
The performance of SYNTAX score versus the coronary angiogram standard evaluation in the prediction of cardiovascular events in a cohort of patients with stable coronary heart disease
Introduction
Coronary artery disease (CAD) is the main cause of death in developed countries (1,2) and also important in Brazil (3). Despite the development of other imaging techniques, coronary angiography remains the gold standard for evaluating CAD. Coronary anatomy combined with clinical data drive the decisions on the treatment for patients with CAD (4,5). In the clinical practice, the severity of stenosis is more commonly based on the visual estimate by the interventional cardiologist. The development of new interventions and devices for myocardial revascularization has required standardized methods of CAD evaluation. Several scores of CAD severity were developed to predict the incidence of adverse outcomes (6-11), but most have not gained clinical utility. The SYNTAX Score (SXscore) (12) was developed for risk stratification of patients submitted to coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) in the SYNTAX Study (13), and is based on the number of lesions and their functional impact, location and complexity. One characteristic of studies comparing surgical or percutaneous coronary interventions and optimal medical treatment is the heterogeneity in the severity of the CAD of the participants. In addition, the lack of an ideal classification of CAD severity, and of the comparison of the complexity of lesions based on pre-treatment angiographic criteria, determines limitations to clinical interpretation. As far as we know, there is no comparative assessment of the performance of the standard CAD severity evaluation and the SXscore in the prediction of cardiovascular events in patients with chronic, stable, CAD submitted to CABG, PCI or medical-therapy (MT). Within this context, the purpose of this cohort study was to evaluate the performance of the SXscore versus the Coronary Angiogram Standard Evaluation (CASE) in the prediction of major cardiovascular outcomes in patients with chronic CAD referred for diagnostic angiography. We present the following article in accordance with the STROBE reporting checklist (14) (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-172/rc).
Methods
Details of this prospective cohort study have been previously reported (15). Methods relevant to this report are described below. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the Hospital de Clínicas de Porto Alegre, which is accredited by the Office of Human Research Protections as an Institutional Review Board, registered under No. 13-0171, and informed consent was taken from all individual participants.
Population
Patients with suspected CAD referred for elective diagnostic coronary angiography in a reference tertiary university-affiliated hospital, with 40 years of age or over, were sequentially enrolled into the study. Patients with acute coronary syndromes, valvular heart disease, aortic diseases (aneurism and dissection), previous coronary revascularization, class III or IV of the NYHA heart failure, chronic renal disease, history of cancer, or severe psychiatric illness were excluded.
Baseline evaluation
Lifestyle, demographic parameters, laboratory determinations, blood pressure, and the diagnosis of hypertension, diabetes and heart failure were assessed as described in the original report of this cohort (15). Three blood pressure measurements were performed using a validated automatic device according to guidelines (16). Diabetes mellitus was defined by the report of a physician’s diagnosis of diabetes or use of medication for diabetes (17), and heart failure (HF) by history and medical records (18).
The assessment of the SXscore was independently done by two certified interventional cardiologists, blinded for clinical features. Scores were calculated prospectively for all coronary lesions ≥50% diameter stenosis in vessels ≥1.5 mm, using the SXscore calculator available at http://www.syntax.com. Subsequently, they were categorized as high (>32), intermediate (23 to 32) and low SXscore (<23) (19) and no significant CAD (reference category). The SXscore was not used for therapeutic decision.
Multivessel CAD was characterized by involvement of more than one epicardial coronary artery or the unprotected left main (20). Considering CASE, left main or multivessel coronary artery disease (LMMCAD) of intermediate and high risk were defined through visual characterization, with reduction of the vessel diameter of at least 50% in vessels ≥1.5 mm, as follows: (I) stenosis of left main CAD; or (II) stenosis of three main vessels (coronary anterior descending, circumflex and right); or (III) stenosis of two main vessels, provided that one of them is the proximal anterior descending artery. The degree of stenosis, on visual analysis, was classified as 0%, lower 20% (wall irregularities), lower than 50% (without significant disease), and higher than 50% (significant disease) (21). For quantification of the LMMCAD lesions, the percentage of obstruction of the left main coronary artery or left anterior descending coronary, whichever was greater, was used. Angiographic visual analysis was independently done by two certified interventional cardiologists and a cardiovascular surgeon, blinded for clinical features. In case of disagreements, a fourth interventionist was consulted and the final decision was reached by consensus of all, with the purpose of minimizing significant potential intra and inter-observer variability.
Assessment of ten-year cardiovascular risk was done by the atherosclerotic cardiovascular disease (ASCVD) risk score (22).
Criteria for treatment allocation
The coronary angiogram was evaluated according to the routine protocol of the Unit of Interventional Cardiology of the Division of Cardiology of our hospital. Patients without coronary artery diameter stenosis above 50% were not considered for surgical or percutaneous treatment (23).
The decision for revascularization in patients with coronary lesions of at least 50% in at least one proximal epicardial coronary artery was additionally based on an objective evidence of myocardial ischemia, or at least one coronary stenosis of at least 70% and classic angina without provocative testing (23). Complex lesions that could be treated by either method, were evaluated by the surgeon, the interventional cardiologist and the clinician. The final allocation of these patients to the therapeutic alternatives was let to the discretion of the attending physicians and patients. Scores of coronary lesion severity were not used to support the decision and the SXscore was not available at the time of decision. Patients submitted to PCI were treated with first generation drug eluting and bare metal stents. Almost all patients treated by CABG received an arterial graft.
Outcomes
The primary endpoint of this study was all-cause death and major adverse cardiac and cerebral events (MACCE), defined by cardiovascular death, nonfatal myocardial infarction, stroke, and late revascularization (not done as a therapeutic option after the result of the angiogram). Individual outcomes were considered secondary endpoints.
Deaths were classified according to the Academic Research Consortium (ARC)-2 (24). Myocardial infarction and revascularization followed by death in the same hospitalization were adjudicated as cardiovascular death. Sudden death was defined, additionally, as cardiovascular death, unless obvious noncardiac causes could be identified. Myocardial infarction was diagnosed by symptoms, and ECG abnormalities suggestive of ischemia (24,25). Stroke was diagnosed by clinical findings and computed tomography. Incident HF was defined by hospitalization. Late revascularization was done either by PCI or CABG. Percutaneous and surgical revascularizations performed until three months after the angiography were defined as index procedures, and those occurring thereafter were considered outcomes.
Follow-up
The follow-up of participants was done by telephone interviews, registered letters, medical records, death certificates, and interviews of next of kin. All data were evaluated by at least two authors independently, with control of quality on data entry to verify amplitude and consistency.
Statistical analysis
The sample for these analyses came from studies planned to evaluate the effectiveness of therapeutic strategies to prevent death and major adverse cardiac and cerebral events in patients with stable CAD diagnosed by elective coronary angiography (26). The sample sizes were not calculated in advance for this analysis.
Quantitative variables were described by mean and standard deviation or median and interquartile range, and qualitative through absolute and relative frequencies. Variables were compared using Analysis of Variance (ANOVA) and the Scheffé test, or the Kruskal-Wallis test followed by the Dunn’s test, in case of quantitative variables, and the Pearson’s chi-squared test or the Fisher’s exact test for qualitative variables.
Exposure was defined by the presence of high SXscore (≥23) and by the presence of LMMCAD. All analyses were stratified by the therapeutic option (medical, PCI, CABG), in order to control for variation in the effectiveness of the methods.
Agreement between the SXscore and CASE was estimated using the kappa statistics. To calculate kappa, Program for epidemiologists for windows (WinPEPI) version 11.43 was used. A kappa ranging below 0.21 was classified as “poor”, from 0.21 to 0.40 was classified as “fair”, from 0.41 to 0.60 as “moderate”, from 0.61 to 0.80 as “good”, and 0.81 to 1.00 as “very good” (27).
The Kaplan-Meier curve was used to assess time until all-cause death, cardiovascular death, myocardial infarction, stroke, late revascularization and MACCE, and tested by the chi-square log-rank test to compare curves between groups. For this purpose, the SXscore was categorized into low (SXscore <23) and intermediate-high (SXscore ≥23).
The association between treatments and outcomes was explored in Cox proportional hazard models and described by Kaplan-Meier survival curves. Analyses were stratified by SXscore and by the presence or absence of LMMCAD and adjusted for the type of treatment and clinical variables.
The receiver-operating characteristic (ROC) curves were used to estimate the predictive performance of each method of assessment of the CAD of the study, which were compared using the DeLong Test by the Program for Epidemiological Analysis of Tabulated Data (EPIDAT) version 3.1. The area under the ROC curves (c-statistics), with 95% CI, sensitivity and specificity for each method, were calculated. Values for the area under the curve below 0.7 suggest no discrimination.
The level of significance was 5%. The data were analyzed with Statistical Package for the Social Sciences (SPSS) version 21.0.
Results
Baseline characteristics and angiographic data
Study flowchart is presented in Figure 1. From 1,028 patients electively submitted to diagnostic coronary angiography, 454 were included in the cohort and were followed up on average for 6±2.0 years (median 5.7 years), from 0.02 to 9.8 years. The use of cardiovascular drugs at the time of the follow-up interview was not substantially different among the treatment arms.
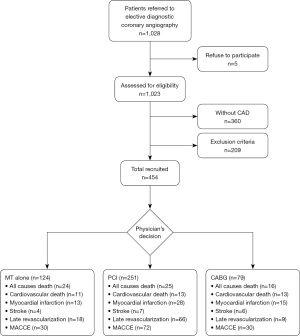
Table 1 shows the baseline clinical and angiographic characteristics according to patient categories classified by the treatment.
Table 1
| Baseline characteristics † | MT alone (n=124) | PCI (n=251) | CABG (n=79) | P value |
|---|---|---|---|---|
| Age (years) | 61.9±10.2 | 60.8±9.4 | 61.7±8.4 | 0.496 |
| Male | 69 (55.6)¶ | 173 (68.9) | 54 (68.4) | 0.032 |
| Race white | 87 (70.2) | 176 (70.1) | 61 (77.2) | 0.449 |
| School degree (years) | 5 [3–8] | 5 [4–9] | 6 [4–10] | 0.227 |
| BMI (kg/m²) | 28.9±5.2b | 28.1±4.3ab | 27.4±4.2a | 0.050 |
| SBP (mmHg) | 141.9±23.5 | 141.1±23.9 | 144.8±20.6 | 0.473 |
| DBP (mmHg) | 79.7±11.9 | 81.5±12.9 | 83.2±11.6 | 0.130 |
| Hypertension | 114 (91.9) | 236 (94.0) | 76 (96.2) | 0.460 |
| Diabetes mellitus | 42 (33.9) | 71 (28.3) | 31 (39.2) | 0.158 |
| Previous myocardial infarction | 45 (36.3) ¶ | 127 (50.6) | 51 (64.6)§ | <0.001 |
| HF | 19 (15.3) | 40 (15.9) | 17 (21.5) | 0.452 |
| LVFE (%) | 62.6±14.1 | 63.2±14.9 | 58.3±15.9 | 0.054 |
| Glucose (mg/dL) | 103.4±33.1 | 106.5±27.8 | 114.7±45.9 | 0.054 |
| Total Cholesterol (mg/dL) | 170.4±47.3 | 170.6±45.3 | 176.6±51.8 | 0.575 |
| HDL-C (mg/dL) | 41.4±11.1 | 39.7±9.9 | 40.7±10.1 | 0.284 |
| Triglycerides (mg/dL) | 119.5 (87.0–173.8) | 125 (90.0–169.0) | 122 (91.0–176.0) | 0.896 |
| Creatinine (mg/dL) | 0.69±0.21 | 0.71±0.21 | 0.72±0.22 | 0.805 |
| hs-CRP (mg/dL) | 2.5 (0.8–5.8) | 2.8 (0.9–7.0) | 2.1 (0.8–5.0) | 0.242 |
| Smoking | 85 (68.5) | 168 (66.9) | 44 (55.7) | 0.129 |
| Current smoking | 16 (12.9) | 40 (15.9) § | 3 (3.8) ¶ | 0.020 |
| Chest pain | 29 (23.4) | 44 (17.5) | 15.0 (19.0) | 0.400 |
| Dyspnea | 44 (35.5) | 68 (27.1) | 26 (32.9) | 0.218 |
| 10-year ASCVD risk | 16.7 (8.2–24.2) | 15.6 (9.1–23.4) | 19.6 (11.2–28.1) | 0.061 |
| SXscore‡ | 4.3 (0–11)a | 8 (5–13)b | 21.5 (13–26.5)c | <0.001 |
| Low SXscore | 117 (94.4) § | 235 (93.6) § | 49 (62.0) ¶ | <0.001 |
| Intermediate SXscore | 5 (4.0) ¶ | 15 (6.0) ¶ | 21 (28.6) § | |
| High SXscore | 2 (1.6) | 1 (0.4) ¶ | 9 (11.4) § | |
| LMMCAD | 20 (16.1) ¶ | 75 (29.9) ¶ | 62 (78.5) § | <0.001 |
| Indication of coronary angiography | ||||
| Suggestive symptoms of CAD | 100 (80.6) | 209 (83.3) | 72 (91.1) | 0.128 |
| With a positive noninvasive test | 50 (40.3) | 86 (34.3) | 38 (48.1) | 0.076 |
| Other complaints | 11 (8.9) | 16 (6.4) | 8 (10.1) | 0.469 |
†, Variables were described by mean ± SD, median (P25–P75) or as number (percentage). ‡, Low SXscore <23; Intermediate SXscore =23–32; High SXscore >32. §, Statistically significant positive association by adjusted residuals test to 5% of significance. ¶, Statistically significant negative association by adjusted residuals test to 5% of significance. a.b.c, Equal letters do not differ by the Scheffé’s or Dunn’s Test at 5% significance. MT, medical-therapy; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HF, heart failure; LVFE, left ventricular fraction ejection; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; ASCVD, atherosclerotic cardiovascular disease; LMMCAD, left main or multivessel CAD; CAD, coronary artery disease; SXscore, SYNTAX score.
Low SXscores were more frequent in patients treated with optimal medical therapy alone or with PCI, while patients who underwent CABG showed intermediate and high scores more frequently. LMMCAD was also more frequent in the CABG group when compared to the other groups.
The agreement between the classification of severe coronary lesions by the descriptive method (LMMCAD) and by the SXscore (≥23) (Kappa statistics) was poor and not significant in patients submitted to medical therapy (Kappa =0.07; 95% CI: −0.11 to 0.26; P=0.178). The agreement was higher and fair in patients treated with CABG (total sample: Kappa =0.29, 95% CI: 0.20 to 0.37, P<0.001; PCI: Kappa =0.18, 95% CI: 0.07 to 0.28, P<0.001 and CABG: Kappa =0.24, 95% CI: 0.11 to 0.38, P=0.001).
Survival and event-free outcomes
Event-free survival Kaplan-Meier curves stratified by types of treatment, SXscore and LMMCAD diagnosis, are shown in Figure 2. Figure 2A,2C,2E,2G shows a significant difference between the groups. In the total sample, patients presenting intermediate or high SXscores had a risk of death 1.94-fold higher when compared to patients in the same group who had low SXscores (Figure 2A). Additionally, patients with LMMCAD showed a risk of death 2.07-fold higher when compared to patients without these lesions (Figure 2E). In analyses stratified by the type of treatment, patients who underwent PCI and had intermediate or high SXscores had a risk of death 3.43-fold higher when compared to patients in the same group who had low SXscores (Figure 2C) and patients with LMMCAD showed a risk of death 2.75-fold higher when compared to patients without the disease (Figure 2G).
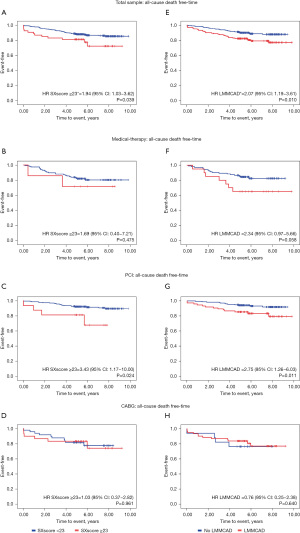
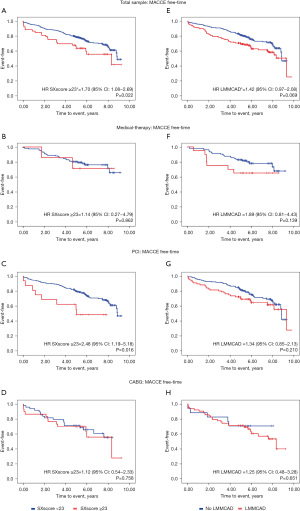
Figure 3 shows Kaplan-Meier curves for time until the occurrence of MACCE stratified by types of treatment, SXscore and LMMCAD diagnosis. Figure 3A,3C shows a significant difference between the groups. In the total sample, patients having an intermediate or high SXscore presented a 70% higher risk of MACCE when compared to those with lower risk (Figure 3A). Patients who underwent PCI, and had intermediate or high SXscores, had a risk of MACCE 2.48-fold higher when compared to patients in the same group who had low SXscores (Figure 3C). Patients with LMMCAD tended to present a higher risk of MACCE in the total sample when compared to those without the multivessel disease (Figure 3E).
Details of the data used in the Figures 2,3 are presented in the Table S1.
Table 2 shows adjusted risks for all-cause death and MACCE in participants with high SXscore and LMMCAD in participants stratified by the type of treatment and in the whole sample. For all-cause death the risk estimated by both scores was not substantially different in analyses stratified by the type of treatment, but was higher for the standard evaluation in the whole sample. For MACCE, LMMCAD had a higher risk than the SXscore in patients treated medically. The inverse occurred in patients treated with PCI, without any difference in patients treated with CABG and in the whole sample.
Table 2
| Treatment | All-cause death | MACCE | |||
|---|---|---|---|---|---|
| HRadjusted† (95% CI) | P value | HRadjusted† (95% CI) | P value | ||
| MT alone | |||||
| SXscore≥23 | 7.93 (0.86–73.3) | 0.068 | 2.99 (0.20–45.80) | 0.431 | |
| LMMCAD | 4.13 (0.96–17.7) | 0.056 | 8.72 (1.73–44.10) | 0.009 | |
| PCI | |||||
| SXscore≥23 | 4.83 (0.41–57.00) | 0.211 | 3.16 (0.97–10.30) | 0.055 | |
| LMMCAD | 3.65 (0.67–19.9) | 0.134 | 1.28 (0.58–2.86) | 0.541 | |
| CABG | |||||
| SXscore≥23 | 0.75 (0.08–7.22) | 0.802 | 1.73 (0.46–6.51) | 0.416 | |
| LMMCAD | 0.66 (0.02–18.6) | 0.808 | 1.36 (0.33–5.56) | 0.670 | |
| Total sample | |||||
| SXscore≥23 | 1.83 (0.52–6.39)‡ | 0.344 | 1.42 (0.97–2.08)‡ | 0.069 | |
| LMMCAD | 2.81 (1.17–6.74)‡ | 0.021 | 1.71 (0.94–3.13)‡ | 0.081 | |
†, adjusted for age, male, BMI, diabetes mellitus, hypertension, previous myocardial infarction, HDL-C, creatinine, LVEF, current smoking, and chest pain. ‡, adjusted for therapeutic method, age, male, BMI, diabetes mellitus, hypertension, previous myocardial infarction, HDL-C, creatinine, LVEF, current smoking, and chest pain. MACCE, major adverse cardiac and cerebral events; MT, medical-therapy; SXscore, SYNTAX score; LMMCAD, left main or multivessel coronary artery disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LVEF, left ventricular fraction ejection.
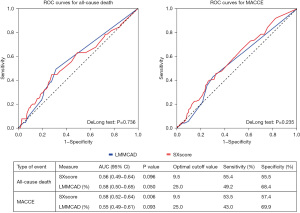
The comparison of the ROC curves of the two methods in predicting all-cause death and MACCE is shown in Figure 4. The area under the curve of both methods represents values below expected (<0.7) and there was no statistically significant difference among them in the prediction of these two endpoints.
Discussion
In this cohort study of patients submitted to elective coronary angiography for diagnosis and therapeutic decisions, the severity of coronary atherosclerosis defined by the CASE and by the SXscore provided similar prediction of the occurrence of cardiovascular events. The performance of both strategies was independent of the therapeutic modalities employed after the examination, and occurred despite the low agreement between the characterization of severity by the different approaches. This may be partially explained by the relatively low increment of the prediction of hard outcomes by both methods. These findings may be useful in the scenario of the therapeutic decision in patients with complex coronary lesions, showing that the traditional method to assess these lesions is not inferior to the more complex Syntax to predict clinical outcomes in patients submitted to any treatment strategy of coronary artery disease. The assessment of the predictive performance of anatomical criteria is particularly important since the publication of the results of the ISCHEMIA trial (28), that together with other studies demonstrated that testing for inducible myocardial ischemia is inferior to anatomic assessment for risk stratifying and managing patients with CHD.
Attempts to stratify the risk of patients with coronary lesions have started almost in parallel with the first descriptions of such lesions. Several scores of CAD severity were developed to predict the incidence of adverse outcomes, such as the SDTML disease classification (Single-, Double-, Triple-vessel and Main Left), the score of Gensini, and others (6-11). Nonetheless, these scores did not gain clinical utility, and the description of the severity of coronary atherosclerosis has been predominantly based on the number, sites, and percentage of coronary occlusion by atherosclerotic plaques.
The SXscore was developed as part of the SYNTAX Study (13) to objectively characterize and quantify the severity and extent of CAD, considering number of lesions and their functional impact, location and complexity, with the intention of stratifying patients for the selection of the best procedure (12). It was originally designed to predict procedural outcome for PCI vs. CABG, and not medical therapy. Subsequent evaluations of this score, both in the SYNTAX trial and in other data sets, demonstrated their predictive capacity for ischemic events in patients undergoing PCI (16,29-31).
Clinical prognostic variables have been combined with the anatomical SXscore to increase the its accuracy to guide the choice between PCI and CABG for patients with multivessel coronary disease, such as the logistic clinical SYNTAX score (31-35), the CABG SYNTAX Score (36,37), the SYNTAX score II (38-41), the SYNTAX score III (42,43), the residual SYNTAX score (44), and the clinical residual SYNTAX score (45). These more complex scores derived from the SYNTAX score have not gained practical application as well, and some of them have been less effective to predict outcomes than other functional predictors, such as the fractional flow reserve (FFR) (46,47). Nonetheless, functional derivations of the SYNTAX score were not assessed in our study, which compared the performance of the anatomical SYNTAX score with the standard evaluation of the coronary lesions. In the comparison of these methods, we showed that after adjustment for traditional demographic and clinical predictors, there was no substantial difference in the prognostic performance of the anatomical SYNTAX score and the standard evaluation of the coronary angiogram.
As far as we know, there is no comparative assessment of the performance of standard CAD severity evaluation and the SXscore in the prediction of cardiovascular events in patients with chronic, stable, CAD submitted to CABG, PCI or MT. As expected with any scoring system, the SXscore and the standard visual assessment have limitations. First, they are a purely anatomic score and do not integrate clinical variables that could be relevant for the patient’s risk stratification. Second, they are subject to interobserver variability, inherent to the visual estimate of the vessel’s stenosis. In addition, none is capable to assess the variation in the coronary anatomy of patients (diameters of the vessel, presence and location of the main branches, myocardial perfusion area, and others) or the impact of the presence or absence of viability beyond the stenosis. Also, these scores are subject to the inability to properly weigh significant differences in the skills of the assessor, experience in performing complex procedures, and impact of novel revascularization techniques or in the improvement of the technology of the devices. These differences between the two methods may be a possible explanation for the low Kappa statistics concordance between them.
This study has limitations that deserve mention, such as the fact that it was carried out in a single center. Another limitation of our study is the relatively small sample size, which may have influenced the estimates of risk ratios in some categories. For the purpose of our study, however, the risks were identified for higher scores in both methods and were not substantially different between them. The period of data collection of our study is not contemporary, but is unlikely that any treatment (stents, surgical technics, drugs) had a differential advance in recent years. The non-randomized design precludes to fully controlling for confounding. On the other hand, the cohort design, including all comers referred for diagnostic coronary angiography, represents more precisely patients with chronic CAD. The assessment of prognosis in patients submitted to all methods of treatment, with clinical outcomes, may be considered a major strength of our study.
Conclusions
The severity of coronary atherosclerosis defined by the CASE and by the SXscore provides similar prediction of the occurrence of cardiovascular events in patients with chronic CAD submitted to clinical, PCI or CABG therapies. The standard evaluation is easier to do and should be preferred in the anatomical stratification of risk in patients with CAD.
Acknowledgments
Funding: This work was supported by Research Support Fund at Hospital de Clínicas de Porto Alegre (FIPE-HCPA), registered under No. 13-0171, and did not receive any other specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-172/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-172/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-172/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-172/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the Hospital de Clínicas de Porto Alegre, which is accredited by the Office of Human Research Protections as an Institutional Review Board, registered under No. 13-0171, and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Timmis A, Townsend N, Gale CP, et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J 2020;41:12-85. [Crossref] [PubMed]
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020;141:e139-596. [Crossref] [PubMed]
- MINISTÉRIO DA SAÚDE. Departamento de Informática do SUS – DATASUS. Informações de Saúde (TABNET). Estatísticas Vitais. Available online: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sim/cnv/obt10uf.def. [Accessed June 20, 2020].
- Nallamothu BK, Spertus JA, Lansky AJ, et al. Comparison of clinical interpretation with visual assessment and quantitative coronary angiography in patients undergoing percutaneous coronary intervention in contemporary practice: the Assessing Angiography (A2) project. Circulation 2013;127:1793-800. [Crossref] [PubMed]
- Marcus ML, Skorton DJ, Johnson MR, et al. Visual estimates of percent diameter coronary stenosis: “a battered gold standard”. J Am Coll Cardiol 1988;11:882-5. [Crossref] [PubMed]
- Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51:606. [Crossref] [PubMed]
- Reeves TJ, Oberman A, Jones WB, et al. Natural history of angina pectoris. Am J Cardiol 1974;33:423-30. [Crossref] [PubMed]
- Burggraf GW, Parker JO. Prognosis in coronary artery disease. Angiographic, hemodynamic, and clinical factors. Circulation 1975;51:146-56. [Crossref] [PubMed]
- Friesinger GC, Page EE, Ross RS. Prognostic significance of coronary arteriography. Trans Assoc Am Physicians 1970;83:78-92. [PubMed]
- Balcon R, Cattell MR, Stone DL, et al. A computer generated index for the assessment of coronary angiography. Acta Med Scand Suppl 1978;615:25-31. [Crossref] [PubMed]
- Ringqvist I, Fisher LD, Mock M, et al. Prognostic value of angiographic indices of coronary artery disease from the Coronary Artery Surgery Study (CASS). J Clin Invest 1983;71:1854-66. [Crossref] [PubMed]
- Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219-27. [PubMed]
- Thuijs DJFM, Kappetein AP, Serruys PW, et al. Percutaneous coronary intervention versus coronary artery grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019;394:1325-34. [Crossref] [PubMed]
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg 2014;12:1500-24. [Crossref] [PubMed]
- Almeida AS, Fuchs SC, Fuchs FC, et al. Effectiveness of Clinical, Surgical and Percutaneous Treatment to Prevent Cardiovascular Events in Patients Referred for Elective Coronary Angiography: An Observational Study. Vasc Health Risk Manag 2020;16:285-97. [Crossref] [PubMed]
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159-219. [Crossref] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S13-28. [Crossref] [PubMed]
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895-e1032. [Crossref] [PubMed]
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. [Crossref] [PubMed]
- Brar SS, Syros G, Dangas G. Multivessel disease: percutaneous coronary intervention for classic coronary artery bypass grafting indications. Angiology 2008;59:83S-8S. [Crossref] [PubMed]
- Malagò R, D'Onofrio M, Tavella D, et al. Diagnostic accuracy in coronary stenosis: comparison between visual score and quantitative analysis (quantitative computed tomographic angiography) in coronary angiography by multidetector computed tomography-coronary angiography and quantitative analysis (quantitative coronary angiography) in conventional coronary angiography. J Comput Assist Tomogr 2010;34:652-9. [Crossref] [PubMed]
- Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S49-73. [Crossref] [PubMed]
- Sousa-Uva M, Neumann FJ, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4-90. [Crossref] [PubMed]
- Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation 2018;137:2635-50. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. [Crossref] [PubMed]
- Fuchs FC, Ribeiro JP, Fuchs FD, et al. Syntax Score and Major Adverse Cardiac Events in Patients with Suspected Coronary Artery Disease: Results from a Cohort Study in a University-Affiliated Hospital in Southern Brazil. Arq Bras Cardiol 2016;107:207-15. [Crossref] [PubMed]
- Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall (1991). 404 p.
- Ferraro R, Latina JM, Alfaddagh A, et al. Evaluation and Management of Patients With Stable Angina: Beyond the Ischemia Paradigm: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;76:2252-66. [Crossref] [PubMed]
- Capodanno D, Di Salvo ME, Cincotta G, et al. Usefulness of the SYNTAX score for predicting clinical outcome after percutaneous coronary intervention of unprotected left main coronary artery disease. Circ Cardiovasc Interv 2009;2:302-8. [Crossref] [PubMed]
- Valgimigli M, Serruys PW, Tsuchida K, et al. Cyphering the complexity of coronary artery disease using the syntax score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am J Cardiol 2007;99:1072-81. [Crossref] [PubMed]
- Serruys PW, Onuma Y, Garg S, et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention 2009;5:50-6. [Crossref] [PubMed]
- Garg S, Sarno G, Garcia-Garcia HM, et al. A new tool for the risk stratification of patients with complex coronary artery disease: the Clinical SYNTAX Score. Circ Cardiovasc Interv 2010;3:317-26. [Crossref] [PubMed]
- Farooq V, Vergouwe Y, Räber L, et al. Combined anatomical and clinical factors for the long-term risk stratification of patients undergoing percutaneous coronary intervention: the Logistic Clinical SYNTAX score. Eur Heart J 2012;33:3098-104. [Crossref] [PubMed]
- Iqbal J, Vergouwe Y, Bourantas CV, et al. Predicting 3-year mortality after percutaneous coronary intervention: updated logistic clinical SYNTAX score based on patient-level data from 7 contemporary stent trials. JACC Cardiovasc Interv 2014;7:464-70. [Crossref] [PubMed]
- Chichareon P, Onuma Y, van Klaveren D, et al. Validation of the updated logistic clinical SYNTAX score for all-cause mortality in the GLOBAL LEADERS trial. EuroIntervention 2019;15:e539-46. [Crossref] [PubMed]
- Farooq V, Girasis C, Magro M, et al. The CABG SYNTAX Score - an angiographic tool to grade the complexity of coronary disease following coronary artery bypass graft surgery: from the SYNTAX Left Main Angiographic (SYNTAX-LE MANS) substudy. EuroIntervention 2013;8:1277-85. [Crossref] [PubMed]
- Takahashi K, Thuijs DJFM, Hara H, et al. Impact of the CABG SYNTAX score on all-cause death at 10 years: a SYNTAX Extended Survival (SYNTAXES) substudy. EuroIntervention 2021;17:75-7. [Crossref] [PubMed]
- Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013;381:639-50. [Crossref] [PubMed]
- Escaned J, Collet C, Ryan N, et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur Heart J 2017;38:3124-34. [Crossref] [PubMed]
- Cid Alvarez AB, Gomez-Peña F, Redondo-Dieguez A, et al. Prognostic impact of the SYNTAX score II in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: analysis of a four-year all-comers registry. EuroIntervention 2019;15:e796-803. [Crossref] [PubMed]
- Modolo R, Chichareon P, van Klaveren D, et al. Impact of non-respect of SYNTAX score II recommendation for surgery in patients with left main coronary artery disease treated by percutaneous coronary intervention: an EXCEL substudy. Eur J Cardiothorac Surg 2020;57:676-83. [PubMed]
- Collet C, Onuma Y, Andreini D, et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J 2018;39:3689-98. [Crossref] [PubMed]
- Sonck J, Miyazaki Y, Collet C, et al. Feasibility of planning coronary artery bypass grafting based only on coronary computed tomography angiography and CT-derived fractional flow reserve: a pilot survey of the surgeons involved in the randomized SYNTAX III Revolution trial. Interact Cardiovasc Thorac Surg 2019;ivz046. Epub ahead of print. [Crossref] [PubMed]
- Farooq V, Serruys PW, Bourantas CV, et al. Quantification of incomplete revascularization and its association with five-year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation 2013;128:141-51. [Crossref] [PubMed]
- Yan L, Li P, Wang Y, et al. The Incremental Prognostic Value of the Clinical Residual SYNTAX Score for Patients With Chronic Renal Insufficiency Undergoing Percutaneous Coronary Intervention. Front Cardiovasc Med 2021;8:647720. [Crossref] [PubMed]
- Ciccarelli G, Barbato E, Toth GG, et al. Angiography Versus Hemodynamics to Predict the Natural History of Coronary Stenoses: Fractional Flow Reserve Versus Angiography in Multivessel Evaluation 2 Substudy. Circulation 2018;137:1475-85. [Crossref] [PubMed]
- Huang J, Emori H, Ding D, et al. Diagnostic performance of intracoronary optical coherence tomography-based versus angiography-based fractional flow reserve for the evaluation of coronary lesions. EuroIntervention 2020;16:568-76. [Crossref] [PubMed]


