Safety and efficacy of transcatheter aortic valve replacement in intermediate risk patients sets the stage for contemporary trials in lower risk groups
Since first performed in 2002 by Alan Cribier (1), transcatheter aortic valve replacement (TAVR) has emerged as an attractive alternative for the treatment of patients with severe symptomatic aortic stenosis (AS) who are inoperable or at high-risk for complications with surgical aortic valve replacement (SAVR), based in large part on the results of the three seminal randomized control trials (2-4). The procedure has indeed been a true transformative technology in the treatment of severe AS, and has expanded the range of options for these complex patients, many of whom had no prior options. As such, there has been a rapid dissemination of the TAVR technology for widespread utilization among these patients.
This issue of the journal features an important meta-analysis of six studies by Arora and colleagues comparing outcomes of “intermediate” risk patients undergoing TAVR versus SAVR (5). The safety and efficacy of performing TAVR in lower-risk patients than those in the initial trials is one of the most important questions currently facing the cardiology community. The authors demonstrated no significant difference with respect to 30-day or 1-year mortality between the two groups. In addition, there was no significant difference in stroke or myocardial infarction between TAVR and SAVR, although the trend seemed to favor the former. Furthermore, there was a higher incidence of pacemaker among patients undergoing TAVR as compared to those treated with SAVR.
The findings presented by the authors have been bolstered by two important studies that were recently presented at the American College of Cardiology meeting in March 2016 (6,7). The first was from the Placement of Aortic Transcatheter Valves (PARTNER) 2 cohort A (PIIA) (6). This was a randomized control trial, conducted at 57 sites in the US and Canada, and patients were stratified in cohorts according to access route [transfemoral (TF) (76.3%) or transthoracic (23.7%)] and were then randomly assigned (in a 1:1 ratio) to undergo either TAVR (n=1,011) or SAVR (n=1,021). TAVR was performed with the second-generation balloon-expandable Sapien XT valve system (Edwards Lifesciences, Irvine, CA, USA). At 2-year follow-up, the rate of all-cause mortality or disabling stroke was 19.3% in the TAVR group and 21.1% in the SAVR group (P=0.25), demonstrating the non-inferiority of TAVR. In the TF cohort, TAVR resulted in a lower rate of mortality or disabling stroke than surgery, whereas in the transthoracic-access cohort, outcomes were similar in the two groups. It must also be noted that TAVR resulted in larger aortic-valve areas than did surgery and also resulted in lower rates of acute kidney injury and severe bleeding; whereas surgery resulted in fewer major vascular complications and less paravalvular aortic regurgitation (PAR). The incidence of moderate/severe PAR with TAVR was 3.7% and 3.4% at 30-day and 1-year follow-up respectively.
The second study was the SAPIEN 3 observational study comprised of 1,077 intermediate risk patients in 51 sites in US or Canada treated with the SAPIEN 3 valve and propensity matched to the SAVR group of PIIA (7). Among the TAVR group, 88% underwent TF implantation. At 1-year follow-up, all-cause mortality in these patients was 13% in the SAVR cohort and 7.4% in the TF cohort (P<0.001), demonstrating superiority of TF-TAVR. The incidence of all strokes and disabling strokes at 1 year was 8.2% and 4.6% (P=0.004), respectively. The incidence of moderate or severe PAR at 1 year was low at 1.5% in the entire cohort (6). While the propensity-matching is not as statistically robust as a true randomized, controlled trial, the PIIA SAVR group is a contemporary surgical group that was selected very carefully and is therefore a reasonable comparator. Furthermore, the results are in-line with the randomized results of PIIA.
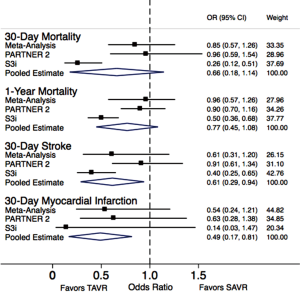
The data from these two recent studies along with the results of the current meta-analysis are truly an attestation of the success of the TAVR technology in a relatively short period of time. In a span of less than 10 years, we have moved from the initial feasibility trials among inoperable patients to those who are operable with only intermediate risk and demonstrated at least similar outcomes compared to SAVR and overall low rates of complications such as death, stroke, and need for permanent pacemaker. In fact, we updated the meta-analysis of key outcomes including mortality, stroke and acute myocardial infarction, after inclusion of data from the PIIA and the SAPIEN 3 observational study as presented in Figure 1. As clearly evident in Figure 1, although there was no significant difference in 30-day and 1-year mortality between the TAVR and SAVR groups, the 30-day stroke and 30-day acute myocardial infarction event rates were more favorable for TAVR as compared to SAVR.
As a result of this evolution in patient selection for TAVR, we have naturally begun to question whether even low-risk populations may share the same benefits of TAVR in comparison with SAVR. To this end, the Nordic Aortic Valve Intervention (NOTION) trial investigators randomized 280 patients >70 years of age and considered low-risk for surgery (STS 2.9%) between TAVR using the first generation CoreValve prosthesis and SAVR. The overall primary composite endpoint of death, stroke, and MI at 1-year was not different between the two groups (13.1% versus 16.3%, P=0.43), and there were statistically insignificant trends toward a lower incidence of each of these outcomes in the TAVR group. It should be noted that rates of pacemaker implantation (34%) and moderate/severe PAR (15.7%) were substantially higher than seen in more recent trials, though this is likely the result of using of the first generation CoreValve and lack of 3D annulus sizing techniques, respectively. The newest generation of valves, both commercially approved (SAPIEN 3, CoreValve Evolut) and in trial (Direct Flow Medical Valve, Boston Scientific Lotus, St Jude Portico, USA), along with pre-procedural 3D annulus sizing and improved understanding of valve implantation have resulted in substantial reductions in both of these issues (8). In addition, the use of cerebral-protection devices may reduce embolic risk among patients undergoing TAVR, increasing the safety of the procedure (9,10). In the surgical arena, minimally invasive surgery with the use of sutureless devices has reduced the invasiveness of surgery and enlarged the post-implantation valve area (11,12). To test the application of the most contemporary TAVR versus SAVR techniques in patients with low risk, both Edwards and Medtronic are in the initial phases of patient enrollment in randomized trials of the SAPIEN-3 and CoreValve Evolut systems, respectively, to answer this important question.
With ongoing improvement in the TAVR device technology, the interest in treating even lower risk patients with TAVR continues to grow, and the current manuscript contributes to the randomized data to demonstrate the safety and sometimes superiority of the transcatheter approach. In moving forward with the application of TAVR to lower risk groups with the newest generation devices, however, we must remember that the excellent results for patients undergoing TAVR thus far has been the result of a multidisciplinary Heart Team evaluation to decide the best treatment route for a given patient. Similarly, the procedures themselves are most often performed with a team of interventional cardiologists, cardiac surgeons, imaging specialists, anesthesiologists, and nurses all involved. We should strive to continue this multidisciplinary approach to decide and enact the best treatment plans for our patients to maintain the highest degree of success.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2485-91. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Arora S, Misenheimer JA, Jones W, et al. Transcatheter versus surgical aortic valve replacement in intermediate risk patients: a meta-analysis. Cardiovasc Diagn Ther 2016;6:241-9. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Vahl TP, Kodali SK, Leon MB. Transcatheter Aortic Valve Replacement 2016: A Modern-Day "Through the Looking-Glass" Adventure. J Am Coll Cardiol 2016;67:1472-87. [Crossref] [PubMed]
- Lansky AJ, Schofer J, Tchetche D, et al. A prospective randomized evaluation of the TriGuard™ HDH embolic DEFLECTion device during transcatheter aortic valve implantation: results from the DEFLECT III trial. Eur Heart J 2015;36:2070-8. [Crossref] [PubMed]
- Samim M, Agostoni P, Hendrikse J, et al. Embrella embolic deflection device for cerebral protection during transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 2015;149:799-805.e1-2.
- Fischlein T, Meuris B, Hakim-Meibodi K, et al. The sutureless aortic valve at 1 year: A large multicenter cohort study. J Thorac Cardiovasc Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Fischlein T, Pfeiffer S, Pollari F, et al. Sutureless Valve Implantation via Mini J-Sternotomy: A Single Center Experience with 2 Years Mean Follow-up. Thorac Cardiovasc Surg 2015;63:467-71. [Crossref] [PubMed]