
The relation of aortic dimensions and obesity in adults with Marfan or Loeys-Dietz syndrome
Introduction
Aortic aneurysms and aortic dissection are often atherosclerotic in origin. However, there is also a broad variety of congenital cardiac anomalies or heritable connective tissue disorders, including Marfan syndrome (MFS) and Loeys-Dietz syndrome (LDS), that predispose patients to weakening of the aortic layers, aortic aneurysm, or aortic dissection or rupture (1,2). According to several studies, the incidence of acute thoracic aortic dissection is on the rise, increasing the burden on the healthcare system (3-6). Existing studies described an incidence increasing over the past decades from 2–3.5/100,000/year to more than 6/100,000/year for a general cohort and up to 15/100,000/year in older individuals (7-12). However, there are also a few contemporary data contradicting recent perceptions of a rising incidence (13).
MFS and LDS are caused by molecular genetic alterations of the FBN1 gene in MFS or the TGFBR1 or TGFBR2 genes in LDS (14,15). In these diseases, life expectancy is highly dependent on the development of complications, especially aortic aneurysm or aortic dissection.
In both MFS and LDS, the aortic root is the most common site of aortic dissection (2). In MFS patients, the aortic root diameter increases in 80% of cases at the sinus of Valsalva, raising the risk of dissection or rupture (2,16) and reducing the average survival to 40 years (2,17). In LDS, aortopathy has an even more aggressive course and the mean age at death has been described as 26 years in the natural course (18).
Due to the risk of aortic dissection or rupture, lifelong medical surveillance is required, risk factors should be identified at an early stage, and appropriate measures should be initiated to prevent complications (1). This applies both to untreated patients and to patients who have undergone aneurysm repair or intervention (19).
The mechanisms that trigger aneurysm formation or dissection are not well understood, but both genetic and environmental risk factors contribute decisively to the pathobiology of these devasting complications (20). As predisposing factors, aortic features such as atrial tortuosity, genetically determined genotype-phenotype correlations as well as cardiovascular risk factors such as arterial hypertension, hypercholesterolemia, diabetes mellitus type II, smoking, and obesity have all been identified (21-24). In MFS and LDS, however, the effect of obesity on the development of aortic aneurysm or dissections is vastly unknown (25).
The bioelectrical impedance analysis (BIA) is a non-invasive exploratory method for assessing body composition. A sinusoidal alternating current of 0.8 mA at a frequency of 50 kHz is passed through the body of the subject in supine position through four surface electrodes placed at the body extremities. BIA measures the resistance (R), reactance (Xc), in other word the impedance (Z), and the phase angle (ϕ). The resistance (R) is defined as the pure ohmic resistance of the electrolytes found in the total body water, to which it is inversely proportional. The reactance Xc, on the other hand, is defined as the resistance created by the cell membranes functioning as capacitors and thus measures the body cell mass (BCM). Therefore, from measured resistance, reactance, impedance, phase angle and anthropometric data, i.e., body weight, height and sex, it possible to calculate fat-free mass and fat mass through prediction equations (26-28).
Thus, BIA allows an exact estimation of the fat and muscle mass of the body and the intracellular and extracellular water content inside and outside the cells (29,30). According to the World Health Organization (WHO), obesity is defined as body fat percentage cut-off values of 25% in men and 35% in women were used (31).
The aim of the current study was to elucidate the relationship between aortic size and body composition in patients with MFS/LDS through assessment by modern BIA, a more sophisticated method than traditional measures such as body mass index (BMI) {body weight (kg)/[body height (m)]2}, and to identify whether or not obesity can serve as a risk marker for potential aortic complications. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-383/rc).
Methods
This joint project of the Department of Cardiac Surgery (Friedrich-Alexander-University Erlangen-Nürnberg, Erlangen, Germany) and the Department for Congenital Heart Disease and Pediatric Cardiology (German Heart Center Munich, Technical University Munich, Munich, Germany) included 51 patients with MFS or LDS. All patients in this explorative, cross-sectional cohort were admitted between June 2020 and May 2022.
MFS or LDS diagnosis was confirmed clinically and/or molecular-genetically by an experienced MFS/LDS specialist. All patients with a confirmed diagnosis of MFS or LDS, who had at least one magnetic resonance imaging (MRI) or computed tomography (CT) scan as part of routine clinical care, and an age >17 years were eligible for inclusion in this study. Included patients were enrolled in the order of presentation at both institutions. Patient inclusion process and classification is described according to the flow diagram in Figure 1. No prior selection for inclusion was performed. Excluded from participation were patients who (I) had implanted cardiac devices [pacemakers or “automatic implantable cardioverter defibrillators (AICD)”]; (II) were pregnant; (III) were cognitively unable to consent to research; (IV) refused consent; or (V) had not received a CT or MRI of their entire aorta.
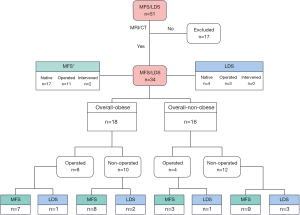
Patient demographics and clinically relevant data were collected by reviewing patient’s medical records. An appropriate form including clinical diagnosis, anthropometric data and clinical parameters (age, sex, weight, height, BMI, molecular genetic test results, medication and operative history) was completed.
Imaging processing and aortic measurements
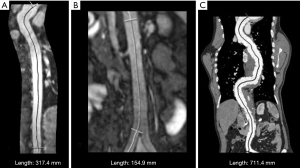
Aortic measurements were performed using MRI or CT scan of patients as part of routine clinical care. Imaging was performed using a 1.5 T scanner (Magnetom Avanto; Siemens Healthineers, Erlangen, Germany) and a third-generation dual-source computed tomography angiograph (Siemens CT Somatom X.ceed, Siemens Healthineers, Forchheim, Germany). All imaging processing was analyzed using SYNGO.VIA software (Syngo.via, Version VB20A, Siemens Healthcare GmbH, Erlangen, Germany). Measurements of the aortic diameter were performed following the practical guide from van Hout et al. (32). By using the “MM Reading” application in Syngo.via, in a first series, for the thoracic aortic anatomy, markers were manually positioned in the axial view, ensuring accurate coverage until a random point distal to the celiac trunk. A reconstruction of the aorta with central line was then generated throughout the length of the aorta (Figure 2A). Thereafter, the length was measured by manually placing markers at the aortic bulb and at the branching of the celiac trunk. In a second series, for the abdominal aortic anatomy, a similar measurement was performed, with the markers placed at the branching of the celiac trunk and at the aortic bifurcation (Figure 2B). In the CT scan, the aorta was scanned in its entirety. Thus, using the “MM Reading” application, the markers were placed manually and the aorta was directly measured from the bulb to the aortic bifurcation (Figure 2C).
Definitions
The aortic length was estimated from the aortic bulb (sinus of Valsalva) to the aortic bifurcation. Besides length, diameters of the aorta at various locations were measured in an edge-to-edge manner at the following seven different levels: aortic bulb (sinus of Valsalva), sinotubular junction, ascending aorta, aortic arch, aortic isthmus, aorta at diaphragmatic level, and abdominal aorta. The term “aortic aneurysm” was chosen for any aortic enlargement measured at a maximum diameter >4.0 cm or for history of aortic dissection or elective aortic repair (33,34).
Bioimpedance analysis of body composition
The multifrequency impedance analyzer “Nutriguard MS”, Data Input GmbH, Pöcking, Germany was used for BIA (Table 1). The “Nutriguard MS” is a validated tool for the assessment of body composition (27). A single BIA of 15 seconds duration was performed per patient, and the obtained results were recorded electronically. Via four body surface electrodes, two on the dorsum of the hand and two on the dorsum of the foot, a sinusoidal alternating current of 0.8 mA at a frequency of 50 kHz was transmitted. Basal metabolic rate, body water, extracellular mass (ECM; a measure of interstitium, bone, and connective tissue), body cell mass (BCM; a measure of muscle and organ cell mass), lean body mass (BCM + ECM; a measure of fat-free body mass), ECM/BCM index, cell-percentage (proportion of BCM in lean mass), body fat (in kg and %), and the phase angle j (quality of lean mass) were estimated. Information about BIA in the normal population as well as in MFS- or LDS-patients were recently published (26). For classification of obesity, the WHO body fat percentage cut-off values of 25% in men and 35% in women were used (31).
Table 1
| Patient-related parameters |
| Age (year) |
| Sex |
| Weight (kg) |
| Height (cm) |
| Body mass index (kg/m2) |
| Molecular-genetically confirmed finding |
| Comorbidities |
| Medication |
| Aortic involvement (+/−) |
| Aortic root diameter (cm) |
| Disease progression/course |
| Aortic dissection |
| Operative aortic or aortic valve replacement |
| BIA-specific parameters |
| Basal metabolic rate |
| Body Water |
| Lean body mass (fat-free mass = BCM + ECM) |
| Extracellular mass (interstitium, bone, connective tissue) |
| Body cell mass (muscle and organ cell mass) |
| ECM/BCM-index |
| Cell-percentage (BCM proportion in lean mass) |
| Body fat (in kg and %) |
| Phase angle j (quality of lean mass) |
BIA, bioelectrical impedance analysis; BCM, body cell mass; ECM, extracellular mass.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice guidelines. The institutional ethic review boards of the Friedrich-Alexander-University Erlangen-Nürnberg (Reference No. 179_21 Bc) and of the Technical University Munich (Reference No. 158/19S) approved the study protocol. Written informed consent, as well as an agreement to an anonymous publication of patient’s data was provided to all participants. Guidelines on good pharmacoepidemiology practice and data protection guidelines were followed.
Statistical analysis
All pseudonymized statistical evaluations of the data were performed using SPSS 28.0 (IBM Inc., Armonk, NY, USA). The study population was characterized using descriptive statistical methods. T, Mann-Whitney and χ2 tests were used to evaluate differences between groups. Associations between metric variables were assessed using Spearman Rank Correlation. Continuous data and categorical/interval-scaled variables were expressed as mean ± standard deviation or as absolute numbers/percentages, respectively. The 95% confidence interval of difference is displayed as (lower-; upper-bound). All occurring P values and tests for significance were performed two-sided. A P value <0.05 was considered significant. Given that the disease entities in this study are rare diseases with high variability, a P value between P=0.05 and <0.1 was considered as a trend.
Results
Study sample, patient characteristics and demographic data
In this project, a total of 51 patients with MFS or LDS (66% female; mean age: 37.7±11.7; range, 17–68 years) were included. Out of these, 34 patients (MFS: n=27; LDS: n=7) had a complete MRI or CT scan of the entire aorta. The mean age of these 34 patients was 37.8±12.6 years, ranging from 18 to 68 years. As for the sex distribution, 22 patients (64.7%) were female. The mean height of the patients was 182.2±10.2 cm (range, 160–203 cm), and the mean weight was 77.2±18.2 kg (range, 54–145 kg). The mean body surface area was 1.97±0.22 m2 (range, 1.55–2.63 m2).
A total of 12 patients underwent one or multiple aortic operations (n=14) and/or interventional aortic treatment (n=2), either prophylactically or after aortic dissection. In MFS and LDS patients, surgery on the ascending aorta and/or aortic stent implantation was performed in 40.7% (n=11) and 42.9% (n=3), respectively. Overall, four patients (11.8%) had developed aortic dissection, of which three (8.8%) had MFS, although these differences did not reach statistical significance. The demographic data and type of intervention in MFS and LDS patients are summarized in Table 2.
Table 2
| Parameter | Overall (N=34) | MFS (n=27) | LDS (n=7) | P value (95% CI) |
|---|---|---|---|---|
| Age (years) | 37.8±12.6 (range, 18–68) | 36.7±11.5 (range, 18–58) | 42.4±16.7 (range, 18–68) | 0.29 (−16.6; 5.1) |
| Female, n (%) | 22 (64.7) | 18 (66.7) | 4 (57.1) | 0.63 (−0.3; 0.5) |
| Height (cm) | 182.2±10.2 (range, 160–203) | 183.6±10.1 (range, 160–203) | 176.7±8.8 (range, 165–188) | 0.11 (−1.6; 15.4) |
| Weight (kg) | 77.2±18.2 (range, 54–145) | 77.8±19.3 (range, 54–145) | 74.9±14.1 (range, 63–101) | 0.70 (−12.9; 18.8) |
| BSA (m2) | 1.97±0.22 (range, 1.55–2.63) | 1.99±0.23 (range, 1.55–2.63) | 1.91±0.18 (range, 1.75–2.27) | 0.39 (−0.11; 0.28) |
| Molecular-genetically confirmed | 30 | 23 | 7 | 0.27 (−0.4; 0.1) |
| Dissections, n (%) | 4 (11.8) | 3 (11.1) | 1 (14.3) | 0.81 (−0.3; 0.2) |
| Aortic operations in 12 patients, n (%) | 14* (41.2) | 11 (40.7) | 3 (42.9) | 0.52 (−0.2; 0.5) |
| Aortic stenting, n (%) | 2 (5.9) | 2 (7.4) | 0 (0) |
*, multiple operations per patient possible. N/n, absolute number; MFS, Marfan syndrome; LDS, Loeys-Dietz syndrome; CI, confidence interval; BSA, body surface area.
Assessment of body composition—BIA measurements
Clinically, the included MFS and LDS patients appeared asthenic by virtue of their body size and slender/long extremities. However, in this cohort of MFS/LDS patients, the percentage of body fat determined by BIA was 31.6%±8.7% (range, 9.5–53.5%). Patients with MFS tended to have more fat (32.8%±8.2%) than patients with LDS [27.1%±9.7%; (−1.6; 13.1); P=0.12]. According to BIA with fat percentage cut-off values of 25% in men and 35% in women, 52.9% (n=18) of the overall study population were obese. Of the obese patients, 72.2% (n=13) were female.
As for the connective tissue content, the mean for the entire cohort was 26.6±4.6 kg. LDS patients had a not statistically significant higher amount of connective tissue (28.1±5.4 kg) than MFS patients [26.2±4.4 kg; (−5.9; 2.0); P=0.16].
The relationship between ECM and BCM in the overall study population was clearly shifted toward ECM. Indeed, the ECM/BCM indices were 1.069, 1.062, and 1.097 for the overall population, MFS patients only, and LDS patients only, respectively. Regarding the remaining BIA parameters, there was no significant difference between MFS and LDS patients (Table 3).
Table 3
| Parameter | Overall (N=34) | MFS (n=27) | LDS (n=7) | P value (95% CI) |
|---|---|---|---|---|
| Body fat (kg) | 25.1±12.3 | 26.3±13.0 | 20.3±8.3 | 0.27 (−4.5; 16.5) |
| Body fat (%) | 31.6±8.7 | 32.8±8.2 | 27.1±9.7 | 0.12 (−1.6; 13.1) |
| ≤25% (M); ≤35% (F) | 16 (7:9) | 12 (5:7) | 4 (2:2) | 0.42 (−0.3; 0.5) |
| >25% (M); >35% (F) | 18 (5:13) | 15 (4:11) | 3 (1:2) | 0.42 (−0.3; 0.5) |
| ECM (kg) | 26.6±4.6 | 26.2±4.4 | 28.1±5.4 | 0.16 (−5.9; 2.0) |
| BCM (kg) | 25.6±6.4 | 25.3±6.4 | 26.4±7.1 | 0.69 (−6.7; 4.5) |
| ECM/BCM index | 1.069±0.16 | 1.062±0.15 | 1.097±0.19 | 0.61 (−0.1; 0.1) |
| Percent cellularity | 48.6±3.8 | 48.7±3.7 | 48.1±4.5 | 0.67 (−2.6; 4.0) |
| Total body water (l) | 38.0±7.6 | 37.7±7.5 | 39.2±8.5 | 0.65 (−8.1; 5.2) |
| Lean body mass (kg) | 52.1±10.5 | 51.5±10.2 | 54.5±11.8 | 0.50 (−12.1; 6.0) |
| Phase angle (°) | 5.4±0.7 | 5.4±0.7 | 5.3±0.8 | 0.71 (−0.5; 0.7) |
BIA, bioelectrical impedance analysis; N/n, absolute number; MFS, Marfan syndrome; LDS, Loeys-Dietz syndrome; CI, confidence interval; ECM, extracellular mass (interstitium, bone, connective tissue); BCM, body cell mass (muscle and organ cell mass).
Measurements of aortic length and diameter
The mean aortic length for the entire cohort was 503.7±58.7 mm. The mean thoracic aortic length and the mean abdominal aortic length were 351.5±52.4 and 152.2±27.4 mm, respectively (Table 4).
Table 4
| Measurements | Overall (N=34) | MFS (n=27) | LDS (n=7) | 95% CI | P value |
|---|---|---|---|---|---|
| Total length of aorta, mm | 503.7±58.7 (range, 378.1–711.9) | 501.4±60.4 (range, 378.1–711.9) | 512.6±55.0 (range, 450.7–622.7) | (−60.0; 40.8) | 0.65 |
| Length thoracic aorta (bulbus to celiac trunk), mm | 351.5±52.4 (range, 251.8–552.3) | 348.5±52.5 (range, 251.8–552.3) | 363.2±54.3 (range, 319–476.2) | (−101.0; 44.0) | 0.51 |
| Length abdominal aorta (celiac trunk to aortic bifurcation), mm | 152.2±27.4 (range, 109.5–236.7) | 152.9±29.1 (range, 109.5–236.7) | 149.4±21.3 (range, 124–184.1) | (−23.7; 29.2) | 0.76 |
| Aortic root 1 | 39.8±6.7 (range, 27–54) | 40.2±7.1 (range, 27–54) | 38.1±4.8 (range, 32–44) | (−3.5; 8.1) | 0.47 |
| Aortic root 2 | 39.3±6.5 (range, 27–51) | 39.7±6.9 (range, 27–51) | 37.9±4.2 (range, 32–43) | (−3.5; 7.7) | 0.50 |
| Aortic root 3 | 38.8±6.6 (range, 23–52) | 39.0±7.2 (range, 23–52) | 38.0±4.3 (range, 31–44) | (−3.7; 7.3) | 0.72 |
| Sinutubular junction 1 | 32.0±4.9 (range, 22–47) | 32.0±4.6 (range, 22–47) | 31.9±6.3 (range, 23–42) | (−4.1; 5.1) | 0.94 |
| Sinutubular junction 2 | 31.9±4.8 (range, 21–45) | 31.9±4.6 (range, 21–45) | 31.9±6.0 (range, 23–42) | (−4.4; 4.8) | 0.98 |
| Ascendens 1 | 29.3±5.1 (range, 21–48) | 29.0±4.0 (range, 21–41) | 30.6±8.4 (range, 28–48) | (−6.1; 2.9) | 0.64 |
| Ascendens 2 | 28.8±5.1 (range, 20–49) | 28.3±3.7 (range, 20–36) | 30.6±8.8 (range, 22–49) | (−6.8; 2.0) | 0.53 |
| Aortic arch 1 | 25.3±7.0 (range, 17–55) | 24.9±7.2 (range, 17–55) | 26.9±6.6 (range, 21–41) | (−8.0; 4.1) | 0.52 |
| Aortic arch 2 | 24.5±5.8 (range, 16–43) | 23.7±5.2 (range, 16–38.6) | 27.4±7.4 (range, 20–43) | (−8.6; 1.1) | 0.13 |
| Aortic isthmus 1 | 24.7±9.4 (range, 17–73) | 24.5±10.2 (range, 17–73.2) | 25.7±5.7 (range, 19–35) | (−9.7; 7.0) | 0.76 |
| Aortic isthmus 2 | 23.6±6.1 (range, 15–50) | 22.9±6.1 (range, 15–49.5) | 26.1±6.3 (range, 18–36) | (−8.6; 1.9) | 0.22 |
| At level of diaphragm 1 | 20.6±4.5 (range, 15–34) | 20.3±4.4 (range, 16–34) | 21.6±5.1 (range, 15–30) | (−4.7; 3.5) | 0.51 |
| At level of diaphragm 2 | 20.4±7.7 (range, 14–58) | 20.3±8.2 (range, 15–58) | 20.7±5.9 (range, 14–29) | (−6.4; 8.2) | 0.91 |
| Abdominal aorta 1 | 22.0±8.9 (range, 14–64) | 21.9±9.7 (range, 14–64) | 22.1±5.0 (range, 16–29) | (−7.9; 7.5) | 0.95 |
| Abdominal aorta 2 | 21.4±7.2 (range, 13–53) | 21.1±7.6 (range, 13–53) | 22.6±5.7 (range, 16–32) | (−7.7; 4.8) | 0.64 |
Aortic root 1, 2 and 3 refer to the aortic bulb. MFS, Marfan syndrome; LDS, Loeys-Dietz syndrome; CI, confidence interval.
Comparing all MFS and LDS patients (n=34), in LDS patients, the entire aorta (512.6±55.0 mm) and the thoracic aorta (363.2±54.3 mm) was not significantly longer than in MFS patients [entire aorta: 501.4±60.4 mm and thoracic aorta: 348.5±52.5 mm; (−60.0; 40.8); P=0.65 and (−101.0; 44.0); P=0.51], while in MFS patients, the abdominal aorta was longer [152.9±29.1 vs. 149.4±21.3 mm for LDS; (−23.7; 29.2); P=0.76]. At the same time, MFS patients were on average (about 7 cm) taller than the LDS patients (Table 4).
When subdividing the entire study population (n=34) into non-obese (n=16) and obese (n=18) according to BIA, in the non-obese patients, the entire aorta tended [513.7±67.1 vs. 494.8±50.3 mm; (−22.4; 59.0); P=0.35] and the thoracic aorta [369.6±64.0 vs. 335.5±33.6; (0.3; 108.8); P=0.05 ] was significantly longer, while in obese patients the abdominal aorta tended to be longer [159.3±31.5 vs. 144.1±19.9 mm; (−36.3; 3.5); P=0.10] (Table 5).
Table 5
| Variables | Total length of aorta | Length thoracic aorta (bulbus to celiac trunk) | Length abdominal aorta (celiac trunk to aortic bifurcation) |
|---|---|---|---|
| Entire population | |||
| Overall (n=34) | 503.7±58.7 (range, 378.1–711.9) | 351.5±52.4 (range, 251.8–552.3) | 152.2±27.4 (range, 109.5–236.7) |
| Obese (n=18) | 494.8±50.3 (range, 378.1–594.8) | 335.5±33.6 (range, 251.8–384.6) | 159.3±31.5 (range, 119.2–236.7) |
| Non-obese (n=16) | 513.7±67.1 (range, 439.5–711.9) | 369.6±64.0 (range, 310–552.3) | 144.1±19.9 (range, 109.5–193.3) |
| P value (95% CI) | 0.35 (−22.4; 59.0) | 0.05 (0.3; 108.8) | 0.10 (−36.3; 3.5) |
| Non-surgically treated patients | |||
| Overall (n=22) | 500.5±38.3 (range, 439.5–594.8) | 347.9±25.7 (range, 310–405.2) | 152.6±31.3 (range, 109.5–236.7) |
| Obese (n=10) | 514.2±45.7 (range, 450.7–594.8) | 348.6±24.5 (range, 311.8–384.6) | 165.6±37.3 (range, 119.2–236.7) |
| Non-obese (n=12) | 489.1±28.0 (range, 439.5–532.3) | 347.4±27.7 (range, 310–405.2) | 141.7±21.2 (range, 109.5–193.3) |
| P value (95% CI) | 0.13 (−56.6; 24.9) | 0.91 (−30.8; 79.0) | 0.07 (−56.0; 0.1) |
Considering only non-surgically treated patients (n=22), the mean aortic length was measured at 500.5±38.3 mm. The mean thoracic aortic length and the mean abdominal aortic length were 347.9±25.7 and 152.6±31.3 mm, respectively (Table 5).
When the non-surgically treated patients were subdivided into obese (n=10) and non-obese (n=12), the entire aorta tended [514.2±45.7 vs. 489.1±28.0; (−56.6; 24.9); P=0.13] and the thoracic aorta [348.6±24.5 vs. 347.4±27.7; (−30.8; 79.0); P=0.91] were, but not statistically significantly longer in the non-obese patients, whereas the abdominal aorta tended to be longer in the obese patients [165.6±37.3 vs. 141.7 ±21.2 mm; (−56.0; 0.1); P=0.07] (Figure 3, Table 5).
Aortic diameters
By analyzing only the non-surgically treated patients (n=22), which comprised 77.3% (n=17) of the patients, 15 MFS patients (88.2%) and 2 LDS patients (40.0%) had an aortic aneurysm according to the definition given above. In this non-surgically treated cohort, the diameters of the aortic bulb, measured at three locations, tended to be larger in MFS than in LDS patients [42.6±5.0, 41.9±4.9, 41.2±4.6 vs. 37.8±5.1, 37.4±4.2, 36.8±4.1 mm; (−1.1; 9.1); P=0.07, (−1.2; 8.4); P=0.07, (−1.5; 7.9); P=0.07, respectively].
Furthermore, the maximal aortic bulb diameter in the non-surgically treated MFS patients (42.3±5.1 mm) tended to be longer than in the non-surgically treated LDS patients [38.8±4.7 mm; (−1.9; 8.3); P=0.09].
Correlations
The body fat content measured by BIA correlated negatively with the thoracic aortic length [R=−0.377; (−0.7; −0.3); P=0.02]. This suggests that the more obese the patient, the shorter his or her thoracic aorta. Similarly, BCM (muscle mass) correlated positively with the total aortic length [R=0.359; (0.1; 0.8); P=0.03] as well as with the thoracic aortic length [R=0.399; (0.2; 0.8); P=0.02]. In addition, ECM (connective tissue) correlated positively with aortic diameters measured at the aortic arch [R=0.511; (0.2; 0.7); P=0.002], aortic isthmus [R=0.565; (0.3; 0.7); P<0.001], and the abdominal aorta [R=0.486; (0.2; 0.7); P=0.004]. Moreover, ECM also correlated positively with the total aortic length [R=0.354; (0.2; 0.8); P=0.04]. Thus, the more connective tissue a patient had, the longer his or her aorta was.
No significant correlation was found between age and aortic length or aortic bulb diameter. However, age did correlate positively with the diameters of aortic arch 1 and 2 [R=0.471; (0.1; 0.7); P=0.02 and R=0.630; (0.3; 0.9); P=0.002], isthmus 1 and 2 [R=0.591; (0.1; 0.7); P=0.004 and R=0.626; (0.2; 0.8); P=0.002], and abdominal aorta 1 and 2 [R=0.510; (0.1; 0.6); P=0.01 and R=0.720; (0.2; 0.8); P<0.001]. Thus, the older the patients were, the wider the diameters of their aortas in several locations. Further, sex had a significant effect on imaging parameters [(−0.7; −0.1); P=0.02], with female patients having a shorter aorta.
Considering only the non-surgically treated patients, a positive correlation was found between bulb diameter and the total aortic length [R=0.672; (0.1; 0.9); P=0.001], as well as the abdominal aortic length [R=0.445; (0.2; 1.2); P=0.04].
Assessment of aortic surgery history
By as for the aortic surgical history of the study population, out of the 34 patients, 12 (35.3%; 66.7% female) had previously undergone aortic (redo-)surgery (n=11) and/or interventional treatment (n=2) as part of prophylactic treatment or treatment of aortic complications.
Out of these patients, 66.7% (n=8) were classified as obese by BIA after surgery.
Of the patients who had already been operated on at the time of the current study, only the preoperative body weight and BMI were considered, so that the possibility of a high BIA based on postoperative weight gain would be excluded (Table 6).
Table 6
| Type | Sex | Age at surgery in years | Acute aortic dissection | Surgery/intervention | Age at BIA | Bodyweight prior operation (kg) | Bodyweight at BIA (kg) | Body fat (%) | Diameter of aorta at surgery (bulb/STJ/ascending) | Length of aorta at surgery (mm) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thoracic | Abdominal | Total | ||||||||||
| LDS | M | 63 | No | Valve sparing root replacement (David procedure) | 63 | 101 | 101 | 23.3 | 44/42/49 | 476.2 | 146.5 | 622.7 |
| MFS | M | 27/39 | Yes | 1. Conduit 25 mm, type Sorin carbon; 2. redo surgery: ascending aorta replacement | 42 | 69 | 74 | 20.0 | 33/30/33 | 389.6 | 132.2 | 521.8 |
| MFS | F | 28 | Yes | Valve sparing root replacement (David procedure) Aortic stent grafting |
35 | 65 | 67 | 29.4 | 32/35/26 | 326.2 | 167.3 | 493.5 |
| MFS | M | 45 | Yes | Aortic stent grafting | 46 | 94 | 94 | 21.5 | 39/41/34 | 552.3 | 159.6 | 711.9 |
| MFS | F | 29 | No | 1. Valve sparing root replacement (David procedure); 2. redo surgery: aortic valve replacement, replacement of the remaining ascending aorta and arch | 40 | 68 | 66 | 43.3 | 32/29/41 | 251.8 | 126.3 | 378.1 |
| MFS | F | 41 | No | Valve sparing root replacement (David procedure) | 41 | 78 | 78 | 41.6 | 27/28/26 | 344.4 | 171.3 | 515.7 |
| MFS | F | 40 | No | Valve sparing root replacement (David procedure) | 53 | 109 | 95 | 41.2 | 37/32/32 | 376.8 | 150.7 | 527.5 |
| MFS | F | 39 | No | Valve sparing root replacement (Yacoub procedure) | 58 | 82 | 93 | 45.0 | 32/31/27 | 295.3 | 171.5 | 466.8 |
| LDS | F | 45/49 | Yes | 1. Aorto-thoracic interposition grafting; 2. thoracic aorta replacement | 54 | 66 | 65 | 35.1 | 38/32/29 | 320.7 | 184.1 | 504.8 |
| MFS | F | 44 | No | Valve sparing root replacement (David procedure) | 45 | 78 | 78 | 38.3 | 52/33/30 | 302.6 | 145.4 | 448.0 |
| MFS | F | 42 | No | Valve sparing root replacement (David procedure) | 43 | 70 | 71 | 38.2 | 51/47/29 | 317.4 | 138.0 | 455.4 |
| MFS | M | 28 | No | Valve sparing root replacement (David procedure) | 30 | 87 | 90 | 26.0 | 34/34/31 | 343.9 | 124.3 | 468.2 |
BIA, bioelectrical impedance analysis; STJ, sinotubular junction; LDS, Loeys-Dietz syndrome; MFS, Marfan syndrome; M, male; F, female.
Discussion
In patients with MFS or LDS, aortic aneurysms and dissections have a tremendous negative effect on life expectancy. To the best of our knowledge, the present study is the first to determine an association between the presence of obesity and aortic diameters at different levels or aortic length, in patients with MFS or LDS. In contrast to previous reports, the present study used BIA, a more sophisticated measurement than traditional measures such as BMI, to diagnose obesity, and MRI- or CT-scans to assess aortic size.
In general, mechanisms that trigger aortic aneurysm formation or dissection are not well understood and remain to be completely elucidated (35). Both genetic and environmental risk factors contribute decisively to the pathobiology of these devastating complications by inducing aortic wall stress, inflammation, proteolytic degradation, and/or autoimmune reactions in the aortic wall (20).
Predisposing factors include arterial hypertension, hypercholesterolemia, diabetes mellitus type II, and smoking (21,22). In addition, obesity has been recognized as a major cardiovascular risk factor for the development of aortic aneurysms. However, like many other features in the development of aneurysms, this risk factor remains poorly understood and understudied (35,36). Central obesity is for the general public an inaccurate surrogate measure for visceral fat, which is associated with a high risk of cardiovascular complications (37,38). Adipose tissue can cause chronic inflammation by secreting inflammatory cytokines, called adipocytokines, as well as proinflammatory cytokines such as interleukin (IL)-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1), which trigger the production of reactive oxygen species and oxidative stress, exacerbating vascular inflammation (35-37). It has been suggested that chronic inflammation of this type is involved in the development of aortic aneurysms (39). In fact, local inflammation caused by the perivascular fat contributes to endothelial dysfunction, atherosclerosis, alteration of adiponectin levels, and metabolic syndrome. With the exception of atherosclerosis, all of these conditions may contribute to the development of aortic aneurysm, particularly in the abdominal aorta (35-37). Moreover, it has been known for some time that obese patients do indeed have a higher risk of acute aortic dissection compared with healthy people (35,40). This has also been reported in the context of inflammatory processes in adipose tissue (41,42).
Stackelberg et al. (43) found, in a study of a Swedish population of 63,655 men and women, aged 46–84 years, that the risk of abdominal aortic aneurysm was 30% higher in individuals with increased waist circumference compared with people with normal-size waist. In their study, there was no association between BMI and risk of abdominal aortic aneurysm. In a sample of 12,000 men, Golledge et al. (44) demonstrated a positive correlation between central obesity, as assessed by anthropometric measurements, and abdominal aortic aneurysm. Iribarren et al. [2007] assessed a population of 104,813 male and female multiethnic subjects, and found no association between sagittal abdominal diameter and incidence of abdominal aortic aneurysm (45).
In the recently published prospective Japan Collaborative Cohort Study, including 103,972 Japanese men and women aged 40–79 years, a positive association was seen between BMI and mortality from thoracic aortic aneurysm, abdominal aortic aneurysm, and total aortic disease, but only among men, not among women (19). No association was seen for aortic dissection (19).
Additional recent studies from international databases indicate a temporal trend in BMI. Indeed, this trend was inversely associated with a temporal trend in age-standardized mortality from thoracic aortic aneurysms and abdominal aortic aneurysms (46). In another prospective study from the UK Biobank, involving 335,308 men and women aged 37 to 73 years, no association between BMI and mortality from aortic aneurysm was found (47).
Many of the resulting assumptions from these studies and databases about the effect of obesity and the association between obesity and development of aortic aneurysm are conflicting. While some studies observed a positive association between obesity and pathogenesis and prognosis of aortic aneurysm, others did not (37,48,49). These discrepancies regarding the association between obesity and development of aortic aneurysm may result from different study populations, methodologies, and study design. Notably, various measurement methods (e.g., BMI, waist circumference, waist-to-hip ratio, or sagittal abdominal diameter) were used for the assessment of obesity (44,50).
In MFS and LDS, the development of aortic aneurysms and aortic dissections are common complications. This is also reflected in the patient cohort under consideration. From the clinical view, 12 of the 34 patients with MFS or LDS in the current study had already undergone aortic surgery or intervention for aortic aneurysm and resulting complications. Indeed, four of these patients had even developed aortic dissection.
In MFS and LDS, the development of aneurysms and aortic dissections is pathoanatomically based on the so called “cystic media necrosis” of the aortic wall, characterized by an accumulation of basophilic ground substance in the media and degenerative changes in collagen, elastin, and vascular smooth muscle cells (51). However, it remains largely unclear whether, in patients with MFS or LDS, obesity is associated with or can contribute to the development of aortic aneurysm or aortic dissection.
It is widely overlooked that Yetman and McCrindle (21) demonstrated for the first time a high prevalence of obesity in MFS patients. In their early study, the authors classified 11 out of 50 MFS patients (22%) as obese, and 36% (n=18) as overweight or obese according to BMI. They concluded that obesity is common and potentially associated with an increased risk of aortic complications in adult MFS. However, this assumption has not been pursued further.
For reliable assessment of body composition, BMI may only be of limited value in MFS and LDS patients, and therefore the incidence of obesity may have been underestimated. By using BIA for the assessment of body composition, our group has recently expanded on the views of Yetman and McCrindle and was able to reveal that obesity is even more widespread in the MFS/LDS population than previously thought (26).
Based on these data, the question emerged of whether an association between obesity, hidden obesity, and aortic diameters existed in MFS or LDS patients, along with the possible influence of obesity on aortic size and histopathological alterations of the aortic wall.
In the current study, BIA-assisted body composition analysis revealed an unexpectedly high body fat content in the 34 included patients with MFS or LDS. While in the normal population, the body fat percentage is less than 25% in women and less than 20% in men, it was 31.6%±8.7% in the whole cohort studied, with Marfan patients more affected than LDS patients.
According to BIA, 52.9% (n=18) of the included patients were obese. Within this same population, obesity was more common in women than in men (72.2%), and the fat content in the female population was significantly higher when compared with the male population.
The proportion of connective tissue was slightly, but not statistically significantly, higher in LDS patients (28.1%±5.4%) than in MFS patients (26.2%±4.4%), and the ECM/BCM index, which characterizes the ratio of connective tissue to musculature (MFS: 1.062; LDS 1.097), prevailed.
On the one hand, these results confirm the data of Yetman and McCrindle (21), regarding the existence of obesity in MFS. On the other hand, the present study shows that this proportion is probably even higher than originally thought. This may be explained by the use of different screening methods for the detection of body fat. Today, modern BIA, as used in the current study, certainly allows a more accurate assessment of obesity than the previously chosen anthropometric methods.
So far, none of the published studies used BIA to assess body composition, particularly body fat, in patients with MFS or LDS, as we did. In the present study, we therefore also tried to clarify whether there is a correlation between aortic diameters at different aortic levels or aortic length in MFS or LDS patients.
Considering the aortic length in the entire collective (n=34), measured as the total length of the aorta from the bulb to the bifurcation, as well as the length of the thoracic aorta and abdominal aorta, it is noticeable that the total aorta tended to be and the thoracic aorta was longer in the LDS patients than in the Marfan patients. This is noteworthy because, on average, the MFS patients were larger in body mass than the LDS patients.
When these patients were analyzed with respect to obesity, it is notable that the abdominal aorta tended to be longer in obese patients than in non-obese patients. In contrast, the total aorta and thoracic aorta tended to be longer in non-obese patients.
Because it must be assumed that the length of the measured aortic segments would have been affected by surgical intervention involving the insertion of synthetic material, the non-surgically treated patients were evaluated separately. In this collective, the total aorta and abdominal aorta were longer in MFS patients than in LDS patients. The distinction between obese and non-obese patients in this collective confirms the previously described finding that the abdominal aorta was longer in obese patients than in non-obese patients, and the total aorta and thoracic aorta were longer in non-obese patients. However, these findings did not reach statistical significance.
Ultimately, it remains speculative whether the measured differences in abdominal aortic length are causally related to demonstrated obesity.
When the aortic bulb of the 22 non-surgically treated patients was measured, aortic ectasia or aneurysm was found in 17 patients (77.3%), depending on the definition chosen. This affected 15 (88%) of the Marfan and 2 (40%) of the LDS patients. Whose mean absolute bulb diameter tended to be larger in MFS (42×42×41 mm) than in LDS (38×37×37 mm).
In contrast, significant correlations were found between body fat content, as measured by BIA, and aortic measurements. Body fat content correlated negatively with thoracic aortic length. Further, muscle mass (as measured by BCM) correlated positively with total and thoracic aortic length. This suggests that the more obese the patient, the shorter the thoracic aorta.
Another significant correlation existed between connective tissue (as measured by ECM) and the diameter of the aortic arch, aortic isthmus, and abdominal aorta. In addition, ECM correlated positively with total aortic length. That is, the more connective tissue a patient had, the longer his or her aorta was.
Another significant correlation was found between age and the diameters of: aortic arches 1 and 2, aortic isthmus 1 and 2, and abdominal aorta 1 and 2. Thus, it can be argued that the older the patients, the larger these diameters. In addition, sex had a significant effect on imaging parameters, with male patients having a longer aorta.
Considering only the non-surgically treated patients, a positive correlation was found between the diameter of the aortic bulb and the total length of the aorta as well as the length of the abdominal aorta.
Limitations
There were several strengths and limitations to our work. The present study is the first to examine the association between aortic dimensions and obesity in MFS and LDS patients using modern technologies for the assessment of body composition (namely, BIA). Several CT- or MRI-based measurements of the aortic diameter and length of the aorta were taken at distinct locations along the aorta corresponding to standardized anatomical positions. The location of these points was chosen to allow controls to be performed during the long-term course of the study. Most differences in measured parameters indicated a trend, and these differences did not reach the level of statistical significance. This may be related to the small number of cases, among other factors.
However, some limitations must be considered when interpreting the current results.
This study is cross-sectional in design and does not examine changes over time. The current study is not powered to enable a conclusive assessment to be made of a potential interaction between obesity and aortic risk in MFS and LDS patients. The question remains whether obesity influences the outcome after surgical or interventional procedures. A subsequent cohort study, following more patients over a longer period, could help to clarify how aortic diseases and obesity interact.
The sample of patients seen at tertiary care centers does not represent the typical population of patients with MFS or LDS seen by general practitioners, internists, and general cardiologists. The prevalence of more severe forms of MFS or LDS in tertiary care centers is likely to be higher than either in community-based hospitals or even in departments of cardiology. Lastly, as the presented data derived from a national study, generalization of the conclusions is debatable when involving patients from different cultures or living different parts of the world.
Conclusions
In conclusion, many patients with MFS or LDS are not slender, but obese, which can be more accurately assessed using BIA rather than the normal anthropometric parameters. In the non-surgically treated cohort, all measured aortic bulb diameters tended to be larger in MFS than in LDS patients. Obesity was not significantly correlated with aortic diameter, but when comparing non-obese and obese patients, the thoracic aorta was significantly longer in non-obese patients, whereas the abdominal aorta was longer in obese patients. As obesity is associated with impaired vascular endothelial function and an increased risk of aortic complications, it may be useful to consider this topic in the clinical counseling of affected patients, in addition to the topic of aortic diameters. Especially since most operated patients were obese and female, it may suggest that obesity could play a role here, as women in general more protected against aortic events in MFS and related diseases. Physicians should systematically screen their MFS and LDS patients for obesity and educate them about the potential risk of aortic complications due to obesity that they face. Patients at risk should be encouraged to engage in preventive health care, which includes preventing and reducing adiposity. Moreover, in order to overcome potential bias, measurements of aortic diameter, performed by a single investigator, were supervised by experience imaging specialists.
Acknowledgments
The authors thank the German Heart Foundation (“Deutsche Herzstiftung e.V.”), the German patient organization “Herzkind e. V.”, and also the German health care insurance AOK-Bayern for the promotion of ACHD research. We explicitly thank Dr. Claudia S. Copeland, New Orleans, USA for the professional editing of the final draft of the manuscript. Furthermore, we would like to thank Tamer Snobar for the artistic design of the illustration of the Aorta (Figure 3).
Funding: We acknowledge financial support by Deutsche Forschungsgemeinschaft and the Friedrich-Alexander-Universität Erlangen-Nürnberg within the funding program “Open Access Publication Funding”.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-383/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-383/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-383/coif). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” was commissioned by the editorial office without any funding or sponsorship. M May corresponds to Matthias May payment or honoraria from Siemens Healthcare GmbH and Bayer AG. HK served as the unpaid Guest Editor of the series, received sponsorship/honoraria from Actelion, Janssen, Bristol-Myers Squibb, and is on the steering board of COMPERA International Steering Board. HK also received research grant/support from Deutsche Herzstiftung and Herzkind e.V. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice guidelines. The study was approved by institutional ethics review boards of the Friedrich-Alexander-University Erlangen-Nürnberg (Reference Nr.: 179_21 Bc) and of the Technical University Munich (Reference Nr: 158/19S). All participants gave written informed consent and agreed to an anonymous publication of their data. Guidelines on good pharmacoepidemiology practice and data protection guidelines were followed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaemmerer AS, Freilinger S, Andonian C, et al. Provision of medical health care for adults with congenital heart disease associated with aortic involvement. Cardiovasc Diagn Ther 2021;11:518-28. [Crossref] [PubMed]
- Franken R, Mulder BJM. The Marfan Syndrome. In: Niwa K, Kaemmerer H. editors. Aortopathy Tokyo: Springer; 2017:217-28.
- Ghanta RK, LaPar DJ, Zhang Q, et al. Obesity Increases Risk-Adjusted Morbidity, Mortality, and Cost Following Cardiac Surgery. J Am Heart Assoc 2017;6:003831. [Crossref] [PubMed]
- Mariscalco G, Wozniak MJ, Dawson AG, et al. Body Mass Index and Mortality Among Adults Undergoing Cardiac Surgery: A Nationwide Study With a Systematic Review and Meta-Analysis. Circulation 2017;135:850-63. [Crossref] [PubMed]
- De Santo LS, Moscariello C, Zebele C. Implications of obesity in cardiac surgery: pattern of referral, physiopathology, complications, prognosis. J Thorac Dis 2018;10:4532-9. [Crossref] [PubMed]
- Liu Y, Zhang B, Liang S, et al. Impact of body mass index on early and mid-term outcomes after surgery for acute Stanford type A aortic dissection. J Cardiothorac Surg 2021;16:179. [Crossref] [PubMed]
- Mészáros I, Mórocz J, Szlávi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest 2000;117:1271-8. [Crossref] [PubMed]
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004;79:176-80. [Crossref] [PubMed]
- Olsson C, Thelin S, Ståhle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006;114:2611-8. [Crossref] [PubMed]
- Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031-7. [Crossref] [PubMed]
- Yu HY, Chen YS, Huang SC, et al. Late outcome of patients with aortic dissection: study of a national database. Eur J Cardiothorac Surg 2004;25:683-90. [Crossref] [PubMed]
- Landenhed M, Engström G, Gottsäter A, et al. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc 2015;4:e001513. [Crossref] [PubMed]
- Melvinsdottir IH, Lund SH, Agnarsson BA, et al. The incidence and mortality of acute thoracic aortic dissection: results from a whole nation study. Eur J Cardiothorac Surg 2016;50:1111-7. [Crossref] [PubMed]
- de la Fuente-Alonso A, Toral M, Alfayate A, et al. Aortic disease in Marfan syndrome is caused by overactivation of sGC-PRKG signaling by NO. Nat Commun 2021;12:2628. [Crossref] [PubMed]
- Yu C, Jeremy RW. Angiotensin, transforming growth factor β and aortic dilatation in Marfan syndrome: Of mice and humans. Int J Cardiol Heart Vasc 2018;18:71-80. [Crossref] [PubMed]
- Saeyeldin A, Zafar MA, Velasquez CA, et al. Natural history of aortic root aneurysms in Marfan syndrome. Ann Cardiothorac Surg 2017;6:625-32. [Crossref] [PubMed]
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355:788-98. [Crossref] [PubMed]
- Aalberts JJ, van den Berg MP, Bergman JE, et al. The many faces of aggressive aortic pathology: Loeys-Dietz syndrome. Neth Heart J 2008;16:299-304. [Crossref] [PubMed]
- Takada M, Yamagishi K, Tamakoshi A, et al. Body Mass Index and Mortality From Aortic Aneurysm and Dissection. J Atheroscler Thromb 2021;28:338-48. [Crossref] [PubMed]
- Amalinei C, Carantu I. Etiology and pathogenesis of aortic aneurysm. 2013;
10.5772/56093 .10.5772/56093 - Yetman AT, McCrindle BW. The prevalence and clinical impact of obesity in adults with Marfan syndrome. Can J Cardiol 2010;26:137-9. [Crossref] [PubMed]
- Grewal N, Gittenberger-de Groot AC. Pathogenesis of aortic wall complications in Marfan syndrome. Cardiovasc Pathol 2018;33:62-9. [Crossref] [PubMed]
- Franken R, El Morabit A, de Waard V, et al. Increased aortic tortuosity indicates a more severe aortic phenotype in adults with Marfan syndrome. Int J Cardiol 2015;194:7-12. [Crossref] [PubMed]
- Gouda P, Kay R, Habib M, et al. Clinical features and complications of Loeys-Dietz syndrome: A systematic review. Int J Cardiol 2022;362:158-67. [Crossref] [PubMed]
- von Kodolitsch Y, Demolder A, Girdauskas E, et al. Features of Marfan syndrome not listed in the Ghent nosology - the dark side of the disease. Expert Rev Cardiovasc Ther 2019;17:883-915. [Crossref] [PubMed]
- Freilinger S, Suleiman MN, Bischoff G, et al. Bioelectrical Impedance Analysis as a Contemporary Biomarker of Obesity in Adults with Marfan- or Loeys-Dietz-Syndrome. RCM. 2022. doi:
10.31083/j.rcm2306215 .10.31083/j.rcm2306215 - Hamilton-James K, Collet TH, Pichard C, et al. Precision and accuracy of bioelectrical impedance analysis devices in supine versus standing position with or without retractable handle in Caucasian subjects. Clin Nutr ESPEN 2021;45:267-74. [Crossref] [PubMed]
- Ward LC. Bioelectrical impedance analysis for body composition assessment: reflections on accuracy, clinical utility, and standardisation. Eur J Clin Nutr 2019;73:194-9. [Crossref] [PubMed]
- Feyrer R, Harig F, Cesnjevar RA, et al. Bioelectrical Impedance Analysis in Cardiac Surgery. J Thorac Cardiovasc Surg 2002;5. doi: xxxxx.
- Watanabe T, Ishida N, Takaoka M, et al. Bioelectrical impedance analysis for perioperative water management in adult cardiovascular valve disease surgery. Surg Today 2021;51:1061-7. [Crossref] [PubMed]
- WHO Expert Committee on Physical Status: the Use, Interpretation of Anthropometry, World Health Organization. Physical status: the use of and interpretation of anthropometry, report of a WHO expert committee. Geneva: World Health Organization; 1995.
- van Hout MJ, Scholte AJ, Juffermans JF, et al. How to Measure the Aorta Using MRI: A Practical Guide. J Magn Reson Imaging 2020;52:971-7. [Crossref] [PubMed]
- Campens L, Demulier L, De Groote K, et al. Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. Am J Cardiol 2014;114:914-20. [Crossref] [PubMed]
- Munden RF, Carter BW, Chiles C, et al. Managing Incidental Findings on Thoracic CT: Mediastinal and Cardiovascular Findings. A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2018;15:1087-96. [Crossref] [PubMed]
- Freilinger S, Suleiman MN, Bischoff G, et al. Bioelectrical Impedance Analysis as a Contemporary Biomarker of Obesity in Adults with Marfan- or Loeys-Dietz-Syndrome. Rev Cardiovasc Med 2022;23. doi: xxxxx. [Crossref]
- Wu Z, Wang Z, Wu H, et al. Obesity is a risk factor for preoperative hypoxemia in Stanford A acute aortic dissection. Medicine (Baltimore) 2020;99:e19186. [Crossref] [PubMed]
- Eckstein HH, Maegdefessel L. Linking obesity with abdominal aortic aneurysm development. Eur Heart J 2020;41:2469-71. [Crossref] [PubMed]
- Apoloni RC, Zerati AE, Wolosker N, et al. Analysis of the Correlation Between Central Obesity and Abdominal Aortic Diseases. Ann Vasc Surg 2019;54:176-84. [Crossref] [PubMed]
- Khan A, Van Iterson EH, Laffin LJ. The obesity paradox in heart failure: What is the role of cardiorespiratory fitness? Cleve Clin J Med 2021;88:449-58. [Crossref] [PubMed]
- Okrzeja J, Karwowska A, Błachnio-Zabielska A. The Role of Obesity, Inflammation and Sphingolipids in the Development of an Abdominal Aortic Aneurysm. Nutrients 2022;14:2438. [Crossref] [PubMed]
- Aizawa K, Sakano Y, Ohki S, et al. Obesity is a risk factor of young onset of acute aortic dissection and postoperative hypoxemia. Kyobu Geka 2013;66:437-44. [PubMed]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005;115:911-9; quiz 920. [Crossref] [PubMed]
- Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752-61. [Crossref] [PubMed]
- Stackelberg O, Björck M, Sadr-Azodi O, et al. Obesity and abdominal aortic aneurysm. Br J Surg 2013;100:360-6. [Crossref] [PubMed]
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation 2007;116:2275-9. [Crossref] [PubMed]
- Iribarren C, Darbinian JA, Go AS, et al. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Ann Epidemiol 2007;17:669-78. [Crossref] [PubMed]
- Sidloff D, Choke E, Stather P, et al. Mortality from thoracic aortic diseases and associations with cardiovascular risk factors. Circulation 2014;130:2287-94. [Crossref] [PubMed]
- Wade KH, Carslake D, Sattar N, et al. BMI and Mortality in UK Biobank: Revised Estimates Using Mendelian Randomization. Obesity (Silver Spring) 2018;26:1796-806. [Crossref] [PubMed]
- Police SB, Thatcher SE, Charnigo R, et al. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 2009;29:1458-64. [Crossref] [PubMed]
- Cronin O, Walker PJ, Golledge J. The association of obesity with abdominal aortic aneurysm presence and growth. Atherosclerosis 2013;226:321-7. [Crossref] [PubMed]
- Hu G, Ding N, Wang Z, et al. The association of body composition with abdominal aortic aneurysm growth after endovascular aneurysm repair. Insights Imaging 2022;13:76. [Crossref] [PubMed]
- Niwa K, Kaemmerer H. Aortopathy. Springer, Tokyo; 2017.



