
Clinical findings, diagnosis and therapy of patent ductus venosus in children: a case series
Introduction
Patent ductus venosus (PDV) is an extremely rare form of congenital portosystemic shunt that results in the diversion of portal blood into the systemic circulation (1). PDV is associated with the potential for life-threatening complications, with early detection and proper management leading to a good prognosis and the prevention of serious complications. The ductus venosus (DV) is an embryonic vascular structure. In utero, the DV connects the umbilical vein to the inferior vena cava (IVC), allowing a portion of the oxygenated umbilical vein blood to bypass the liver and return to the heart. Spontaneous closure of the DV begins immediately after birth, with complete functional closure usually occurring by 18 days of age (2,3). Failure of DV closure after birth leads to PDV. PDV results in portal venous blood bypassing the liver and directly entering the systemic circulation, decreasing hepatic blood flow and increasing blood volume and toxic substances in the systemic circulation. Diagnosis of PDV primarily depends on imaging examinations. However, because of the diversity of clinical symptoms and insufficient knowledge of this condition, clinicians often fail to perform targeted examinations, resulting in missed diagnoses and misdiagnoses. Only a few cases of PDV have been reported, appearing in small case series, case reports, and literature reviews (4,5). Literature regarding radiological findings in patients with PDV is limited, and no reports exist concerning the secondary radiological findings of PDV. In this study, we summarized the clinical findings, secondary radiological findings, and surgical methods for 9 patients with PDV to improve early diagnosis and guide treatment. We present the following case in accordance with the AME Case Series reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-179/rc).
Methods
Patients
This study is a single-center retrospective case series study, which was approved by the institutional review board of Hunan Children’s Hospital, and the requirement for informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institute’s database was searched to retrieve the clinical, laboratory, and radiological data of patients with PDV who attended the hospital between May 2013 and December 2020. Consecutive patients with PDV were identified and enrolled based on the following inclusion criteria: (I) PDV confirmed by computed tomography (CT) and ultrasound according to the criteria described by Blanc et al. (1) (i.e., an intrahepatic shunt running at the depth of the Arantius sulcus between the left and caudate lobes from the proximal part of the left branch of the portal vein to the terminal part of the left hepatic vein) and (II) sufficient image quality to allow accurate interpretation of radiological features. Patients with a PDV diameter <3 mm and aged <2 weeks were excluded from this study due to a higher likelihood of the PDV closing spontaneously. In all, 9 patients with PDV were enrolled in this study (7 males and 2 females; median age 1.6 years, age range 16 days to 16.5 years).
Examinations and analysis
All patients underwent abdominal contrast-enhanced CT. CT examinations were performed using a Philips Brilliance 64 CT scanner with a tube voltage of 120 kV and a tube current of 200–250 mA. Iodixanol (320 mg I/mL) was used as the radiocontrast agent at a dose of 1.5–2.0 mL/kg body weight and an injection rate of 1.0–2.5 mL/s. Scans were obtained in the arterial phase 25 s after injection and in the portal vein phase 45 s after injection. All images from all patients were transferred to an imaging workstation (Philips Brilliance V4.5.4.50030) for postprocessing. To obtain clear depiction of the lesions, the main image processing methods used were sagittal, coronal position, maximum intensity projection (MIP), and volume rendering (VR). All patients underwent ultrasound and/or CT angiography of the cardiovascular system. Brain magnetic resonance imaging (MRI) was performed in 7 patients. Two pediatric radiologists (YHX and KJ, with 20 and 25 years’ experience, respectively) retrospectively reviewed all the images and reached a consensus for each patient. The data were counted and analyzed.
Treatment and follow-up
Two patients (patients 7 and 9) without coexisting malformations underwent surgical ligation of PDV in our hospital. Because these 2 patients had coagulation disorders and 1 presented with recurrent decreases in the platelet count associated with hypersplenism, we chose to ligate the PDV with a surgical procedure instead of percutaneous closure to avoid heparin-induced thrombocytopenia that could aggravate their coagulation disorders. Pulmonary artery and portal vein manometry were performed during the operation. A PDV banding test was performed before ligation, which indicated that the portal venous pressure increased transiently after banding and recovered 15 min later. The pulmonary artery pressure remained stable. The PDV was ligated (double ligation) in 1 step without clinical deterioration in 2 patients. Patient 3 underwent PDV ligation in another institute (details unclear). Patients 7 and 9 were reviewed in our hospital 1 year after their procedures. The remaining 6 patients were discharged from our hospital without surgical intervention for PDV, and were followed up by telephone for 2 weeks to 3 years until February 2022.
Statistical analysis
Demographic data were summarized using descriptive statistics. Descriptive statistics for categorical variables were reported as frequency and percentage.
Results
Clinical findings
The clinical characteristics and laboratory tests for all 9 patients are summarized in Table 1. The diameter of the PDV ranged from 4.0 to 17.5 mm. The initial clinical presentations of PDV varied, but jaundice and respiratory symptoms were the most common. Laboratory tests showed that 5 of 9 patients had hypoxemia, 2 had hyperammonemia, 7 had hyperbilirubinemia, 6 had abnormal coagulation function, 4 had abnormal myocardial enzymes, 8 had hepatic dysfunction, and 3 had renal dysfunction.
Table 1
| Case | Sex/age | Diam (mm) | Symptoms | Hypoxemia | NH4 (μmol/L) | Hyperbilirubinemia | Coagulation function | Myocardial enzyme | HD | RD |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/16 d | 7.0 | Icterus and cough | Yes | 60 | Yes | Abnormal | Abnormal | Yes | Yes |
| 2 | M/18 d | 5.0 | Cyanosis | Yes | 55 | Yes | Abnormal | Normal | Yes | Yes |
| 3 | M/25 d | 4.5 | Icterus | Yes | 86 | Yes | Abnormal | Normal | Yes | Normal |
| 4 | M/30 d | 7.1 | Icterus | Normal | 78 | Yes | Normal | Normal | Yes | Normal |
| 5 | F/1.6 y | 9.5 | Cough and fever | Yes | 46 | Yes | Normal | Abnormal | Yes | Normal |
| 6 | M/4.2 y | 4.0 | Cough and wheeze repeated | Normal | 60 | Normal | Normal | Normal | Yes | Normal |
| 7 | M/4.9 y | 10.0 | recurrent respiratory infections | Yes | 39 | Yes | Abnormal | Abnormal | Yes | Yes |
| 8 | M/10.2 y | 17.5 | Hemoptysis, recurrent respiratory infections | Normal | 41 | Normal | Abnormal | Normal | Normal | Normal |
| 9 | M/16.5 y | 14.5 | Yellowing sclera and skin for two years | Normal | 26 | Yes | Abnormal | Abnormal | Yes | Normal |
F, female; M, male; d, day; y, year; Age, age at diagnosis; Diam, diameter of PDV; HD, hepatic dysfunction; RD, renal dysfunction; PDV, patent ductus venosus. The reference range for NH4 is 18–72 μmol/L.
Imaging findings and coexisting malformations
The imaging findings and coexisting malformations of the 9 patients are summarized in Table 2. The direct imaging sign of PDV was a vascular structure connecting the left branch of the portal vein (LPV) to the IVC, running at the depth of the Arantius sulcus. Secondary imaging findings of PDV (Figure 1) included an enlarged livers (n=8), especially the left lobe, and multiple nodules in the liver (n=1). Three patients were found to have hypoperfusion in the right lobe of the liver on abdominal contrast-enhanced CT. The spleen was enlarged in 8 patients but shrunk in 1. A dilated LPV and atrophic right branch of the portal vein (RPV) were observed in all patients. The main portal vein was dilated in 8 patients and shrunk in 1. A dilated right heart and pulmonary artery were observed in all patients. Abnormal renal imaging was found in 2 patients. Complications and coexisting malformations included hepatic encephalopathy on brain MRI in 4 patients (Figure 2). A further 7 patients had other malformations, including congenital heart disease (CHD), vascular abnormalities, and congenital hypospadias; CHD and vascular abnormalities were the most common, found in 5 and 6 patients, respectively. The most common types of CHD associated with PDV were atrial septal defect (ASD) and patent ductus arteriosus (PDA).
Table 2
| Case | Liver | Spleen | PV | Heart dilation | PA | Renal imaging | Brain MRI | Coexisting malformations |
|---|---|---|---|---|---|---|---|---|
| 1 | Enlarged; hypoperfusion in right lobe | Small; CE reduced | MPV and RPV small; LPV and UV cystic dilated | Right heart | Dilated | CE of kidneys is reduced | NA | ASD; PDA; multiple arteriovenous fistulas |
| 2 | Enlarged | Enlarged | MPV and LPV dilated; thrombus in LPV; RPV small | Right heart | Dilated | Diffuse lesion of kidneys | Normal | ASD; PDA; VSD; Congenital hypospadias |
| 3 | Multiple nodules; hypoperfusion in right lobe | Enlarged | MPV and LPV dilated; RPV small | Right heart | Dilated | Normal | Normal | Hepatic arteriovenous fistula |
| 4 | Left lobe enlarged | Enlarged | MPV and LPV dilated; RPV small | Right heart | Dilated | Normal | Normal | ASD; PDA; Abernethy II malformation |
| 5 | Left lobe enlarged | Enlarged | MPV and LPV dilated; RPV small | Right heart | Dilated | Normal | HE | ASD; PDA; multiple skin hemangiomas |
| 6 | Enlarged | Enlarged | MPV and LPV dilated; RPV small | Right heart | Dilated | Normal | NA | Congenital hypospadias |
| 7 | Enlarged; hypoperfusion in right lobe | Enlarged | MPV and LPV slightly dilated; RPV small | Right heart | Dilated | Normal | HE | No |
| 8 | Left lobe enlarged | Enlarged | MPV dilated; LPV cystic dilated; RPV small | Whole heart | Dilated | Normal | HE | ASD; cerebrovascular malformation; bronchial artery-pulmonary fistula |
| 9 | Enlarged | Enlarged | MPV dilated; LPV cystic dilated; RPV small | Right heart | Dilated | Normal | HE | No |
CE, contrast enhancement; NA, not available; PV, portal vein; MPV, main portal vein; RPV, right branch of the portal vein; LPV, left branch of the portal vein; PA, pulmonary artery; UV, umbilical vein; HE, hepatic encephalopathy; ASD, atrial septal defect; PDA, patent ductus arteriosus; VSD, ventricular septal defect.
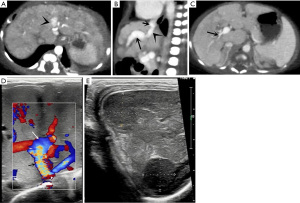

Treatment and follow-up
Two patients (patients 7 and 9) successfully underwent surgical ligation of the PDV without clinical deterioration during the procedure. There were no serious complications after surgery: patient 7 was hospitalized for 24 days (14 days before surgery and 10 days after), while patient 9 was hospitalized for 21 days (9 days before surgery and 12 days after); 1 year after their procedures, patients 7 and 9 showed normal exercise tolerance and weight gain. The laboratory parameters of myocardial enzymes and liver and kidney function were normal. The frequency of recurrent respiratory infections decreased in patient 7, and the jaundice in patient 9 disappeared. In the patient 3 who returned six months after the surgery to our hospital for examination, serum bilirubin, alanine aminotransferase, and aspartate aminotransferase were decreased.
Because PDV was associated with complex CHD and other malformations in 3 patients (patients 1, 2, and 5), their parents abandoned treatment, and these 3 patients died within 1 to 2 weeks after discharge. Another patient (patient 4), in whom PDV was associated with ASD, PDA, and Abernethy II malformation, was transferred to another hospital; this patient did not undergo surgery for PDV (no other details are available) and died 15 months after discharge. The remaining 2 patients (patients 6 and 8) were followed up by telephone for 3 years after discharge from our hospital. The parents of these patients reported that they were hospitalized 2–3 times a year at a local hospital for coughing, wheezing, fever, and other problems, with the frequency increasing each year; however, at the time of writing, these 2 patients have still not undergone surgical treatment for PDV. Patient 8 underwent surgical treatment for cerebrovascular malformation in another hospital 1 year ago.
Discussion
Clinical symptoms and laboratory tests
PDV is more common in boys (5,6), and, in our study, the male to female ratio was 7:2. The clinical presentations of PDV are highly diverse (7-10). In the present study, the initial symptoms and signs varied, but jaundice and respiratory symptoms were the most common. In younger patients, jaundice was often the initial presentation, whereas recurrent respiratory infections were more common in older patients. A previous report suggests that PDV is a very rare cause of cholestatic jaundice (6). Symptoms of patients with PDV can appear at different ages; patients may show severe symptoms in infancy or appear initially asymptomatic, with symptoms slowly developing with age.
Hepatic dysfunction secondary to PDV has been reported previously (11,12). In the present study, 89% (8/9) of patients had hepatic dysfunction. Hepatic dysfunction was an important initial presentation of PDV and may be secondary to a reduction in blood flow in the portal vein that deprives hepatocytes of nutrients and causes hepatic dysfunction, including in protein synthesis (12). Hyperammonemia can be caused by hepatocellular insufficiency or abnormal shunting of the portal blood from the intestine directly into the systemic circulation (13), and hepatic encephalopathy occurs with hyperammonemia. The incidence of hyperammonemia in patients with PDV has been reported to be high. In a study of 8 patients with PDV, all 8 were reported to have hyperammonemia (14). However, in the present study, hyperammonemia was detected in only 2 (22%) patients, and the serum ammonia concentrations of 4 patients with hepatic encephalopathy were within the normal range. Hypoxemia, hyperbilirubinemia, and coagulation dysfunction associated with PDV have been reported previously (5,6,14). In the present study, the incidence of hypoxemia, hyperbilirubinemia, and coagulation dysfunction was 56%, 78%, and 67%, respectively. Myocardial damage caused by PDV is rarely reported. In the present study, abnormal myocardial enzymes were detected in 4 (44%) patients. All 4 patients had a larger PDV diameter, and a significantly dilated pulmonary artery. We speculate that pulmonary hypertension leads to increased right ventricular afterload and contributes to myocardial damage. Previous studies have shown that congenital portosystemic shunt can lead to proliferative glomerulonephritis (15,16). In the present study, renal dysfunction was detected in 3 patients, 2 of whom exhibited decreased renal enhancement.
Imaging findings
Imaging examinations can lead to an accurate diagnosis based on the specific anatomic position of the PDV. The direct imaging sign of PDV is a vascular structure from the anterior inferior to the posterior position connecting the LPV to the IVC, running at the depth of the Arantius sulcus. PDV can be mistaken for the hepatic veins if its diameter is close to that of the hepatic veins on CT images; however, the PDV communicates with the LPV and its density is higher than that of the hepatic vein in the early stage of portal phase imaging, which enables discrimination of the PDV from the hepatic vein.
It is important to evaluate secondary imaging signs of PDV. PDV can lead to an increase in blood volume returning to right heart and then into the pulmonary artery, causing dilation of the right heart and pulmonary artery. In the present study, all patients had a dilated right atrium, right ventricle, and pulmonary artery. These signs were not specific for the diagnosis of PDV, but may be the first to be detected. This is because the initial presentation of patients is often precipitated by respiratory symptoms, and chest imaging examinations are therefore performed first. In fact, in several patients in this study, dilatation of the pulmonary artery was first observed, followed by further examinations to confirm PDV. Patient 7 was admitted several times for cough, and chest CT revealed obvious dilatation of the right heart and pulmonary artery for unknown reasons; however, abdominal examinations were not performed, leading to several missed diagnoses. Thus, PDV should be considered as a possible cause of unexplained dilatation of the right heart and pulmonary artery. PDV was previously reported to coexist with hypoplasia of the intrahepatic portal venous system (5,11). In the present study, all patients had a dilated LPV and an atrophic RPV. We believe that the PDV increased blood flow in the LPV, while decreasing flow in the RPV, leading to the dilatation of the LPV and atrophy of the RPV. This sign is rarely observed in other types of portosystemic shunt, and is a relatively characteristic indirect manifestation of PDV, which is of great value in identifying PDV. Atrophy of the RPV may result in decreased blood flow to the right lobe of the liver and manifests as decreased perfusion on CT images. Hypoperfusion in the right lobe of the liver was observed in 3 patients during portal phase imaging in the present study. In addition, enlargement of the liver and spleen was common, with liver enlargement manifesting as either whole liver or left lobe enlargement.
Complications and coexisting malformations
Complications of portosystemic shunt depend on the shunt ratio and age. When the shunt ratio is small, there may be no symptoms, whereas a large shunt ratio can lead to multiple systemic symptoms, such as pulmonary hypertension, hepatopulmonary syndrome, high-output heart failure, membranous proliferative glomerulonephritis, gastrointestinal bleeding, and hepatic encephalopathy (7,17,18). Hepatic encephalopathy due to cerebral effects of circulating toxins, which normally undergo first-pass metabolism in the liver, is a critical problem in patients with PDV. The age of onset of encephalopathy is variable and related, in part, to the volume and duration of the shunt. A previous study reported (19) that when the shunt ratio is <30%, symptoms associated with portosystemic shunt may not develop throughout the lifetime of the individual; however, when the shunt ratio exceeds 30%, hepatic encephalopathy could develop at any time, and when the shunt ratio exceeds 60%, the risk of hepatic encephalopathy is increased. In the present study, brain MRI revealed hepatic encephalopathy in 4 patients; unfortunately, the shunt ratio was not measured, but we found that the diameter of the PDV was larger in these patients, the smallest being 9.5 mm; in addition, these 4 patients were older, with a minimum age of 1.6 years. It is important to note that although hepatic encephalopathy is present, there may be no neurological symptoms or only mild symptoms. Indeed, 2 patients in the present study had no neurological symptoms, even though brain MRI suggested hepatic encephalopathy. Therefore, in the case of patients with a larger PDV diameter and older age, we suggest brain MRI should be performed to detect hepatic encephalopathy, regardless of the presence of neurological symptoms.
Patients with PDV may have other anatomical deformities (20). In the present study, 78% (7/9) of patients with PDV had other malformations involving multiple systems. CHD and vascular abnormalities were the most common, with 5 and 6 cases, respectively. The most common types of CHD associated with PDV were ASD and PDA. There is a recognized association between extrahepatic shunts and CHD (21). We believe that PDV is also associated with CHD. A review of the literature indicated that up to 25% of patients with PDV have CHD (20). In the present study, all patients had dilation of the right atrium and ventricle, so we speculate that PDV increased the blood volume returning to the right heart, increasing the pressure in the right heart and pulmonary artery, thereby delaying or impeding the closure of the ASD and PDA. These coexisting malformations are more likely to be detected and diagnosed, leading to missed diagnoses of PDV. Patient 8 in our study underwent ASD repair, residual ASD repair, and cerebrovascular malformation surgery in 3 different institutions, but PDV was missed each time. Finally, the patient was admitted to our institute because of hemoptysis, with chest CT demonstrating a dilated pulmonary artery and heart. Further examinations were performed to determine the cause, which led to the discovery of PDV.
Treatment
PDV rarely closes spontaneously and is the most likely of the intrahepatic shunts to require surgical closure or a catheter-based intervention (22). The treatment of PDV is inconsistent, with different therapeutic strategies being applied, including conservative management and surgical treatment, such as coil embolization, surgical ligation, Teflon banding, and liver transplant (5,6,23-26). In the present study, 2 patients underwent surgical treatment of PDV in our hospital. Because these 2 patients had coagulation disorders and 1 of them presented with recurrent decreases in the platelet count associated with hypersplenism, we chose to ligate the PDV with a surgical procedure instead of percutaneous closure to avoid heparin-induced thrombocytopenia that could result in the aggravation of their conditions. The adequacy of the portal venous system must be assessed before closure is attempted, both through imaging of the portal venous system and measurement of the rise in portal venous pressure with temporary occlusion of the PDV (27,28). Before ligation, we performed a PDV banding test, which indicated that portal pressure increased transiently after banding and recovered 15 min later. In both patients, the PDV was ligated in 1 step without clinical deterioration. In addition, there were no serious complications after the operation. Although a small RPV was observed in both patients, this might not have been truly hypoplastic. We speculate that a large PDV resulted in an insufficient opening of the portal branches by transferring most of the portal blood to the IVC. Hence, the portal pressure decreased spontaneously when the portal branches gradually opened after PDV banding. One year after their procedures, both patients exhibited normal exercise tolerance and weight gain. The laboratory parameters of myocardial enzymes and liver and kidney function were normal. The frequency of recurrent respiratory infections had decreased in 1 patient, and the other patient was no longer jaundiced. Unfortunately, 4 patients in this study died before surgical intervention. The time of symptom onset in these 4 patients was at an early age, and all 4 were complicated with CHD and other vascular malformations. Of these patients, 2 did not undergo surgical intervention of PDV for external reasons, and telephone follow-up of these patients indicates that their general condition is worsening.
Limitations
This study has some limitations. First, only a small number of PDV cases were included. Second, the study was a single-center study. Finally, only a few patients received surgical intervention.
Conclusions
In conclusion, PDV is a rare vascular malformation that can lead to multi-system damage. The clinical symptoms, signs and laboratory findings of PDV are diverse. The diagnosis of PDV depends on imaging examinations, with secondary radiological signs playing an important role in early diagnosis and preoperative evaluation. Complications and coexisting malformations are common and should not be missed during the preoperative evaluation. Early surgical closure of PDV is recommended.
Acknowledgments
We would like to thank N. Korszniak and J. Gray for their help in polishing our paper.
Funding: This study was supported by Hunan Provincial Science and Technology Department General Program (grant numbers: 2022JJ30320).
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-179/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-179/dss
Peer Review File: Available at https://dx.doi.org/10.21037/cdt-22-179
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-179/coif). YX reports that this study was supported by Hunan Provincial Science and Technology Department General Program (grant numbers: 2022JJ30320). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study is a single-center retrospective case series study, which was approved by the institutional review board of Hunan Children’s Hospital, and the requirement for informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blanc T, Guerin F, Franchi-Abella S, et al. Congenital portosystemic shunts in children: a new anatomical classification correlated with surgical strategy. Ann Surg 2014;260:188-98. [Crossref] [PubMed]
- Kondo M, Itoh S, Kunikata T, et al. Time of closure of ductus venosus in term and preterm neonates. Arch Dis Child Fetal Neonatal Ed 2001;85:F57-9. [Crossref] [PubMed]
- Fugelseth D, Lindemann R, Liestøl K, et al. Ultrasonographic study of ductus venosus in healthy neonates. Arch Dis Child Fetal Neonatal Ed 1997;77:F131-4. [Crossref] [PubMed]
- Ikeda S, Yamaguchi Y, Sera Y, et al. Surgical correction of patent ductus venosus in three brothers. Dig Dis Sci 1999;44:582-9. [Crossref] [PubMed]
- Yoshimoto Y, Shimizu R, Saeki T, et al. Patent ductus venosus in children: a case report and review of the literature. J Pediatr Surg 2004;39:E1-5. [Crossref] [PubMed]
- Kamali L, Moradi M, Ebrahimian S, et al. Patent ductus venosus in an infant with direct hyperbilirubinemia. Clin Case Rep 2019;7:1430-4. [Crossref] [PubMed]
- Konstas AA, Digumarthy SR, Avery LL, et al. Congenital portosystemic shunts: imaging findings and clinical presentations in 11 patients. Eur J Radiol 2011;80:175-81. [Crossref] [PubMed]
- Torigoe M, Maeshima K, Takeshita Y. Congenital intrahepatic portosystemic venous shunt presenting with paraparesis as the initial symptom. Intern Med 2013;52:2439-42. [Crossref] [PubMed]
- Ohno T, Muneuchi J, Ihara K, et al. Pulmonary hypertension in patients with congenital portosystemic venous shunt: a previously unrecognized association. Pediatrics 2008;121:e892-9. [Crossref] [PubMed]
- Lautz TB, Shah SA, Superina RA. Hepatoblastoma in Children With Congenital Portosystemic Shunts. J Pediatr Gastroenterol Nutr 2016;62:542-5. [Crossref] [PubMed]
- Jacob S, Farr G, De Vun D, et al. Hepatic manifestations of familial patent ductus venosus in adults. Gut 1999;45:442-5. [Crossref] [PubMed]
- Uchino T, Endo F, Ikeda S, et al. Three brothers with progressive hepatic dysfunction and severe hepatic steatosis due to a patent ductus venosus. Gastroenterology 1996;110:1964-8. [Crossref] [PubMed]
- Broere D, van Gemert WG, Kneepkens CM, et al. A 6-year-old boy with hyperammonaemia: partial N-acetylglutamate synthase deficiency or portosystemic encephalopathy? Eur J Pediatr 2000;159:905-7. [Crossref] [PubMed]
- Kamimatsuse A, Onitake Y, Kamei N, et al. Surgical intervention for patent ductus venosus. Pediatr Surg Int 2010;26:1025-30. [Crossref] [PubMed]
- Schaeffer DF, Laiq S, Jang HJ, et al. Abernethy malformation type II with nephrotic syndrome and other multisystemic presentation: an illustrative case for understanding pathogenesis of extrahepatic complication of congenital portosystemic shunt. Hum Pathol 2013;44:432-7. [Crossref] [PubMed]
- Karashima S, Hattori S, Nakazato H, et al. Membranoproliferative glomerulonephritis in congenital portosystemic shunt without liver cirrhosis. Clin Nephrol 2000;53:206-11. [PubMed]
- Law YM, Mack CL, Sokol RJ, et al. Cardiopulmonary manifestations of portovenous shunts from congenital absence of the portal vein: pulmonary hypertension and pulmonary vascular dilatation. Pediatr Transplant 2011;15:E162-8. [Crossref] [PubMed]
- Gong Y, Zhu H, Chen J, et al. Congenital portosystemic shunts with and without gastrointestinal bleeding - case series. Pediatr Radiol 2015;45:1964-71. [Crossref] [PubMed]
- Uchino T, Matsuda I, Endo F. The long-term prognosis of congenital portosystemic venous shunt. J Pediatr 1999;135:254-6. [Crossref] [PubMed]
- Poeppelman RS, Tobias JD. Patent Ductus Venosus and Congenital Heart Disease: A Case Report and Review. Cardiol Res 2018;9:330-3. [Crossref] [PubMed]
- Massin M, Verloes A, Jamblin P. Cardiac anomalies associated with congenital absence of the portal vein. Cardiol Young 1999;9:522-5. [Crossref] [PubMed]
- Paganelli M, Lipsich JE, Sciveres M, et al. Predisposing Factors for Spontaneous Closure of Congenital Portosystemic Shunts. J Pediatr 2015;167:931-935.e12. [Crossref] [PubMed]
- Xiao Y, Li W, Deng X, et al. Ligation of patent ductus venosus in a child with pulmonary arterial hypertension and hypersplenism: A case report. Medicine (Baltimore) 2020;99:e21849. [Crossref] [PubMed]
- Chacko A, Kock C, Joshi JA, et al. Patent ductus venosus presenting with cholestatic jaundice in an infant with successful trans-catheter closure using a vascular plug device. Indian J Radiol Imaging 2016;26:377-82. [Crossref] [PubMed]
- Marx M, Huber WD, Crone J, et al. Interventional stent implantation in a child with patent ductus venosus and pulmonary hypertension. Eur J Pediatr 2001;160:501-4. [Crossref] [PubMed]
- Kamata S, Kitayama Y, Usui N, et al. Patent ductus venosus with a hypoplastic intrahepatic portal system presenting intrapulmonary shunt: a case treated with banding of the ductus venosus. J Pediatr Surg 2000;35:655-7. [Crossref] [PubMed]
- Franchi-Abella S, Branchereau S, Lambert V, et al. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr 2010;51:322-30. [Crossref] [PubMed]
- Bernard O, Franchi-Abella S, Branchereau S, et al. Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis 2012;32:273-87. [Crossref] [PubMed]


