
Prognostic value of N-terminal pro-form B-type natriuretic peptide (NT-proBNP) in patients with congenital heart disease undergoing cardiac surgery: a systematic review and meta-analysis of cohort studies
Introduction
Congenital heart disease (CHD) is defined as clinically significant structural malformations of the heart and/or intrathoracic great vessels appearing in embryonic stage (1). Currently, CHD is the most common birth defects worldwide and the primary cause of perinatal mortality (2,3), with birth prevalence about 8.9‰ in China (4) and 10‰ worldwide (5) Owing to remarkable success in pediatric cardiology and open-heart surgery, the number of adult patients with CHD is gradually increasing over the past few decades and will increase further over time. However, most cardiac surgical repairs are palliative rather than curative (6-8). Lots of CHD survivors undergoing heart surgery still have an elevated risk of cardiovascular events, such as arrhythmias and heart failure. In this case, to develop a prognostic tool for the risk of adverse events in patients with CHD undergoing cardiac surgery over the long term is necessary; it will be helpful for early identification and prevention and timely intervention of adverse events in patients with CHD undergoing cardiac surgery, and will help to prolong survival and enhance the quality of life of CHD patients.
As a well-recognized marker of heart failure in the general population, N-terminal pro-form B-type natriuretic peptide (NT-proBNP) has gained lots of interest over the last few years (6). NT-proBNP is an inactive by-product of B-type natriuretic peptide (BNP), which is synthesized by the ventricular myocytes and released into the circulation. It is a marker of volume expansion, increased myocardial wall stress, and cardiac pressure overload (9). NT-proBNP can be facilely measured with commercial kits (10). The reference levels of NT-proBNP’s vary with age and to the type of assay method (11).
With the application of NT-proBNP in the diagnosis and management of pediatric patients with various CHD phenotypes (6,12-15), quite a few studies have focused on the prognostic value of NT-proBNP in CHD patients undergoing cardiac surgery. However, the findings were inconsistent and sometimes conflict (16-20). At present, only one meta-analysis on this topic has been performed (21). The meta-analysis merely focused on patients with aortic stenosis (AS) undergoing transcatheter aortic valve implantation (TAVI) and evaluated the correlation of NT-proBNP with mortality. A comprehensive and timely meta-analysis of the prognostic value of NT-proBNP in congenital heart surgery is still lacking.
Therefore, we aimed to perform a comprehensive and updated meta-analysis about the prognostic value of NT-proBNP in CHD patients undergoing cardiac surgery. The results of our study could be helpful to facilitate heart team’s decision. Besides, our findings will help clinicians make more informed decisions in the management of patients undergoing cardiac surgery for correction/palliation of CHD. We present the following article in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting checklist and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-155/rc) (22,23).
Methods
Search strategy
Two authors (D Xing and Z Gong) independently identified studies published in English in PubMed, Web of Science Database, and Embase by September 2021. Relevant studies assessing the prognostic value of NT-proBNP in patients with CHDs undergoing cardiac surgery were identified. The following search terms were used: (I) congenital heart disease, congenital heart anomalies, congenital heart defect, congenital cardiac malformation, congenital heart malformation, congenital cardiac disease, congenital cardiac defect, congenital cardiac anomalies, congenital cardiovascular disease, cardiovascular defect, cardiovascular malformation, and cardiovascular anomalies; (II) NT-proBNP, N-terminal pro-BNP, N-terminal pro-brain natriuretic peptide, N-terminal pro-B-type natriuretic peptide, proBNP, amino-terminal pro-brain natriuretic peptide, aminoterminal pro-B-type natriuretic peptide, N-terminal prohormone B-type natriuretic peptide, BNP, B-type natriuretic peptide, brain natriuretic peptide, N-terminal proBNP, and natriuretic peptide. Afterwards, the authors manually searched and reviewed reference lists of relevant reviews and identified studies.
Exposures and outcomes of interest
In our study, the exposure of interest was NT-proBNP level. It was collected within ±3 months of the echocardiogram (17). The concentration of NT-proBNP above the average concentration was identified as high NT-proBNP level, otherwise, was low NT-proBNP level. The outcomes of interest were mortality, cardiovascular events, and other postoperative outcomes. Outcome of mortality was defined as all-cause mortality after congenital heart surgery. Defined as a combined endpoint, cardiovascular events included heart failure, arrhythmia (requiring treatment), thromboembolic events (pulmonary embolism, myocardial infarction, or ischemic cerebrovascular accident), and cardiac reinterventions (percutaneous or surgical) (24). Other postoperative outcomes included prolonged intensive care unit (ICU) stay (ICU stay >24 h), prolonged mechanical ventilation (mechanical ventilation time >48 h), hospitalization (for cardiac reasons), and acute kidney injury (AKI).
Study selection
We have expanded our inclusion criteria specifically to avoid omitting any studies when screening titles and abstracts. First, studies published in English reporting the prognostic value of NT-proBNP in CHD patients undergoing cardiac surgery were included. Afterwards, a full-text review of all selected studies was performed. Studies that met the following criteria were included: they were (I) cohort studies, (II) regarded individuals with CHD undergoing cardiac surgery as the study subjects, (III) had sufficient data to extract and estimate relative risk and their 95% confidence intervals (CIs), including the risk ratio (RR), odds ratio (OR), hazard ratio (HR), and data to calculate them, and (IV) were published in English. Conversely, studies were excluded if they (I) were case reports, conference abstracts, review papers, qualitative studies, experimental studies, case-control studies, or cross-sectional studies; (II) were duplicate publications. When there was more than one study concerning the same population of CHD, we only included the most comprehensive or recent published one.
Study quality assessment
All included studies were assessed for study quality using the Newcastle-Ottawa Scale (NOS) of cohort studies, and the quality of the included studies was independently assessed by two authors (D Xing and Z Gong). NOS assesses the quality of each study through three modules comprising a total of 8 items, each rated 1–9 stars. The NOS for cohort studies consists of the following three modules: (I) the selection of cohorts, (II) the comparability of cohorts, and (III) the evaluation of outcomes. The first and third modules have a total of seven items with a total score of seven stars; the second module has one item with a total score of two stars. Studies with a score of ≥6 (median) stars were considered to have high-quality methodological studies.
Data extraction
Two of our authors (D Xing and T Wang) independently completed the extraction of data from each included study using standardized forms, with a third reviewer involved in mediating disagreements when consensus could not be reached. The data we extracted includes: the first author and year of publication, study period, geographic region, study design, participants, sample size, age composition, type of CHD, type of surgery, assay method of NT-proBNP, assay time of NT-proBNP, controlled confounders, reported outcomes, and follow-up time. If both unadjusted and adjusted values were present in the study, we extracted the adjusted values preferentially.
Statistical analysis
RR was used as the measurement of prognostic value of NT-proBNP in CHD patients undergoing cardiac surgery. Separate meta-analyses were performed for three outcomes including mortality, cardiovascular events, and other postoperative outcomes. Random effects models were used to calculate the pooled RRs and their corresponding 95% CIs. Cochran’s Q statistic (P<0.10 was considered to have significant heterogeneity) and I2 statistic (I2>50% was considered to have significant heterogeneity) were used to quantitatively detect heterogeneity between studies.
Subgroup analysis was used to explore possible mediators of heterogeneity, and we analyzed the following variables: geographic region (e.g., European countries, non-European countries), type of CHD [e.g., AS, tetralogy of Fallot, systemic right ventricle, transposition of the great arteries (TGA), multiple types of CHD], type of surgery (e.g., TAVI, Fallot repair, Mustard or Senning, multiple types of cardiac surgery), assay method of NT-proBNP (e.g., electrochemiluminescence, Immulite 2500, information not available), whether adjusted for confounding factors (e.g., adjusted versus unadjusted), and follow-up time (e.g., <1 year, 1–8 years). Then, a Q test for heterogeneity was applied to compare the subgroup differences under the random effects model (P<0.05 implied statistically significant differences). Sensitivity analyses were also conducted by comparing the pooled risk estimates from random-effects model (REM) and fixed-effects model (FEM). Given the limited number of included studies for other postoperative outcomes, subgroup and sensitivity analyses were conducted only for mortality and cardiovascular events. Begg’s rank correlation test was used to evaluate publication bias (P<0.05 indicated statistically significant differences). R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria), Comprehensive Meta Analysis Version 2.2 and Review Manager Version 5.3 (The Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) were used for all analyses.
In addition, for studies showed levels of NT-proBNP in the form of logn (n=2, e, 10), only qualitative descriptions were performed rather than quantitatively combined analysis.
Results
Study characteristics
Two authors (D Xing and Z Gong) independently searched through a search strategy, supplemented by manual searches, and obtained a total of 3,876 studies. Among them, after screening titles and abstracts, 3,716 were deleted because of duplication, irrelevance to this study, or because it was a review. Then, after a full-text review of 160 studies, 128 were excluded. Finally, a total of 32 studies were included in the systematic review, of which 18 studies were included in the meta-analysis (17-20,24-37) and 14 were not (38-51) (Figure 1).
The characteristics of the 32 included studies were summarized in Table 1, with 14 published after 2016 (17,18,24-28,37-42,51) and 18 published before 2016 (19,20,29-36,43-50). The studies were published between 2008 and 2021 involving a total of 7,571 participants. Twenty-one (65.6%) studies (18-20,24,26-31,33-37,40,41,45-47,49) were conducted in the European countries and 11 (34.4%) studies (17,25,32,38,39,42-44,48,50,51) in non-European countries. All studies sampled CHD patients from clinic-based population. Eight (25.0%) studies were targeted on children, while the others were targeted on adults. Sixteen (50.0%) studies included patients with AS, one study included patients with systemic right ventricle, one study included patients with TGA, two studies included patients with tetralogy of Fallot (TOF), while the remaining studies included multiple types of CHD patients. Regarding type of surgery, TAVI was reported in 19 (59.4%) studies, Mustard or Senning in two studies, Fallot repair in one study, open-heart surgery in one study, multiple types of surgery in seven studies, while the information was not available in two studies. For the assay method of NT-proBNP, electrochemiluminescence immunoassay (ECLIA) was used in 20 (62.5%) studies, competitive enzyme immunoassay in two studies, chemiluminescence immunoassay (Immulite 2500) in two studies, enzyme-linked immunosorbent assay (ELISA) in two studies, while the method used in the remaining studies was unclear. Twenty-eight (87.5%) studies measured NT-proBNP levels at pre-operation, one study measured NT-proBNP levels at post-operation, and three studies measured NT-proBNP levels both at pre-operation and post-operation. All but 11 studies (34.4%) adjusted or matched for age, sex, and other possible confounders. For the follow-up time, 16 studies (50.0%) were <1 year, 11 studies (34.4%) were 1–8 years, while the others were unclear.
Table 1
| First author, Year | Study period | Geographic region | Study design | Participants | Sample size | Age composition | Type of CHD | Type of surgery | Assay method of NT-proBNP | Assay time of NT-proBNP | Controlled confounders | Outcomes | Follow-up time | Quality scores |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ito 2020 | 2012 | Minnesota, America | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 130; NEG: 131 | Adults only | AS | TAVI | NA | Pre-operation | Echocardiography parameters | Mortality | 2.7 years | 8 |
| Parker 2019 | 2010–2014 | United States and Canada, Americas | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 522 | Children only | Multiple | Multiple | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation, post-operation | STS score, RACHS-1 category | Mortality | 30 days | 8 |
| Lin 2019 | 2004 | China, Asia | Retrospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 148; NEG: 181 | Children only | Multiple | Multiple | Competitive enzyme immunoassay; ReLIA II, Shenzhen, China | Pre-operation | Age, gender, weight, cardiopulmonary bypass time, aorta cross clamp time, and RACHS-1 category | Prolonged ICU stay | NA | 8 |
| Seoudy 2020 | 2010–2018 | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 126; NEG: 378 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | Age and gender | Mortality | 1.8 years | 7 |
| Burke 2018 | 2015–2016 | United States, Americas | Retrospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 46; NEG: 96 | Adults only | AS | TAVI | NA | Pre-operation | None | Mortality, cardiovascular events, prolonged ICU stay | 30 days | 7 |
| Vale 2018 | 2008–2014 | Portugal, Europe | Retrospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 151 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Post-operation | STS score, ejection fraction, major bleeding, and comorbidities | Mortality | 1 year | 7 |
| Qu 2017 | 2014 | China, Asia | Retrospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 363 | Children only | Multiple | Multiple | Competitive enzyme immunoassay; ReLIA II, Shenzhen, China | Pre-operation | RACHS-1 category | Prolonged ICU stay, prolonged mechanical ventilation | 3 days | 7 |
| Popelová 2017 | 2005–2015 | Czech Republic, Europe | Retrospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 87 | Adults only | TGA | Mustard or Senning | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | None | Mortality | 6.4 years | 6 |
| Baldenhofer 2017 | 2009–2011 | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 100 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | None | Cardiovascular events | 1 year | 6 |
| Stundl 2017 | 2009–2014 | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 113; NEG: 348 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | None | Mortality, cardiovascular events | 1 year | 7 |
| Baggen 2017 | 2011–2013 | the Netherlands, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 297; NEG: 298 | Adults only | Multiple | Multiple | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | Age and sex | Cardiovascular events | 3.5 years | 7 |
| Westhoff-Bleck 2016 | 2003–2013 | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 81 | Adults only | TOF | Fallot repair | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | None | Cardiovascular events | 6.9 years | 7 |
| Stähli 2015 | 2008–2012 | Switzerland, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 122; NEG: 122 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | Age and gender | Mortality | 9 months | 7 |
| Lindman 2015 | 2000–2014 | United States, Americas | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 345 | Adults only | AS | TAVI | NA | Pre-operation | Sex, glomerular filtration rate, diabetes mellitus, obstructive lung disease, NYHA class, and aortic mean gradient | Mortality | 1.9 years | 7 |
| Krau 2015 | 2011–2013 | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 154; NEG: 163 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | None | Mortality | 2 years | 7 |
| Bucholz 2015 | NA | United States, Americas | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 51; NEG: 51 | Children only | Multiple | Multiple | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation, post-operation | Age, preoperative estimated GFR percentile, hospital site, RACHS-1 category, and CPB time | AKI, prolonged ICU stay | NA | 6 |
| Sinning 2015 | 2010–2013 | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 310 | Adults only | Multiple | TAVI | Immulite 2500; Siemens Medical Solutions Diagnostics, La Garenne Colombes, France | Pre-operation | None | Mortality | 1 year | 7 |
| Frogoudaki 2014 | NA | Greece, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 76 | Adults only | Multiple | Multiple | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | Neutrophil gelatinase-associated lipocalin | Mortality, cardiovascular events | 1.7 years | 6 |
| Borz 2014 | 2006–2012 | France, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 118; NEG: 118 | Adults only | Multiple | TAVI | Immulite 2500; Siemens Medical Solutions Diagnostics, La Garenne Colombes, France | Pre-operation | None | Mortality, cardiovascular events, AKI | 1 year | 7 |
| Seiffert 2014 | NA | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 347 | Adults only | AS | TAVI | NA | Pre-operation | Age and sex | Mortality | 1 year | 7 |
| Ribeiro 2014 | NA | Canada, Americas | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 148; NEG: 185 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | Sex, chronic atrial fibrillation, chronic obstructive pulmonary disease, estimated glomerular filtration, and stroke volume index | Mortality | 4 years | 7 |
| Westhoff-Bleck 2013 | 2003–2013 | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 116 | Adults only | Systemic right ventricle | Mustard or Senning | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | NYHA class, tricuspid regurgitation, systemic ventricular function, and history of tachyarrhythmia | Mortality, cardiovascular events, hospitalisation | 7.3 years | 7 |
| Elhmidi 2013 | 2007–2010 | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 123; NEG: 250 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | None | Mortality | 1 year | 7 |
| Lange 2012 | 2007–2010 | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 420 | Adults only | AS | TAVI | NA | Pre-operation | Stroke, STS score, and ejection fraction | Mortality | 6 months | 7 |
| Rérez-Piaya 2011 | 2007–2008 | Spain, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 68 | Children only | Multiple | Open-heart surgery | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | Age and RACHS-1 category | Prolonged ICU stay | 2 days | 7 |
| Spargias 2011 | 2007–2010 | Greece, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 27; NEG: 54 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | None | Mortality | 1.4 years | 6 |
| Pfister 2010 | 2008–2009 | Germany, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | EG: 15; NEG: 16 | Adults only | Multiple | TAVI | NA | Pre-operation | None | Mortality | 2 months | 6 |
| Carmona 2008 | 2004–2005 | Brazil, Americas | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 46 | Children only | Multiple | NA | ELISA; Biomedica, Vienna, Austria | Pre-operation | Age and gender | Mortality | NA | 6 |
| Røsjø 2014 | 2009–2012 | Norway, Europe | Prospective cohort study | EG: patients with AS needed to surgery; NEG: Age, body mass index and gender-matched healthy control subjects | EG: 57; NEG: 13 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | Age, body mass index, and gender | Mortality | 3.5 years | 8 |
| Hirono 2014 | 2008–2012 | Japan, Asia | Prospective cohort study | EG: patients with TOF after surgical repair; NEG: Age-matched healthy control patients | EG: 58; NEG: 53 | Children only | TOF | NA | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | Age | Reoperation | NA | 7 |
| Seoudy 2021 | 2010–2019 | the Netherlands, Europe | Prospective cohort study | EG: patients from hospital with high NT-proBNP level; NEG: patients from hospital with low NT-proBNP level | Total: 349 | Adults only | AS | TAVI | ECLIA; Roche Diagnostics, Mannheim, Germany | Pre-operation | Age, gender, congenital diagnosis, NYHA class, cardiac medication and systemic ventricular function | Mortality | 1 year | 8 |
| Green 2021 | 2010–2014 | United States, Americas | Retrospective cohort study | EG: patients with AS needed to surgery; NEG: Age, body mass index and gender-matched healthy control subjects | Total: 162 | Children only | Multiple | Multiple | ELISA | Pre-operation, post-operation | STS score | Hospitalisation | NA | 7 |
NT-proBNP, N-terminal pro-form B-type natriuretic peptide; CHD, congenital heart diseases; EG, exposed group; NEG: non-exposed group; AS, aortic stenosis; TAVI, transcatheter aortic valve implantation; NA, not available; ECLIA, electrochemiluminescence; STS score, Society of Thoracic Surgeons score; RACHS-1 category, Risk Adjustment for Congenital Heart Surgery-1 category; ICU, intensive care unit; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; NYHA class, New York Heart Association functional class; GFR, glomerular filtration rate; CPB, cardiopulmonary bypass; AKI, acute kidney injury; ELISA, enzyme-linked immunosorbent assay.
Out of the 32 included studies, 24 studies reported on mortality (17 of them were able to extract the information needed for the meta-analysis); nine studies reported on cardiovascular events (eight of them provided sufficient information for meta-analysis); 11 studies reported on other postoperative outcomes (three of them provided sufficient information for meta-analysis), specifically, two reported on AKI, five studies reported on prolonged ICU stay, one study reported on prolonged mechanical ventilation, two studies reported on hospitalisation, and one study reported on reoperation.
All included studies were cohort studies, so the NOS of cohort studies was used to assess study quality. Each study had a score of ≥6, as shown in Table 1.
NT-proBNP levels and risk of mortality
Risk estimate between NT-proBNP levels and mortality in patients with CHDs undergoing cardiac surgery are summarized in Figure 2. Overall, CHD patients at high NT-proBNP levels yielded a significantly increased risk of mortality compared with those at low NT-proBNP levels (RR =1.14; 95% CI: 1.08–1.20; n=17). However, significant heterogeneity was detected (I2=90%; P<0.1). The Begg’s rank correlation test did not find potential evidence of publication bias (z=1.36, P=0.20). Sensitivity analysis was performed by comparing the pooled risk estimates of mortality from REM and FEM; result showed that the risk estimate of mortality associated with the high level of NT-proBNP based on the two methods were not significantly different (REM: RR =1.14, 95% CI: 1.08–1.20; FEM: RR =1.02, 95% CI: 1.01–1.03).
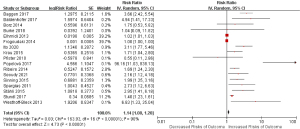
Subgroup analyses for risk estimates between NT-proBNP levels and mortality in patients with CHD undergoing cardiac surgery were summarized in Table 2. Overall, the variables “geographic region” (χ2=6.29, P=0.01), “type of CHD” (χ2=15.20, P<0.01), and “assay method of NT-proBNP” (χ2=6.90, P=0.03) could serve as moderators of heterogeneity, however, neither of the variables “type of surgery” nor “whether adjusted for confounding factors” could explain the source of heterogeneity (all P>0.05). Specifically, the risk of mortality associated with a higher level of NT-proBNP increased further when data focused on European countries (RR =1.10; 95% CI: 1.05–1.16), studies included patients with AS (RR =1.81; 95% CI: 1.38–2.39), studies measured NT-proBNP by ECLIA (RR =1.11; 95% CI: 1.05–1.16), studies followed up less than or equal to 1 year (RR =1.59; 95% CI: 1.19–2.12), and studies followed up 1 to 8 years (RR =2.66; 95% CI: 1.54–4.57).
Table 2
| Subgroup variables | No. of studies | Pooled RR (95% CI) | I2 (%) | P for heterogeneity | Test for subgroup differences | |
|---|---|---|---|---|---|---|
| χ2 | P | |||||
| Geographic region | 17 | 6.29 | 0.01 | |||
| Europe | 14 | 1.10 (1.05, 1.16) | 91.0 | <0.01 | ||
| Non-Europe | 3 | 2.09 (1.27, 3.45) | 46.0 | 0.16 | ||
| Type of CHD | 15.20 | <0.01 | ||||
| AS | 10 | 1.81 (1.38, 2.39) | 88.0 | <0.01 | ||
| TGA | 1 | 96.16 (11.03, 838.13) | – | – | ||
| Systemic right ventricle | 1 | 6.83 (1.33, 35.04) | – | – | ||
| Multiple | 5 | 1.64 (0.81, 3.32) | 92.0 | <0.01 | ||
| Type of surgery | 3.74 | 0.15 | ||||
| TAVI | 13 | 1.79 (1.39, 2.30) | 86.0 | <0.01 | ||
| Mustard or Senning | 2 | 23.20 (1.75, 307.82) | 73.0 | 0.06 | ||
| Multiple | 2 | 1.88 (0.53, 6.70) | 97.0 | <0.01 | ||
| Assay method of NT-proBNP | 6.90 | 0.03 | ||||
| ECLIA | 12 | 1.11 (1.05, 1.16) | 92.0 | <0.01 | ||
| Immulite 2500 | 2 | 1.96 (1.27, 3.01) | 0.0 | 0.84 | ||
| NA | 3 | 1.56 (0.46, 5.26) | 54.0 | 0.12 | ||
| Whether adjusted for confounding factors | 1.12 | 0.29 | ||||
| Adjusted | 7 | 2.34 (1.37, 3.99) | 93.0 | <0.01 | ||
| Unadjusted | 10 | 1.68 (1.23, 2.28) | 86.0 | <0.01 | ||
| Follow-up time | 2.67 | 0.10 | ||||
| <1 year | 9 | 1.59 (1.19, 2.12) | 84.0 | <0.01 | ||
| 1–8 years | 8 | 2.66 (1.54, 4.57) | 93.0 | <0.01 | ||
NT-proBNP, N-terminal pro-form B-type natriuretic peptide; RR, risk ratio; CI, confidence interval; CHD, congenital heart disease; AS, aortic stenosis; TGA, transposition of the great arteries; TAVI, transcatheter aortic valve implantation; ECLIA, electrochemiluminescence immunoassay; NA, not available.
There were five (43,45,46,48,49) studies dealing with NT-proBNP levels as log-transformed data. Carmona et al. (48) assessed children younger than 18 months with CHD undergoing cardiac surgery and suggested that preoperative NT-proBNP level was associated with higher overall mortality. Lindman et al. (43), Seiffert et al. (45), and Røsjø et al. (49) assessed adults older than 70 years with AS undergoing TAVI and showed that preoperative NT-proBNP levels were associated with a significantly higher risk of mortality, while Lange et al. (46). did not find a significant association between preoperative NT-proBNP levels and risk of mortality in AS patients over 70 years old and undergoing TAVI.
NT-proBNP levels and risk of cardiovascular events
Risk estimate between NT-proBNP levels and cardiovascular events in patients with CHDs undergoing cardiac surgery are summarized in Figure 3. Overall, CHD patients at high NT-proBNP levels had a significantly higher risk of cardiovascular events compared with those at low NT-proBNP levels (RR =2.02; 95% CI: 1.26–3.24; n=8). However, significant heterogeneity was detected (I2=87%; P<0.1). The Begg’s rank correlation test did not find potential evidence of publication bias (z=0.99, P=0.32). Sensitivity analysis was performed by comparing the pooled risk estimates of mortality from REM and FEM. The results showed that the risk estimate of cardiovascular events associated with the high level of NT-proBNP based on the two methods was not significantly different (REM: RR =2.02; 95% CI: 1.26–3.24; FEM: RR =1.01, 95% CI: 1.01–1.02).

Subgroup analyses for risk estimates between NT-proBNP levels and cardiovascular events in patients with CHD undergoing cardiac surgery were summarized in Table 3. Overall, none of the variables “geographic region”, “type of CHD”, “type of cardiac surgery”, and “whether adjusted for confounding factors” could explain the source of heterogeneity (all P>0.05). Specifically, when data focused on European countries (RR =1.82; 95% CI: 1.13–2.92), studies adjusted for confounding factors (RR =2.17; 95% CI: 1.83–5.69), the risk of cardiovascular events associated with NT-proBNP levels increased further, and studies were followed-up 1 year to 8 years (RR =2.67; 95% CI: 1.09–6.59).
Table 3
| Subgroup variables | No. of studies | Pooled RR (95% CI) | I2 (%) | P for heterogeneity | Test for subgroup differences | |
|---|---|---|---|---|---|---|
| χ2 | P | |||||
| Geographic region | 2.71 | 0.10 | ||||
| Europe | 7 | 1.82 (1.13, 2.92) | 87.0 | <0.01 | ||
| Non-Europe | 1 | 4.59 (1.69, 12.44) | – | – | ||
| Type of CHD | 6.03 | 0.11 | ||||
| AS | 3 | 2.36 (0.90, 6.16) | 80.0 | <0.01 | ||
| TOF | 1 | 5.49 (1.75, 17.23) | – | – | ||
| Systemic right ventricle | 1 | 6.83 (1.33, 35.04) | – | – | ||
| Multiple | 3 | 1.35 (0.65, 2.77) | 92.0 | <0.01 | ||
| Type of surgery | 4.97 | 0.17 | ||||
| TAVI | 4 | 1.73 (0.87, 3.44) | 76.0 | <0.01 | ||
| Fallot repair | 1 | 5.49 (1.75, 17.23) | – | – | ||
| Mustard or Senning | 1 | 6.83 (1.33, 35.04) | – | – | ||
| Multiple | 2 | 1.65 (0.60, 4.59) | 96.0 | <0.01 | ||
| Whether adjusted for confounding factors | 0.00 | 0.97 | ||||
| Adjusted | 3 | 2.17 (1.83, 5.69) | 94.0 | <0.01 | ||
| Unadjusted | 5 | 2.11 (1.05, 4.24) | 78.0 | <0.01 | ||
| Follow-up time | 0.57 | 0.45 | ||||
| <1 year | 4 | 1.73 (0.87, 3.44) | 76.0 | <0.01 | ||
| 1–8 years | 4 | 2.67 (1.09, 6.59) | 92.0 | <0.01 | ||
NT-proBNP, N-terminal pro-form B-type natriuretic peptide; RR, risk ratio; CI, confidence interval; CHD, congenital heart disease; AS, aortic stenos; TOF, Tetralogy of Fallot; TAVI, transcatheter aortic valve implantation.
NT-proBNP levels and risk of other postoperative outcomes
Risk estimate between NT-proBNP levels and other postoperative outcomes in patients with CHD undergoing cardiac surgery are summarized in Figure 4. Overall, CHD patients at high NT-proBNP levels did not yield a significantly increased risk of other postoperative outcomes compared with those at low NT-proBNP levels (RR =1.73; 95% CI: 0.86–3.47; n=3). However, significant heterogeneity was detected (I2=71%; P<0.1), and no publication bias was detected by Begg’s rank correlation test (z=0.52, P=0.60). Subgroup and sensitivity analysis were not performed due to the limited number of included studies.

There were four (42,44,47,50) studies dealing with NT-proBNP levels as log-transformed data. These studies all assessed children younger than 7 years with CHD undergoing cardiac surgery. Qu et al. (42), Bucholz et al. (44), and Rérez-Piaya et al. (47) all reported that NT-proBNP levels associated with a significantly higher risk of prolonged ICU stay in pediatric CHD patients, and Bucholz et al. (44), showed a significant relationship between NT-proBNP levels and AKI. Hirono et al. (50) demonstrated a significant increased risk estimate between preoperative NT-proBNP level and reoperation in children corrected TOF.
Discussion
In our study, by utilizing data from 32 published studies involving 7,571 CHD individuals, we provided evidence on the prognostic value of NT-proBNP in CHD patients undergoing cardiac surgery. Compared with those at low NT-proBNP levels, CHD patients at high NT-proBNP levels had significantly increased risks of mortality and cardiovascular events, whereas the risk of other postoperative outcomes was comparable between the two groups. As far as we know, our study is the most comprehensive and up-to-date meta-analysis assessing the prognostic value of NT-proBNP in congenital heart surgery.
To date, only one meta-analysis has been performed on the prognostic value of NT-proBNP in CHD patients undergoing cardiac surgery. Based on 16 eligible studies involving a total of 3,679 patients who underwent TAVA for AS, Takagi et al. indicated that high levels of baseline NT-proBNP predict increased mortality after TAVI for AS (OR =1.88) (21). Our study provides supportive evidence for this. Compared with those at low levels of NT-proBNP, patients with CHD undergoing cardiac surgery at high levels of NT-proBNP yielded a significantly increased risk of mortality (RR =1.14); risk estimates were further increased when the data focused only on studies performed in AS patients (RR =1.81) and studies using TAVI as a surgical method (RR =1.79). On the one hand, the sample size of our study is large than that of the previous meta-analysis, which can help to increase the power of our results. On the other hand, in the previous meta-analysis, only the relative risk for mortality associated with high levels of baseline NT-proBNP was estimated. The non-fatal outcomes such as cardiovascular events, which are common and important in CHD patients, are not reported. Unique to our study, the impact of prognostic value of baseline NT-proBNP for risk of cardiovascular events and other postoperative outcomes in CHD patients undergoing cardiac surgery were evaluated. Results showed that high levels of preoperative NT-proBNP were linked with a statistically significant increment in cardiovascular events; this suggests that regular postoperative follow-up of patients with CHD undergoing cardiac surgery to assess their long-term cardiovascular risk might be necessary, which can be helpful for early identification and timely intervention for cardiovascular events and, therefore, decrease in incidence of cardiovascular-related mortality.
Limitations
There are several limitations in our study. First, current literature did not reach an agreement on reference intervals of NT-proBNP, because the existence of several degraded peptides in the sample affected differently commercial methods (52). Hence, results in individual patients should be viewed dialectically. Second, significant heterogeneity was detected between the included cohort studies. To explore heterogeneity sources, subgroup analyses have been carried out according to geographic region, type of CHD, type of surgery, assay method of NT-proBNP, and whether adjusted for confounding factors. Although we found several heterogeneity moderators, such as geographic region, type of CHD, and assay method of NT-proBNP, there was still some evidence of heterogeneity among subgroups. However, the heterogeneity was inevitable and expected given the numerous differences in socioeconomic situation, study population, follow-up time, methodology, and ethnic background. Nevertheless, we believe the effect of heterogeneity to be small in consideration of the steady summary estimates across the subgroup and sensitivity analyses. Besides, significant unmeasured confounding factors may influence the observed prognostic value of NT-proBNP in CHD patients undergoing cardiac surgery in our meta-analysis. Though none of them have sufficiently controlled all latent confounding factors, such as gender, age, body mass index, complications (e.g., diabetes mellitus, lung disease), more than 60% of the studies have adjusted for more than one potential confounding factors. Furthermore, subgroup analysis indicated that the risk estimates of mortality and cardiovascular events in subjects with a higher level of NT-proBNP were comparable between studies adjusted for confounding factor and studies without any adjustment. Besides, because the ability of cohort studies to verify cause and effect is weaker than that of randomized controlled trials, this study only included cohort studies and not randomized controlled trials, so the results of this meta-analysis may have certain limitations. Lastly, this systematic review and meta-analysis merely included studies published in English, so added research in other languages is needed to confirm the findings.
Strengths
Our study has several strengths. First, we included the latest literature, which means that our findings have good implications for current practice. We evaluated, for the first time, the risks of the comprehensive prognosis associated with NT-proBNP levels in CHD patients undergoing cardiac surgery, such as mortality, cardiovascular events, and other postoperative outcomes. Furthermore, the results of the subgroup analyses can address specific questions about geographic region, type of CHD, and assay method of NT-proBNP, and the results of the sensitivity analysis indicated the robustness of prognostic value of NT-proBNP levels in congenital heart surgery. Finally, due to the larger sample size of this meta-analysis, the statistical power was improved, making the composite risk estimates more realistic and reliable.
Our finding of significant associations between the high level of NT-proBNP and future risk of mortality and cardiovascular events has important clinical and public health implications. Our findings will help clinicians make more informed decisions in the management of patients undergoing cardiac surgery for correction/palliation of CHD. Until more definitive data are available, the measurement of NT-proBNP cannot replace echocardiography, but may serve as a complementary low-cost, simple, promising, and noninvasive tool in the perioperative management of patients with CHD. Further investigation, especially large-scale well-controlled studies on sequential NT-proBNP measurement, is crucially necessary to address the prognostic value of NT-proBNP in congenital heart surgery. Besides, the issue on risks of effect of NT-proBNP in long-term follow-up of CHD patients after surgery is worthy of systematic induction.
Conclusions
Our study showed that CHD patients undergoing cardiac surgery at high NT-proBNP levels had elevated risks of mortality and cardiovascular events when compared with those at low NT-proBNP levels. Further large-scale and well-controlled studies are needed to confirm our findings.
Acknowledgments
We thank all our colleagues working in Department of Epidemiology and Health Statistics, Xiangya School of Public Health, Central South University. And we thank the editors and reviewers for their constructive suggestions.
Funding: The research was supported by National Natural Science Foundation Program (No. 81973137), Hunan Provincial Key Research and Development Program (No. 2018SK2062), and the Fundamental Research Funds for the Central Universities of Central South University (No. 2020zzts797).
Footnote
Provenance and Peer Review: While submitted as a standard submission to the journal, this article is selected as part of the special series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” published in Cardiovascular Diagnosis and Therapy, with joint decision from the editorial office and Guest Editors (Yskert von Kodolitsch, Harald Kaemmerer, Koichiro Niwa). The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist and MOOSE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-155/rc
Data Sharing Statement: https://cdt.amegroups.com/article/view/10.21037/cdt-22-155/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-155/coif). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” was commissioned by the editorial office without any funding or sponsorship. All authors are supported by National Natural Science Foundation Program (No. 81973137), Hunan Provincial Key Research and Development Program (No. 2018SK2062), and the Fundamental Research Funds for the Central Universities of Central South University (No. 2020zzts797). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mitchell SC, Korones SB, Berendes HW. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation 1971;43:323-32. [Crossref] [PubMed]
- Krasuski RA, Bashore TM. Congenital Heart Disease Epidemiology in the United States: Blindly Feeling for the Charging Elephant. Circulation 2016;134:110-3. [Crossref] [PubMed]
- Saxena A, Mehta A, Sharma M, et al. Birth prevalence of congenital heart disease: A cross-sectional observational study from North India. Ann Pediatr Cardiol 2016;9:205-9. [Crossref] [PubMed]
- Zhao QM, Ma XJ, Ge XL, et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet 2014;384:747-54. [Crossref] [PubMed]
- Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J 2004;147:425-39. [Crossref] [PubMed]
- Eindhoven JA, van den Bosch AE, Jansen PR, et al. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol 2012;60:2140-9. [Crossref] [PubMed]
- Oliver JM, Gallego P, Gonzalez AE, et al. Risk factors for excess mortality in adults with congenital heart diseases. Eur Heart J 2017;38:1233-41. [Crossref] [PubMed]
- Stout KK, Broberg CS, Book WM, et al. Chronic Heart Failure in Congenital Heart Disease: A Scientific Statement From the American Heart Association. Circulation 2016;133:770-801. [Crossref] [PubMed]
- Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007;50:2357-68. [Crossref] [PubMed]
- Soldin SJ, Soldin OP, Boyajian AJ, et al. Pediatric brain natriuretic peptide and N-terminal pro-brain natriuretic peptide reference intervals. Clin Chim Acta 2006;366:304-8. [Crossref] [PubMed]
- Teixeira RP, Neves AL, Guimarães H. Cardiac biomarkers in neonatology: BNP/NTproBNP, troponin I/T, CK-MB and myoglobin – a systematic review. Journal of Pediatric and Neonatal Individualized Medicine 2017;6:e060219. (JPNIM).
- Afshani N, Schülein S, Biccard BM, et al. Clinical utility of B-type natriuretic peptide (NP) in pediatric cardiac surgery--a systematic review. Paediatr Anaesth 2015;25:115-26. [Crossref] [PubMed]
- Cantinotti M, Giovannini S, Murzi B, et al. Diagnostic, prognostic and therapeutic relevance of B-type natriuretic hormone and related peptides in children with congenital heart diseases. Clin Chem Lab Med 2011;49:567-80. [Crossref] [PubMed]
- Knirsch W, Häusermann E, Fasnacht M, et al. Plasma B-type natriuretic peptide levels in children with heart disease. Acta Paediatr 2011;100:1213-6. [Crossref] [PubMed]
- Koch A, Zink S, Singer H. B-type natriuretic peptide in paediatric patients with congenital heart disease. Eur Heart J 2006;27:861-6. [Crossref] [PubMed]
- Giordana F, D'Ascenzo F, Nijhoff F, et al. Meta-analysis of predictors of all-cause mortality after transcatheter aortic valve implantation. Am J Cardiol 2014;114:1447-55. [Crossref] [PubMed]
- Ito S, Miranda WR, Jaffe AS, Oh JK. Prognostic Value of N-Terminal Pro-form B-Type Natriuretic Peptide in Patients With Moderate Aortic Stenosis. Am J Cardiol 2020;125:1566-70. [Crossref] [PubMed]
- Stundl A, Lünstedt NS, Courtz F, et al. Soluble ST2 for Risk Stratification and the Prediction of Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation. Am J Cardiol 2017;120:986-93. [Crossref] [PubMed]
- Krau NC, Lünstedt NS, Freitag-Wolf S, et al. Elevated growth differentiation factor 15 levels predict outcome in patients undergoing transcatheter aortic valve implantation. Eur J Heart Fail 2015;17:945-55. [Crossref] [PubMed]
- Borz B, Durand E, Godin M, et al. Does residual aortic regurgitation after transcatheter aortic valve implantation increase mortality in all patients? The importance of baseline natriuretic peptides. Int J Cardiol 2014;173:436-40. [Crossref] [PubMed]
- Takagi H, Hari Y, Kawai N, et al. Meta-Analysis of Impact of Baseline N-TerminalPro-Brain Natriuretic Peptide Levels on SurvivalAfter Transcatheter Aortic Valve Implantation for Aortic Stenosis. Am J Cardiol 2019;123:820-6. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Baggen VJ, van den Bosch AE, Eindhoven JA, et al. Prognostic Value of N-Terminal Pro-B-Type Natriuretic Peptide, Troponin-T, and Growth-Differentiation Factor 15 in Adult Congenital Heart Disease. Circulation 2017;135:264-79. [Crossref] [PubMed]
- Burke WT, Trivedi JR, Flaherty MP, et al. Acute Heart Failure at the Time of Transcatheter Aortic Valve Replacement Does Not Increase Mortality. Innovations (Phila) 2018;13:47-50. [Crossref] [PubMed]
- Popelová JR, Tomková M, Tomek J. NT-proBNP predicts mortality in adults with transposition of the great arteries late after Mustard or Senning correction. Congenit Heart Dis 2017;12:448-57. [Crossref] [PubMed]
- Baldenhofer G, Laule M, Mockel M, et al. Mid-regional pro-adrenomedullin (MR-proADM) and mid-regional pro-atrial natriuretic peptide (MR-proANP) in severe aortic valve stenosis: association with outcome after transcatheter aortic valve implantation (TAVI). Clin Chem Lab Med 2017;55:275-83. [Crossref] [PubMed]
- Westhoff-Bleck M, Kornau F, Haghikia A, et al. NT-proBNP Indicates Left Ventricular Impairment and Adverse Clinical Outcome in Patients With Tetralogy of Fallot and Pulmonary Regurgitation. Can J Cardiol 2016;32:1247.e29-36. [Crossref] [PubMed]
- Stähli BE, Gebhard C, Saleh L, et al. N-terminal pro-B-type natriuretic peptide-ratio predicts mortality after transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2015;85:1240-7. [Crossref] [PubMed]
- Sinning JM, Wollert KC, Sedaghat A, et al. Risk scores and biomarkers for the prediction of 1-year outcome after transcatheter aortic valve replacement. Am Heart J 2015;170:821-9. [Crossref] [PubMed]
- Frogoudaki A, Andreou C, Parissis J, et al. Clinical and prognostic implications of plasma NGAL and NT-proBNP in adult patients with congenital heart disease. Int J Cardiol 2014;177:1026-30. [Crossref] [PubMed]
- Ribeiro HB, Urena M, Le Ven F, et al. Long-term prognostic value and serial changes of plasma N-terminal prohormone B-type natriuretic peptide in patients undergoing transcatheter aortic valve implantation. Am J Cardiol 2014;113:851-9. [Crossref] [PubMed]
- Westhoff-Bleck M, Podewski E, Tutarel O, et al. Prognostic value of NT-proBNP in patients with systemic morphological right ventricles: a single-centre experience. Int J Cardiol 2013;169:433-8. [Crossref] [PubMed]
- Elhmidi Y, Bleiziffer S, Piazza N, et al. The evolution and prognostic value of N-terminal brain natriuretic peptide in predicting 1-year mortality in patients following transcatheter aortic valve implantation. J Invasive Cardiol 2013;25:38-44. [PubMed]
- Spargias K, Polymeros S, Dimopoulos A, et al. The predictive value and evolution of N-terminal pro-B-type natriuretic peptide levels following transcutaneous aortic valve implantation. J Interv Cardiol 2011;24:462-9. [Crossref] [PubMed]
- Pfister R, Wahlers T, Baer FM, et al. Utility of NT-pro-BNP in patients undergoing transapical aortic valve replacement. Clin Res Cardiol 2010;99:301-7. [Crossref] [PubMed]
- Seoudy H, Lambers M, Winkler V, et al. Elevated high-sensitivity troponin T levels at 1-year follow-up are associated with increased long-term mortality after TAVR. Clin Res Cardiol 2021;110:421-8. [Crossref] [PubMed]
- Parker DM, Everett AD, Stabler ME, et al. The Association Between Cardiac Biomarker NT-proBNP and 30-Day Readmission or Mortality After Pediatric Congenital Heart Surgery. World J Pediatr Congenit Heart Surg 2019;10:446-53. [Crossref] [PubMed]
- Lin F, Zheng L, Cui Y, et al. Prognostic value of perioperative NT-proBNP after corrective surgery for pediatric congenital heart defects. BMC Pediatr 2019;19:497. [Crossref] [PubMed]
- Seoudy H, Kuhn C, Frank J, et al. Prognostic implications of N-terminal pro-B-type natriuretic peptide in patients with normal left ventricular ejection fraction undergoing transcatheter aortic valve implantation. Int J Cardiol 2020;301:195-9. [Crossref] [PubMed]
- Vale NC, Campante Teles R, Madeira S, et al. Post-procedural N-terminal pro-brain natriuretic peptide predicts one-year mortality after transcatheter aortic valve implantation. Rev Port Cardiol 2018;37:67-73. (Engl Ed). [Crossref] [PubMed]
- Qu J, Liang H, Zhou N, et al. Perioperative NT-proBNP level: Potential prognostic markers in children undergoing congenital heart disease surgery. J Thorac Cardiovasc Surg 2017;154:631-40. [Crossref] [PubMed]
- Lindman BR, Breyley JG, Schilling JD, et al. Prognostic utility of novel biomarkers of cardiovascular stress in patients with aortic stenosis undergoing valve replacement. Heart 2015;101:1382-8. [Crossref] [PubMed]
- Bucholz EM, Whitlock RP, Zappitelli M, et al. Cardiac biomarkers and acute kidney injury after cardiac surgery. Pediatrics 2015;135:e945-56. [Crossref] [PubMed]
- Seiffert M, Sinning JM, Meyer A, et al. Development of a risk score for outcome after transcatheter aortic valve implantation. Clin Res Cardiol 2014;103:631-40. [Crossref] [PubMed]
- Lange R, Bleiziffer S, Mazzitelli D, et al. Improvements in transcatheter aortic valve implantation outcomes in lower surgical risk patients: a glimpse into the future. J Am Coll Cardiol 2012;59:280-7. [Crossref] [PubMed]
- Pérez-Piaya M, Abarca E, Soler V, et al. Levels of N-terminal-pro-brain natriuretic peptide in congenital heart disease surgery and its value as a predictive biomarker. Interact Cardiovasc Thorac Surg 2011;12:461-6. [Crossref] [PubMed]
- Carmona F, Manso PH, Vicente WV, et al. Risk stratification in neonates and infants submitted to cardiac surgery with cardiopulmonary bypass: a multimarker approach combining inflammatory mediators, N-terminal pro-B-type natriuretic peptide and troponin I. Cytokine 2008;42:317-24. [Crossref] [PubMed]
- Røsjø H, Dahl MB, Bye A, et al. Prognostic value of circulating microRNA-210 levels in patients with moderate to severe aortic stenosis. PLoS One 2014;9:e91812. [Crossref] [PubMed]
- Hirono K, Sekine M, Shiba N, et al. N-terminal pro-brain natriuretic peptide as a predictor of reoperation in children with surgically corrected tetralogy of fallot. Circ J 2014;78:693-700. [Crossref] [PubMed]
- Green MD, Parker DM, Everett AD, et al. Cardiac Biomarkers Associated With Hospital Length of Stay After Pediatric Congenital Heart Surgery. Ann Thorac Surg 2021;112:632-7. [Crossref] [PubMed]
- Panteghini M, Clerico A. Understanding the clinical biochemistry of N-terminal pro-B-type natriuretic peptide: the prerequisite for its optimal clinical use. Clin Lab 2004;50:325-31. [PubMed]



