoraco-abdominal aortic aneurysm repair (11,12). As familiarity with CET has accumulated, its indications have expanded from merely elective thoraco-abdominal aneurysm repair to acute aortic dissections, with the aim of curtailing downstream reintervention rates, especially in younger patients and those presenting with aortic arch dilatation or connective tissue diseases (8). Depending on the length of the prosthesis employed, the ET procedure has potential to initiate false lumen thrombosis and obliteration (11-15), although the sealing effect produced is incomplete and may expose the patient to persistent false lumen perfusion, and therefore the risk for ongoing expansion and delayed rupture.
Despite these benefits, the CET procedure may be accompanied by various graft-related complications, including graft occlusion and kinking, and neurological sequalae such as stroke and spinal cord injury (14,16,17). Clot formation around the free end of the graft can precipitate peripheral thromboembolic events due to graft flapping and entrapment of the free end of the graft within the true or false lumen occurs exclusively with the elephant graft configuration (12,18). Thirty-day mortality is reported at 5–14% for patients undergoing stage I procedures, and 0–10% early mortality following stage II procedures (11,12,14,17,18). Perhaps of greater concern is the considerable inter-stage mortality, ranging from 3–13%, and the significant 45% of “non-returners” who underwent the first-stage procedure but did not undergo second-stage completion (19). Stroke and paraplegia rates with the CET procedure are 4.5% and 2.0%, respectively (20).
These limitations combined with the advent of endovascular treatment strategies have driven the development and widespread adoption of an innovative hybrid one-stage procedure, the so-called stented or FET procedure.
Rationale and indications for the FET procedure
The FET procedure (Figure 2) (21) permits the simplified hybrid single-stage repair of complex concomitant aortic arch and descending aortic disease under circulatory arrest, affording patients the advantages of a combined conventional and endovascular approach. It represented a major paradigm change from CET when the concept was described by Kato and colleagues in the mid-1990s (5), although the FET procedure was introduced in 2003 by Karck and colleagues (22). This coincided with the availability of novel ET prostheses advanced antegradely via the distal aortic arch, combining a covered nitinol or stainless steel stent sutured to the distal end of a conventional vascular graft, alongside developments in the field of endovascular stent-graft technology (19). The antegrade delivery of the stent-graft is particularly valuable in patients in whom second-stage endovascular completion via a retrograde approach may be complicated by unfavourable peripheral access (20). The distal aspect of the FET stent-graft functions as artificial distal anastomosis by generating an effective seal to encourage thrombotic exclusion of peri-graft space, whilst the proximal aspect of the FET prosthesis is sutured to the aortic arch in zone II or another prosthesis, thereby preventing proximal endoleak and stent-graft migration (5). Progressive thrombotic occlusion of the peri-graft space is vital for reduction of aneurysm wall stress, and may inhibit ongoing aortic expansion (22). The aortic repair is completed by anastomosis of the arch vessels using the island technique or branched anatomic or extra-anatomic approaches (23).
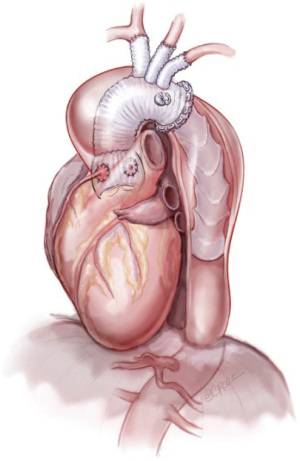
Surgery for acute type A aortic dissection is highly challenging, even in expert hands. The goal in such emergent circumstances is primarily targeted to saving the patient’s life. The application of a CET or FET is dependent largely on the operating surgeon’s experience with these techniques, patient-related anatomical factors, and the ready availability of radiological and vascular surgical expertise. Furthermore, indications for CET or FET are not well delineated and may vary considerably between institutions, or even between individual surgeons. Certainly, the FET procedure demands greater experience than CET and the aortic surgeon must negotiate a learning curve associated with the time-consuming nature of the operation, the attention requisite for optimal cerebral and myocardial protection and the challenges of achieving a satisfactory descending aortic and left subclavian artery anastomoses within a deep surgical field.
FET in acute type A dissection
FET is particularly appealing in the treatment of acute type A aortic dissection because of its ability to treat malperfusion by encouraging true lumen expansion, and potentially reducing longer-term adverse remodelling within the descending aorta (24-29). In the setting of acute type A dissection, the FET technique is indicated as an adjunct to total arch replacement in the presence of distal aortic malperfusion, complex primary and re-entry tears affecting the distal arch and proximal descending thoracic aorta, aneurysms of the distal arch or proximal descending thoracic aorta, rupture of the distal arch or descending thoracic aorta, and severe arch disruption precluding safe anastomosis (26). In malperfusion scenarios, the FET technique enables restoration of the patency of the compressed true lumen by stabilising arch entry tears and excluding tears within the proximal descending thoracic aorta which maintain false lumen pressurisation (26). Additionally, the FET prosthesis provides an excellent proximal landing zone for prospective stent-graft implantation [thoracic endovascular aortic repair (TEVAR)] in the downstream aorta, thus avoiding the need for a difficult and hazardous anastomosis in a fragile distal aortic arch (26). Distal anastomotic new entry is a primary contributor towards false lumen patency after acute type A aortic dissection, alongside non-resection of a primary intimal tear and distal re-entry tears (30), and is associated with a greater incidence of distal aortic events. The reinforcement of the distal anastomosis with Teflon strips to prevent suture leak represents another potential benefit when employing a FET, and serves as an effective preventative measure against distal anastomotic new entry (30). Since a substantial 60–90% of patients with false lumen patency develop aneurysms or rupture of the descending aorta, or require later reintervention (26,31-35), the beneficial effects on descending aortic morphology associated with FET implantation may contribute to a reduction in descending aortic aneurysm formation, an important feature since endovascular repair is not always feasible, which can enhance long-term survival by reducing aorta-related mortality and complex distal reinterventions (26). Nevertheless, the facility provided by FET for eventual TEVAR makes it an attractive treatment modality for acute type A dissection. The FET operation is certainly more technically demanding, and should ideally be offered by dedicated high-volume aortic centres (36). Finally, FET may not be applicable in the most systemically unwell patients with poor prognosis who may not survive to receive the full benefit of the FET procedure as a prophylactic operation to mitigate the risk of future distal aortic events and re-intervention.
FET in acute type B aortic dissection
Whilst primary TEVAR is recognised as the first-line treatment strategy in patients with complicated acute type B dissection, it is associated with a risk of retrograde ascending aortic dissection with an attendant mortality approaching 40% (37). In contrast, the FET approach eliminates the risk of retrograde aortic dissection by placement of an aortic graft within the aortic arch and ascending aorta (38). In situations where an endovascular approach is anatomically contraindicated, including in the presence of a proximal aortic arch landing zone diameter >40 mm, concomitant aortic arch aneurysm, severe aortic arch angulation or left subclavian artery dissection with additional distal intimal tears (26,39,40), open aortic repair was traditionally undertaken via a left thoracotomy with prohibitively high mortality. The FET procedure has since evolved as a valid alternative offering reduced in-hospital and longer-term mortality, combined with excellent rates of false lumen thrombosis (41).
FET in chronic aortic disease
Patients undergoing a more proximal aortic repair for acute type A aortic dissection despite a patent primary entry tear in the distal aortic arch have a greater likelihood of developing post-dissection aneurysmal formation in the descending aorta. A FET may be employed in these patients for exclusion of the aneurysmal aortic segment (42-45). Similarly, false lumen aneurysmal dilatation occurs in approximately 73% of chronic type B aortic dissections (46). FET has been proposed as the most suitable treatment option for patients with chronic type B aortic dissection (47), who tend to be younger and have a greater prevalence of connective tissue disease. Accordingly, aortic wall fragility inherent in these patients renders them more susceptible to endovascular stent-graft-induced aortic injury and retrograde aortic dissection (48). FET is also more advantageous than CET in chronic type B aortic dissection since it can be performed as a single-stage procedure eliminating the risk of interval aortic rupture between stages, and CET-related graft kinking (49,50).
Concomitant aneurysms of arch and descending thoracic aorta may be electively addressed using a FET as a single-stage approach or with completion TEVAR. Several European societies have currently recommended the FET procedure for aortic arch replacement over isolated arch replacement and the CET (51), cognisant of the underlying arch pathology’s potential to provoke disease progression in the more distal non-intervened aorta (51,52). Whilst the CET achieves satisfactory outcomes for chronic degenerative and post-dissection aneurysms, a major limitation is the incomplete repair of the distal descending aorta. In contrast, the FET technique has been demonstrated to moderate further aortic dilatation and promote aortic wall remodelling (53). By depressurising the aneurysm wall, FET implantation reduces wall stress and is believed to reduce aneurysm size (54). The FET procedure is being increasingly advocated as a prophylactic strategy in younger patients presenting electively with chronic aortic dissection, connective tissue disorders and aortitis requiring proximal aortic replacement with modest aortic arch dilatation, in anticipation of the greater likelihood for distal reintervention in these populations (50).
An important concern surrounding FET utilisation in chronic aortic dissection is related to the higher incidence of distal stent-graft-induced new entry compared to that in acute dissection (55). This serious phenomenon results from the development of a new intimal tear due to the stent-graft portion of the FET prosthesis (56), causing persistent false lumen perfusion and thereby impacting negatively on aortic remodelling and leading to unintended distal aortic reintervention and even aortic rupture (57). Compliance mismatch with disturbances in stress-strain distributions between the stent-covered aorta and uncovered aorta could theoretically induce a flap perforation and a new intimal tear (58). Furthermore, the thickened and non-pliable intimal flap in chronic dissections combined with aortic tortuosity may generate a kink in the junction between the aortic arch and descending aorta and produce a pseudo-coarctation of the stent-graft into a compressed true lumen (50). FET stent-graft over-sizing and spring-back force, and aortic wall fragility are reported risk factors for distal stent-graft-induced new entry (56,58). Indeed, accurate stent-graft sizing in chronic aortic dissection may be complicated since the true lumen is usually compressed by the false lumen separated by a rigid intimal flap.
Current FET prostheses
The two FET prostheses commercially available in Europe are the E-vita Open Neo (JOTEC GmbH, Hechingen, Germany) (Figure 3) and the Thoraflex Hybrid (Vascutek, Inchinnan, Scotland, UK) (Figure 4) stent-graft systems. Both devices are available in different sizes and with different delivery systems.

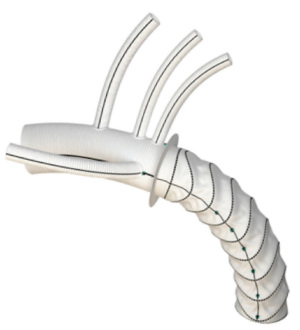
The E-vita Open, originally the Essen I prosthesis, was introduced as the first commercially available hybrid prothesis in 2005, consisting of polyester fabric covering an endoskeleton of nitinol z-stents and a proximal non-stented Dacron prosthesis (59). The E-vita Open Plus became available in 2008, allowing supra-aortic vessel implantation en bloc via a Carrel patch technique and incorporating a sewing collar for distal aortic anastomosis in zone 2/3. In 2012, a branched E-vita FET device permitted a zone 1/2/3 collar anastomosis and sequential supra-aortic vessel anastomosis, followed in 2020 by the E-via Open Neo, a next-generation FET device with a tetra-furcate proximal graft configuration enabling a zone 0/1 collar anastomosis (60). Proximalisation of the aortic arch to zone 0 by collar anastomosis in zone 0/1 can facilitate performance of the FET procedure by less experienced surgeons, eliminates the risk of recurrent laryngeal nerve palsy, significantly reduces the incidence of paraplegia, and aids with haemostasis processes (60).
The Thoraflex Hybrid FET device was introduced in 2012, integrating a distal nitinol-ringed self-expanding stent-graft and proximal unstented, quadruple-branched and gelatin-impregnated polyester woven graft (28,61,62). Separating these two conjoined sections is a sewing collar which facilitates the distal aortic anastomosis (63). Three branches of the Thoraflex Hybrid prosthesis allow individual arch vessel implantation, a valuable feature when the arch vessel origins are distant from each other, using an arch-first approach which can reduce lower body and myocardial ischaemia times (64). Earlier lower body reperfusion can commence via the fourth branch on completion of the distal aortic anastomosis (63,64).
Compared to the E-vita Open Plus device, the Thoraflex Hybrid system possesses a simpler deployment system, has a gelatin-sealed vascular prosthesis, and its nitinol ring arrangement exerts less radial force compared to the z-stent design of the E-vita Open Plus (64). Both FET devices have radio-opaque markers for identification on subsequent imaging studies.
Surgical technique
Several descriptions of aortic arch replacement utilising a FET are presented in the literature (26,62,63,65-71). One technique utilising a four-branched FET prosthesis is described here.
Following median sternotomy, the aortic arch vessels are identified and mobilised. An 8-mm Dacron graft is anastomosed to the innominate artery for arterial inflow, although the axillary artery and ascending aorta may also be used according to surgeon preference and patient anatomy. Cardiopulmonary bypass is established after cannulation of the right atrium. A retrograde cardioplegia cannula is placed in the coronary sinus. Cardiopulmonary bypass and systemic cooling to 26 ℃ are then initiated and a left ventricular vent is placed via the right superior pulmonary vein. The ascending aorta is cross-clamped and cardioplegic arrest is obtained by antegrade and retrograde cardioplegia administration.
When the desired nasopharyngeal temperature is reached, circulatory arrest is commenced, and the innominate and left common carotid arteries are clamped and transected approximately 1 cm from their origins which are then ligated. The left subclavian artery remains clamped to avoid steal from the left vertebral system. The aortic cross-clamp is released and the aortic arch is transected just distal to the left subclavian artery. Selective antegrade cerebral perfusion (72) is initiated with near infrared spectroscopy monitoring, maintaining a flow rate of 10 mL/kg/min and a right radial artery pressure of 60–80 mmHg. Alternatively, an additional perfusion cannula may be introduced into the left common carotid artery for bilateral cerebral perfusion. The proximal descending aorta is reinforced with an external Teflon strip using four pledgeted 3/0 polypropylene sutures. In cases of aortic dissection, the false lumen must be obliterated.
The diameter of the FET prosthesis is selected as 90–100% of the diameter of the descending aorta in acute dissection, avoiding over-sizing, and its length determined by the distance between the anastomosis site and the projected level of the aortic valve in the descending aorta. The stent-graft component of the FET prosthesis is pre-curved and delivered into the descending aorta (73). The stent-graft is deployed using trans-oesophageal echocardiography to confirm satisfactory positioning and expansion, which may be optimised by balloon catheter. The distal anastomosis is completed with 3/0 polypropylene incorporating the FET sewing collar. If available, a fourth branch of the FET prosthesis is then cannulated to enable antegrade lower body perfusion, and systemic rewarming is commenced. The proximal FET graft is then anastomosed to the ascending aorta. Arch reconstruction is performed by sequentially reimplanting the left subclavian, left common carotid and innominate arteries using a 5/0 polypropylene suture into the third, second and first branches of the FET prosthesis, respectively, before tying off each vessel at its origin. The patient is then weaned off cardiopulmonary bypass.
Importantly, effective FET surgery mandates close collaboration between all members of the aortic team, including cardiac and vascular surgeons, radiologists, anaesthetists, perfusionists and nurses.
Clinical outcomes with FET
Contemporary data from observational studies and meta-analyses published within the preceding decade on aortic arch replacement using the FET technique are now discussed.
Early mortality and long-term survival
Since the earliest experience of FET implantation by Kato et al. who reported 0% surgical mortality in 10 patients with aneurysms or acute dissections involving the distal aortic arch (5), FET has been associated with acceptable early and long-term mortality outcomes. Reduced early mortality and better overall survival has been observed in patients undergoing implantation of a FET compared to a CET (52). Moulakakis et al. conducted a systematic review and meta-analysis investigating the efficacy of hybrid techniques for aortic arch pathologies in 46 studies with 2,272 patients undergoing aortic debranching or FET, and determined a pooled estimate for 30-day/in-hospital mortality of 9.5% in patients receiving a FET (74). A meta-analysis in 2013 by Tian et al. demonstrated a pooled in-hospital mortality of 8.3% across 17 studies including 1,675 patients undergoing a FET procedure for acute or chronic dissection or aneurysmal disease (53). Longer-term mortality was acceptable with weighted 1-year survival 85.6% (range, 70–97%) and 5-year survival 71.5% (range, 63–88%). Di Eusanio et al. performed a review of 13 observational studies in 2014 involving 598 patients with acute type A dissection, obtaining an average weighted in-hospital/30-day mortality of 10.0%, ranging from 0% to 27.7% (75). One- and 5-year year survival ranged from 79% to 100% and from 68% to 96%, respectively. A 2015 meta-analysis addressing the safety and efficacy of FET in acute type A aortic dissection by Lin et al. demonstrated an in-hospital mortality of 8% across 11 studies encompassing 881 patients (76). Early mortality ranged between 6.4–15.8% in a 2015 review by Ma et al., utilising the E-vita Open Plus, Thoraflex Hybrid, Cronus and J Graft Open FET prostheses in heterogenous populations (77). Late survival was reported at 69–85% at 5 years with the E-vita Open Plus and 77%±7% at 1 year using the Thoraflex Hybrid prostheses in this analysis, with the variations in survival reflective of patient selection criteria and intra-operative cerebral and myocardial protection. In-hospital mortality was reported at 1.8–17.2% in a position paper from the Vascular Domain of the European Association for Cardio-Thoracic Surgery (EACTS) (39). A large multi-centre study in 2016 reporting on the outcomes of FET using the E-vita prosthesis documented in-hospital mortality rates of 17.1% and 13.2% in patients with acute dissection and chronic aneurysmal disease, respectively (78). Takagi et al. analysed FET outcomes in acute type A aortic dissection in a 2016 meta-analysis, revealing an early mortality of 9.2% amongst 15 studies including 1,279 patients, and overall mortality beyond 1 year of 13.0% (79). Using the E-vita Open plus hybrid device in 94 patients with mainly chronic aortic dissections and degenerative aneurysms, a French group described a peri-operative mortality rate of 11.7%, with a 1-year survival rate of 98% amongst 83 surviving patients (80). The Essen group in 2020 reported a 12% 30-day mortality in 307 patients undergoing FET implantation with the E-vita Open prosthesis, 55% of whom had acute dissections (81). Patients with acute dissection, chronic dissection and thoracic aneurysms achieved 5-year survival rates of 67%, 74% and 65%, respectively in the Essen series, corresponding to an overall 8-year survival of 60% (81). A 2020 meta-analysis incorporating 35 studies and 3,154 patients by Preventza et al. found a variously reported operative, 30-day and in-hospital mortality ranging from 0% to 21.6% (82). A systematic review published in 2020 confirmed a mortality of 10.2% in 4,178 patients undergoing FET, mostly for acute dissection, with 5-year survival 82.0% (83).
Neurological complications
Despite the clinical benefits afforded by the FET technique, adverse neurological events including stroke and spinal cord injury are devastating complications for patients with lifelong physical, psychological and financial implications. Spinal cord injury is multifactorial and attributed to spinal cord ischaemia from incomplete revascularisation of the left subclavian artery, occlusion of the thoracic intercostal arteries by the FET stent-graft and prolonged intra-operative systemic hypotension.
Cerebrovascular events occurred in 6.2% and irreversible spinal cord injury in 5.0% of patients undergoing FET in the early meta-analysis by Moulakakis et al. (74). Reduced overall stroke and spinal cord injury rates of 3% and 4%, respectively, were observed amongst 881 patients with acute dissection undergoing FET in the meta-analysis by Lin and colleagues (76). Takagi and colleagues similarly demonstrated rates of stroke at 4.8% and spinal cord injury at 3.5% amongst 15 studies in their meta-analysis enrolling 1,279 patients with acute dissection specifically undergoing FET (79). Leontyev and colleagues noted an incidence of stroke and spinal cord injury at 7.7% and 7.5%, respectively, in their large case series incorporating 509 patients undergoing FET using the E-vita prosthesis (78). They advised a two-staged procedure with endovascular completion is appropriate for more distal aortic lesions, rather than a single procedure with a FET stent-graft landing zone lower than T10 (78). Di Eusanio et al. reported an acceptable stroke rate of 4.8% (range, 0–12%) and spinal cord injury rate of 4.3% (range, 0–13.8%) in their review of existing case series (75). Interestingly, the review by Ma et al. revealed a 2.6% stroke risk and 3.5% spinal cord injury risk with the E-vita Open Plus, compared to an 8.0% stroke risk and 4.0% spinal cord injury risk associated with the Thoraflex Hybrid devices (77). The EACTS position paper on the FET reported aggregate rates of stroke at 2.5–20% and spinal cord injury at 0–21%, and emphasised the significantly higher incidence of spinal cord injury in patients undergoing FET compared with CET (39). In the French experience, Verhoye et al. had a 4% spinal cord injury rate and 9% stroke rate in elective patients receiving the E-vita Open Plus prosthesis (80). Permanent stroke occurred in 7% of patients in the Essen series of 307 patients undergoing E-vita Open FET implantation, with a paraplegia rate of 3% (81). A recent meta-analysis by Preventza and colleagues found a 7.6% and 4.7% pooled estimate for overall stroke and overall spinal cord injury, respectively, amongst 35 studies comprising 3,145 patients (82). The authors suggest that a FET stent-graft landing zone at T8 or beyond, or stent-graft length ≥15 cm, are significant risk factors for the development of spinal cord injury (82). Similarly, in the systematic review by Tian et al. in 2020, permanent neurological deficit developed in 7.7% of the heterogenous group of 4,178 patients in 37 studies undergoing FET implantation, with spinal cord injury occurring in 6.5% (83).
Aortic remodelling and late re-intervention
False lumen thrombosis occurs more predictably in patients undergoing FET replacement of the aortic arch compared to a CET procedure, and a greater extent of descending aortic remodelling is evident after FET implantation (26). Pooled average aortic remodelling with partial/complete thrombosis of the peri-stent descending thoracic aorta was noted in 88.9% of patients undergoing FET surgery for acute type I aortic dissection in the review by Di Eusanio et al. (75), compared to 33.3–77.8% in patients receiving conservative management (84). They reported a 92.3% (range, 71.8–100%) pooled average for 1-year freedom from reintervention. In their review, Ma et al. reported a requirement for late reintervention in 2–27% undergoing FET implantation with the E-vita Open Plus prosthesis and 14.1% with the Thoraflex Hybrid prosthesis (77). In the International E-vita Open registry, complete false lumen thrombosis was demonstrated in 94% of patients with acute dissection, 86% with chronic dissection and 94% with aneurysms (85). In their experience with the Thoraflex Hybrid prosthesis in the first 100 patients in 2013, the Hannover group reported a 1-year freedom from distal re-operation at 73%±8% (86). Using the same FET system, distal aortic procedures were necessary in 5/38 (13%) patients with acute and chronic dissections and aneurysms under the Munich group (87). Pre-discharge computed tomography (CT) angiography encouragingly confirmed complete thrombosis of the descending aortic peri-graft space in 11 patients undergoing implantation of a Thoraflex Hybrid FET in the initial experience of the Bologna group (88). Similarly, Shrestha et al. reported outcomes in their first 100 patients with acute and chronic aortic dissections and aneurysms treated with the Thoraflex Hybrid prosthesis, noting early false lumen thrombosis in patients presenting with acute aortic dissection in conjunction with favourable remodelling within the stented aortic segment (61). However, 22% of patients underwent secondary reintervention on the downstream aorta, of which 32% were not planned as part of a two-stage repair strategy. Furthermore, late reintervention was less frequent in patients undergoing a FET procedure for acute dissection, compared to chronic dissection and degenerative aneurysmal disease (61). The authors propose that the Thoraflex Hybrid prosthesis provides beneficial remodelling effects on mid-term follow-up.
A meta-analysis comprising 1,279 patients undergoing a FET procedure for acute type A aortic dissection showed a reintervention rate of 9.6%, accompanied by a false lumen thrombosis in 96.8% (79). The FET procedure induces reverse aortic remodelling, whilst encouraging false lumen thrombosis and true lumen expansion, as evidenced by results from the French group using the E-vita Open Plus device (80). At 1-year post-operatively, they discovered significant reductions in the diameters of the stented aortic segment and downstream aortic segment up to the coeliac trunk, with stable dimensions of the more distal aorta (80). With the largest single-centre experience with the E-vita Open FET, the Essen group demonstrated greater positive aortic remodelling occurring in 90% of patients with acute dissection, compared to 78% in chronic dissections (81). This correlated with 5- and 10-year freedom from reintervention rates of 87% and 74%, respectively, in the acute dissection cohort. These findings support the E-vita Open prosthesis as a viable long-term solution for complex pathology involving the aortic arch. Finally, the updated systematic review by Tian et al. demonstrated 1-, 3- and 5-year freedom from reintervention was 93.9%, 89.3% and 86.8%, respectively, for 4,178 patients included in 37 studies examining FET implantation in a mixture of elective and emergency indications (83).
The beneficial downstream aortic remodelling induced by FET, with increased true lumen/false lumen ratios, is also elegantly reflected in aortic luminal volumetric changes. Evidence suggests that false lumen thrombosis occurs more frequently and at a greater rate along the stent-graft portion of the FET than in more distal aortic segments (89,90). Dohle et al. demonstrated that within the first post-operative year after FET implantation for acute type A aortic dissection, 90% of patients displayed positive or stable aortic remodelling in the stent-graft portion of the thoracic aorta (increasing to 92% after 1 year), 65% in the aortic segment between the distal FET anastomosis and the coeliac trunk (65% after 1 year), and 58% between the coeliac trunk and aortic bifurcation (increasing to 62% after 1 year) (90). The authors observed a corresponding 13% volume increase in true lumen volume, with a 44% reduction in false lumen volume, in the stent-graft portion of the thoracic aorta within the first post-operative year after FET for acute type A aortic dissection, whilst both true and false lumen volumes remained stable beyond 1 year (90). Interestingly, no significant volumetric changes occurred post-operatively in the downstream aorta between the distal FET and aortic bifurcation. A similar volumetric analysis by Usai et al. revealed significant increases in true lumen volumes measured between the left subclavian artery and coeliac trunk in 20 patients following FET implantation for acute type A dissection at mid-term follow-up of 24 months, with associated non-significant changes in the false lumen volumes (91).
Future directions in FET surgery
Aortic arch surgery is a rapidly evolving discipline within the cardiac surgery specialty with significant potential on its horizons. Over the last quarter of a century alone, sophisticated FET procedures have been borne out of the urge to attain an elegant yet definitive solution for challenging aortic arch pathology, combined with innovative refinements in surgical technology and energetic industry-driven advances in the development of hybrid FET devices.
Subsequent iterations of aortic arch replacement procedures will embrace the opportunity for open stent-grafting with branched endoprostheses. Just as the CET procedure was enhanced with the addition of a surgeon-deployed descending aortic stent-graft, so current FET devices could be further augmented with branched stent-grafts optimised for hybrid supra-aortic vessel reconstruction. Extra-anatomic bypasses originating from the main-stent graft could facilitate re-attachment of the left-sided supra-aortic vessels and thus obviate the need for more difficult anastomosis in confined spaces (92). Several modifications of the FET technique incorporating fenestrations at various locations on the superior arch aspect of the FET prosthesis have been described (27,93,94). These aim to enhance perfusion of the supra-aortic branches without complex arch vessel reconstruction and can minimise neurological complications and also shorten operative times.
Advances in stent-graft design integrating newer materials offering greater compliancy and stimulating aortic remodelling could positively impact the natural history of the non-intervened downstream aorta, and perhaps mitigate distal re-operation. The novel Ascyrus Medical Dissection Stent (AMDS) has been developed as a partially uncovered hybrid aortic arch graft which affords coverage of the descending thoracic aorta to treat malperfusion, whilst permitting uninterrupted perfusion of the supra-aortic vessels and their subsequent re-intervention if necessary (95-97). In contrast to established aortic dissection surgery entailing extensive resection of diseased aorta, the AMDS device emphasises occlusion of the false lumen at the distal anastomosis with expansion and re-pressurisation of the true lumen (95-97). In the mid-term, the AMDS has been proposed as a safe and reproducible single-stage therapy for malperfusion in acute aortic dissection, and may well transform the course of modern aortic arch dissection surgery.
Progress in FET surgery will necessitate structured evaluation processes to allow accurate discrimination of the intended benefits and attendant limitations of the diverse procedures available, and determine the ideal patient populations for their application. Currently, no well-powered randomised trial exists to substantiate the superiority of existing FET techniques over more conservative approaches for arch management, in terms of long-term survival and freedom from late aortic re-operation for example. This should represent an important task for the worldwide aortic surgical community and could help to broaden the indications for FET procedures.
Conclusions
The surgical management of complex, multi-segment aortic disease concomitantly involving the aortic arch and descending aorta is challenging. The FET procedure provides the opportunity for hybrid single-stage aortic repair utilising an open surgical approach for implantation of a descending aortic stent-graft that functions as an ideal landing zone to accommodate a TEVAR stent-graft if anticipated. The FET procedure is currently indicated in acute type A and type B aortic dissection, particularly in association with malperfusion syndromes where it facilitates true lumen expansion and false lumen obliteration, in addition to chronic degenerative aneurysmal disease of the aortic arch and descending aorta. The evidence presented supports the FET operation as a technically feasible treatment for complex aortic disease with acceptable mortality. Despite the not insignificant risks of neurological sequelae, the FET technique offers well-selected patients the benefits of a single-stage intervention with durable longer-term performance and favourable influence on distal aortic remodelling.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Frozen Elephant Trunk”. The article has undergone external peer review.
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-330/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-330/coif). The series “Frozen Elephant Trunk” was commissioned by the editorial office without any funding or sponsorship. MB served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Crawford ES, Crawford JL, Stowe CL, et al. Total aortic replacement for chronic aortic dissection occurring in patients with and without Marfan's syndrome. Ann Surg 1984;199:358-62. [Crossref] [PubMed]
- Crawford ES, Svensson LG, Coselli JS, et al. Surgical treatment of aneurysm and/or dissection of the ascending aorta, transverse aortic arch, and ascending aorta and transverse aortic arch. Factors influencing survival in 717 patients. J Thorac Cardiovasc Surg 1989;98:659-73; discussion 673-4. [Crossref] [PubMed]
- Crawford ES, Coselli JS, Svensson LG, et al. Diffuse aneurysmal disease (chronic aortic dissection, Marfan, and mega aorta syndromes) and multiple aneurysm. Treatment by subtotal and total aortic replacement emphasizing the elephant trunk operation. Ann Surg 1990;211:521-37. [Crossref] [PubMed]
- Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using "elephant trunk" prosthesis. Thorac Cardiovasc Surg 1983;31:37-40. [Crossref] [PubMed]
- Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94:II188-93. [PubMed]
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63. [Crossref] [PubMed]
- Velasquez CA, Zafar MA, Saeyeldin A, et al. Two-Stage Elephant Trunk approach for open management of distal aortic arch and descending aortic pathology in patients with Marfan syndrome. Ann Cardiothorac Surg 2017;6:712-20. [Crossref] [PubMed]
- Tanaka A, Estrera AL. Elephant Trunk: Argument for All Arches. Semin Cardiothorac Vasc Anesth 2016;20:322-6. [Crossref] [PubMed]
- Svensson LG. Rationale and technique for replacement of the ascending aorta, arch, and distal aorta using a modified elephant trunk procedure. J Card Surg 1992;7:301-12. [Crossref] [PubMed]
- Di Bartolomeo R, Murana G, Di Marco L, et al. Frozen versus conventional elephant trunk technique: application in clinical practice. Eur J Cardiothorac Surg 2017;51:i20-8. [Crossref] [PubMed]
- Heinemann MK, Buehner B, Jurmann MJ, et al. Use of the "elephant trunk technique" in aortic surgery. Ann Thorac Surg 1995;60:2-6; discussion 7. [Crossref] [PubMed]
- Schepens MA, Dossche KM, Morshuis WJ, et al. The elephant trunk technique: operative results in 100 consecutive patients. Eur J Cardiothorac Surg 2002;21:276-81. [Crossref] [PubMed]
- Toda K, Taniguchi K, Masai T, et al. Arch aneurysm repair with long elephant trunk: a 10-year experience in 111 patients. Ann Thorac Surg 2009;88:16-22. [Crossref] [PubMed]
- LeMaire SA, Carter SA, Coselli JS. The elephant trunk technique for staged repair of complex aneurysms of the entire thoracic aorta. Ann Thorac Surg 2006;81:1561-9; discussion 1569. [Crossref] [PubMed]
- Svensson LG, Kim KH, Blackstone EH, et al. Elephant trunk procedure: newer indications and uses. Ann Thorac Surg 2004;78:109-16; discussion 109-16. [Crossref] [PubMed]
- Kouchoukos NT. Complications and limitations of the elephant trunk procedure. Ann Thorac Surg 2008;85:690-1; author reply 691-2. [Crossref] [PubMed]
- Safi HJ, Miller CC 3rd, Estrera AL, et al. Staged repair of extensive aortic aneurysms: long-term experience with the elephant trunk technique. Ann Surg 2004;240:677-84; discussion 684-5. [Crossref] [PubMed]
- Safi HJ, Miller CC 3rd, Estrera AL, et al. Staged repair of extensive aortic aneurysms: morbidity and mortality in the elephant trunk technique. Circulation 2001;104:2938-42. [Crossref] [PubMed]
- Karck M, Chavan A, Hagl C, et al. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2003;125:1550-3. [Crossref] [PubMed]
- Miyamoto Y. Elephant trunk technique for hybrid aortic arch repair. Gen Thorac Cardiovasc Surg 2014;62:135-41. [Crossref] [PubMed]
- Yan TD, Field M, Tian DH, et al. Aortic root and total arch replacement with frozen elephant trunk procedure, using a Thoraflex Hybrid Graft. Ann Cardiothorac Surg 2013;2:667-8. [PubMed]
- Karck M, Kamiya H. Progress of the treatment for extended aortic aneurysms; is the frozen elephant trunk technique the next standard in the treatment of complex aortic disease including the arch? Eur J Cardiothorac Surg 2008;33:1007-13. [Crossref] [PubMed]
- Hagl C, Pichlmaier M, Khaladj N. Elephant trunks in aortic surgery: fresh and frozen. J Thorac Cardiovasc Surg 2013;145:S98-102. [Crossref] [PubMed]
- Hoffman A, Damberg ALM, Schälte G, et al. Thoracic stent graft sizing for frozen elephant trunk repair in acute type A dissection. J Thorac Cardiovasc Surg 2013;145:964-969.e1. [Crossref] [PubMed]
- Uchida N, Katayama A, Tamura K, et al. Frozen elephant trunk technique and partial remodeling for acute type A aortic dissection. Eur J Cardiothorac Surg 2011;40:1066-71. [Crossref] [PubMed]
- Di Bartolomeo R, Pantaleo A, Berretta P, et al. Frozen elephant trunk surgery in acute aortic dissection. J Thorac Cardiovasc Surg 2015;149:S105-9. [Crossref] [PubMed]
- Roselli EE, Rafael A, Soltesz EG, et al. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J Thorac Cardiovasc Surg 2013;145:S197-201. [Crossref] [PubMed]
- Shrestha M, Pichlmaier M, Martens A, et al. Total aortic arch replacement with a novel four-branched frozen elephant trunk graft: first-in-man results. Eur J Cardiothorac Surg 2013;43:406-10. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [Crossref] [PubMed]
- Tamura K, Chikazawa G, Hiraoka A, et al. The prognostic impact of distal anastomotic new entry after acute type I aortic dissection repair. Eur J Cardiothorac Surg 2017;52:867-73. [Crossref] [PubMed]
- Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50. [Crossref] [PubMed]
- Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35. [Crossref] [PubMed]
- Kimura N, Tanaka M, Kawahito K, et al. Influence of patent false lumen on long-term outcome after surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2008;136:1160-6, 1166.e1-3.
- Kirsch M, Soustelle C, Houël R, et al. Risk factor analysis for proximal and distal reoperations after surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2002;123:318-25. [Crossref] [PubMed]
- Tan ME, Morshuis WJ, Dossche KM, et al. Long-term results after 27 years of surgical treatment of acute type a aortic dissection. Ann Thorac Surg 2005;80:523-9. [Crossref] [PubMed]
- Mariscalco G, Maselli D, Zanobini M, et al. Aortic centres should represent the standard of care for acute aortic syndrome. Eur J Prev Cardiol 2018;25:3-14. [Crossref] [PubMed]
- Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation 2009;120:S276-81. [Crossref] [PubMed]
- Di Eusanio M, Pantaleo A, Cefarelli M, et al. Frozen elephant trunk surgery in type B aortic dissection. Ann Cardiothorac Surg 2014;3:400-2. [PubMed]
- Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg 2015;47:759-69. [Crossref] [PubMed]
- Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78. [Crossref] [PubMed]
- Weiss G, Tsagakis K, Jakob H, et al. The frozen elephant trunk technique for the treatment of complicated type B aortic dissection with involvement of the aortic arch: multicentre early experience. Eur J Cardiothorac Surg 2015;47:106-14. [Crossref] [PubMed]
- Di Bartolomeo R, Di Marco L, Armaro A, et al. Treatment of complex disease of the thoracic aorta: the frozen elephant trunk technique with the E-vita open prosthesis. Eur J Cardiothorac Surg 2009;35:671-5; discussion 675-6. [Crossref] [PubMed]
- Tsagakis K, Pacini D, Di Bartolomeo R, et al. Multicenter early experience with extended aortic repair in acute aortic dissection: is simultaneous descending stent grafting justified? J Thorac Cardiovasc Surg 2010;140:S116-20; discussion S142-S146. [Crossref] [PubMed]
- Tsagakis K, Pacini D, Di Bartolomeo R, et al. Arch replacement and downstream stent grafting in complex aortic dissection: first results of an international registry. Eur J Cardiothorac Surg 2011;39:87-93; discussion 93-4. [Crossref] [PubMed]
- Pacini D, Tsagakis K, Jakob H, et al. The frozen elephant trunk for the treatment of chronic dissection of the thoracic aorta: a multicenter experience. Ann Thorac Surg 2011;92:1663-70; discussion 1670. [Crossref] [PubMed]
- Fattori R, Montgomery D, Lovato L, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv 2013;6:876-82. [Crossref] [PubMed]
- Kim JB, Sundt TM 3rd. Best surgical option for arch extension of type B aortic dissection: the open approach. Ann Cardiothorac Surg 2014;3:406-12. [PubMed]
- Cochennec F, Tresson P, Cross J, et al. Hybrid repair of aortic arch dissections. J Vasc Surg 2013;57:1560-7. [Crossref] [PubMed]
- Shrestha M, Martens A, Krüger H, et al. Total aortic arch replacement with the elephant trunk technique: single-centre 30-year results. Eur J Cardiothorac Surg 2014;45:289-95; discussion 295-6. [Crossref] [PubMed]
- Roselli EE, Bakaeen FG, Johnston DR, et al. Role of the frozen elephant trunk procedure for chronic aortic dissection. Eur J Cardiothorac Surg 2017;51:i35-9. [Crossref] [PubMed]
- Czerny M, Pacini D, Aboyans V, et al. Current options and recommendations for the use of thoracic endovascular aortic repair in acute and chronic thoracic aortic disease: an expert consensus document of the European Society for Cardiology (ESC) Working Group of Cardiovascular Surgery, the ESC Working Group on Aorta and Peripheral Vascular Diseases, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2021;59:65-73. [Crossref] [PubMed]
- Shrestha M, Beckmann E, Krueger H, et al. The elephant trunk is freezing: The Hannover experience. J Thorac Cardiovasc Surg 2015;149:1286-93. [Crossref] [PubMed]
- Tian DH, Wan B, Di Eusanio M, et al. A systematic review and meta-analysis on the safety and efficacy of the frozen elephant trunk technique in aortic arch surgery. Ann Cardiothorac Surg 2013;2:581-91. [PubMed]
- Pichlmaier MA, Teebken OE, Khaladj N, et al. Distal aortic surgery following arch replacement with a frozen elephant trunk. Eur J Cardiothorac Surg 2008;34:600-4. [Crossref] [PubMed]
- Hiraoka A, Iida Y, Furukawa T, et al. Predictive factors of distal stent graft-induced new entry after frozen elephant trunk procedure for aortic dissection. Eur J Cardiothorac Surg 2022;62:ezac325. [Crossref] [PubMed]
- Jubouri M, Kayali F, Saha P, et al. Incidence of Distal Stent Graft Induced New Entry vs. Aortic Remodeling Associated With Frozen Elephant Trunk. Front Cardiovasc Med 2022;9:875078. [Crossref] [PubMed]
- Kreibich M, Siepe M, Berger T, et al. Downstream thoracic endovascular aortic repair following zone 2, 100-mm stent graft frozen elephant trunk implantation. Interact Cardiovasc Thorac Surg 2022;34:1141-6. [PubMed]
- Wada T, Yamamoto H, Kadohama T, et al. Aortic remodeling mismatch: A potential risk factor of late distal stent graft-induced new entry after frozen elephant trunk deployment. JTCVS Tech 2021;8:46-8. [Crossref] [PubMed]
- Jakob H, Tsagakis K, Leyh R, et al. Development of an integrated stent graft-dacron prosthesis for intended one-stage repair in complex thoracic aortic disease. Herz 2005;30:766-8. [Crossref] [PubMed]
- Jakob H, Idhrees M, Bashir M. From E-VITA open plus to E-VITA NEO and E-NOVIA. J Card Surg 2021;36:1814-7. [Crossref] [PubMed]
- Shrestha M, Kaufeld T, Beckmann E, et al. Total aortic arch replacement with a novel 4-branched frozen elephant trunk prosthesis: Single-center results of the first 100 patients. J Thorac Cardiovasc Surg 2016;152:148-159.e1. [Crossref] [PubMed]
- Di Bartolomeo R, Murana G, Di Marco L, et al. Is the frozen elephant trunk frozen? Gen Thorac Cardiovasc Surg 2019;67:111-7. [Crossref] [PubMed]
- Di Bartolomeo R, Cefarelli M, Folesani G, et al. Frozen elephant trunk surgery using the Vascutek Thora-flex hybrid prosthesis. Ann Cardiothorac Surg 2013;2:660-2. [PubMed]
- Bozso SJ, White A, Nagendran J, et al. Hybrid aortic arch and frozen elephant trunk reconstruction: bridging the gap between conventional and total endovascular arch repair. Expert Rev Cardiovasc Ther 2018;16:209-17. [Crossref] [PubMed]
- Di Marco L, Pantaleo A, Leone A, et al. The Frozen Elephant Trunk Technique: European Association for Cardio-Thoracic Surgery Position and Bologna Experience. Korean J Thorac Cardiovasc Surg 2017;50:1-7. [Crossref] [PubMed]
- Preventza O, Al-Najjar R, Lemaire SA, et al. Total arch replacement with frozen elephant trunk technique. Ann Cardiothorac Surg 2013;2:649-52. [PubMed]
- Tsagakis K, Jakob H. Which Frozen Elephant Trunk Offers the Optimal Solution? Reflections From Essen Group. Semin Thorac Cardiovasc Surg 2019;31:679-85. [Crossref] [PubMed]
- Murzi M, Farneti PA, Rizza A, et al. Hybrid Approach in Acute and Chronic Aortic Disease. Medicina (Kaunas) 2021;58:49. [Crossref] [PubMed]
- Roselli EE, Tong MZ, Bakaeen FG. Frozen elephant trunk for DeBakey type 1 dissection: the Cleveland Clinic technique. Ann Cardiothorac Surg 2016;5:251-5. [Crossref] [PubMed]
- Di Bartolomeo R, Pellicciari G, Cefarelli M, et al. Frozen elephant trunk surgery using the E-vita open plus prosthesis. Ann Cardiothorac Surg 2013;2:656-9. [PubMed]
- Kazui T. Total arch replacement with separated graft technique and selective antegrade cerebral perfusion. Ann Cardiothorac Surg 2013;2:353-7. [PubMed]
- Kazui T, Inoue N, Yamada O, et al. Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment. Ann Thorac Surg 1992;53:109-14. [Crossref] [PubMed]
- Shimamura K, Kuratani T, Matsumiya G, et al. Long-term results of the open stent-grafting technique for extended aortic arch disease. J Thorac Cardiovasc Surg 2008;135:1261-9. [Crossref] [PubMed]
- Moulakakis KG, Mylonas SN, Markatis F, et al. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg 2013;2:247-60. [PubMed]
- Di Eusanio M, Castrovinci S, Tian DH, et al. Antegrade stenting of the descending thoracic aorta during DeBakey type 1 acute aortic dissection repair. Eur J Cardiothorac Surg 2014;45:967-75. [Crossref] [PubMed]
- Lin HH, Liao SF, Wu CF, et al. Outcome of frozen elephant trunk technique for acute type A aortic dissection: as systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e694. [Crossref] [PubMed]
- Ma WG, Zheng J, Sun LZ, et al. Open Stented Grafts for Frozen Elephant Trunk Technique: Technical Aspects and Current Outcomes. Aorta (Stamford) 2015;3:122-35. [Crossref] [PubMed]
- Leontyev S, Tsagakis K, Pacini D, et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: results of a multicentre study. Eur J Cardiothorac Surg 2016;49:660-6. [Crossref] [PubMed]
- Takagi H, Umemoto T. ALICE Group. A Meta-Analysis of Total Arch Replacement With Frozen Elephant Trunk in Acute Type A Aortic Dissection. Vasc Endovascular Surg 2016;50:33-46. [Crossref] [PubMed]
- Verhoye JP, Belhaj Soulami R, Fouquet O, et al. Elective frozen elephant trunk procedure using the E-Vita Open Plus prosthesis in 94 patients: a multicentre French registry. Eur J Cardiothorac Surg 2017;52:733-9. [Crossref] [PubMed]
- Jakob H, Idhrees M, Bashir M. Frozen elephant trunk with straight vascular prosthesis. Ann Cardiothorac Surg 2020;9:164-9. [Crossref] [PubMed]
- Preventza O, Liao JL, Olive JK, et al. Neurologic complications after the frozen elephant trunk procedure: A meta-analysis of more than 3000 patients. J Thorac Cardiovasc Surg 2020;160:20-33.e4. [Crossref] [PubMed]
- Tian DH, Ha H, Joshi Y, et al. Long-term outcomes of the frozen elephant trunk procedure: a systematic review. Ann Cardiothorac Surg 2020;9:144-51. [Crossref] [PubMed]
- Zierer A, Voeller RK, Hill KE, et al. Aortic enlargement and late reoperation after repair of acute type A aortic dissection. Ann Thorac Surg 2007;84:479-86; discussion 486-7. [Crossref] [PubMed]
- Jakob H, Tsagakis K. International E-vita open registry. Ann Cardiothorac Surg 2013;2:296-9. [PubMed]
- Ius F, Fleissner F, Pichlmaier M, et al. Total aortic arch replacement with the frozen elephant trunk technique: 10-year follow-up single-centre experience. Eur J Cardiothorac Surg 2013;44:949-57. [Crossref] [PubMed]
- Pichlmaier MA. Thoraflex hybrid graft – the Munich experience. Second Aortic Live Symposium. Essen, Germany; September 11-13, 2014.
- Di Bartolomeo R, Di Marco L, Cefarelli M, et al. The Bologna experience with the Thoraflex™ hybrid frozen elephant trunk device. Future Cardiol 2015;11:39-43. [Crossref] [PubMed]
- Kozlov BN, Panfilov DS, Saushkin VV, et al. Distal aortic remodelling after the standard and the elongated frozen elephant trunk procedure. Interact Cardiovasc Thorac Surg 2019;29:117-23. [Crossref] [PubMed]
- Dohle DS, Tsagakis K, Janosi RA, et al. Aortic remodelling in aortic dissection after frozen elephant trunk†. Eur J Cardiothorac Surg 2016;49:111-7. [Crossref] [PubMed]
- Usai MV, Ibrahim A, Oberhuber A, et al. Quantification of volume changes in the descending aorta after frozen elephant trunk procedure using the Thoraflex hybrid prosthesis for type A aortic dissection. J Thorac Dis 2021;13:60-6. [Crossref] [PubMed]
- Phung DHS, Nguyen TS, Vo HL, et al. A novel modification of frozen elephant trunk technique: unique protocol from one institution. Eur Rev Med Pharmacol Sci 2021;25:4738-45. [PubMed]
- Song SB, Wu XJ, Sun Y, et al. A modified frozen elephant trunk technique for acute Stanford type A aortic dissection. J Cardiothorac Surg 2020;15:322. [Crossref] [PubMed]
- Okamura H, Kitada Y, Miyagawa A, et al. Clinical outcomes of a fenestrated frozen elephant trunk technique for acute type A aortic dissection. Eur J Cardiothorac Surg 2021;59:765-72. [Crossref] [PubMed]
- Bozso SJ, Nagendran J, MacArthur RGG, et al. Dissected Aorta Repair Through Stent Implantation trial: Canadian results. J Thorac Cardiovasc Surg 2019;157:1763-71. [Crossref] [PubMed]
- Bozso SJ, Nagendran J, Chu MWA, et al. Midterm Outcomes of the Dissected Aorta Repair Through Stent Implantation Trial. Ann Thorac Surg 2021;111:463-70. [Crossref] [PubMed]
- Montagner M, Heck R, Kofler M, et al. New Hybrid Prosthesis for Acute Type A Aortic Dissection. Surg Technol Int 2020;36:95-7. [PubMed]


