
Clinical aspects and targeted inspiratory muscle training in children and adolescents with Fontan circulation: a randomized controlled trial
Introduction
Univentricular heart (UVH) encompasses a variety of complex congenital heart defects (CHD) in which there is anatomically or functionally only one ventricle, instead of two. Typical examples are tricuspid atresia, mitral atresia, hearts with two atrio-ventricular valves opening into one ventricle (e.g., double inlet left ventricle, double inlet right ventricle), hearts with hypoplastic ventricles (e.g., pulmonary atresia with intact ventricular septum, hypoplastic left heart syndrome), or other rare cardiac defects (e.g., heterotaxy syndromes). Associated are often abnormalities of the great arteries and veins (e.g., transposition of great arteries, systemic or pulmonary vein anomalies, coarctation of the aorta) as well as abnormalities of the atrioventricular (AV) valves (1,2).
Surgical treatment of UVH dates back to the mid-1940s, when the Blalock-Taussig shunt, primarily at that time developed to treat tetralogy of Fallot (TOF), was also used to improve pulmonary perfusion in other cyanotic patients. Other palliative measures for the surgical treatment of UVH followed (3).
The first complete separation of the systemic and pulmonary circulation was performed in 1968 by F. Fontan and E. Baudet in a 12-year-old girl with tricuspid atresia and pulmonary stenosis (4). Through this operation, the systemic and pulmonary circulations are connected in series without a subpulmonary ventricle in between. The driving force for perfusion of the pulmonary circulation is the transpulmonary gradient of a few mmHg, measured as the difference between the elevated central venous pressure and the left atrial pressure. This “passive” pulmonary perfusion is supported by the diastolic suction of the systemic ventricle and by the intrathoracic suction during inspiration (5).
Since the introduction of the Fontan-Operation, worldwide advanced and modified forms of this operation have been applied to many thousands of patients with a morphological or functional UVH and app. 70,000 of them are currently alive (6-8).
Nevertheless, despite all advances in modern medicine and decreasing mortality, patients after Fontan surgery are not cured by this procedure, but invariably have chronic heart disease due to multiple anatomical and functional residua and sequelae (9). Major complications include cardiac arrhythmias, thromboembolism, systemic ventricular dysfunction, AV-valve regurgitation, hepatic dysfunction, increasing cyanosis due to veno-venous collaterals between the systemic and pulmonary venous circulation, protein-losing enteropathy, plastic bronchitis and “Late-Fontan-failure”, a progressive chronic low-cardiac-output syndrome (8,10-12).
Without any doubt, with regard to their exercise tolerance and exercise duration, patients with UVH benefit from Fontan surgery compared to the preoperative situation. But, as known from various studies, the physical performance of patients after any type of Fontan operation or total cavopulmonary connection (TCPC) is only 50–60% of peers (13,14) caused by hemodynamics and peripheral factors such as impaired muscle perfusion, skeletal muscle weakness, impaired muscle function and a high prevalence of respiratory muscle dysfunction (15,16). In adulthood, only 10% show a normal or slightly impaired exercise capacity (17).
Current recommendations for Fontan children include physical activity programs in both aerobic and strength exercise. Regularity, with a duration of at least 30 minutes per day, exercise should be encouraged through activities involving games. Exercise training, physical activity and muscle training result in an increased amount of skeletal muscles and peak exercise capacity (17,18).
Due to the high prevalence of health and psychiatric diagnosis as well as limitations in exercise capacity after Fontan surgery, it is important to use early adapted physical training as well as preventive and/or rehabilitative measures to improve exercise capacity and cardiopulmonary function to favorably control the course of the disease and to reduce morbidity and mortality (17,19).
The results of a meta-analysis show that IMT is an effective training for patients with chronic heart failure, improves ventilatory efficiency and respiratory muscle strength. Further, IMT increases inspiratory endurance, reduces dyspnea, delays fatigue and increases exercise capacity (20). Targeted inspiratory breathing, which can be performed by providing resistance during inhalation with a breathing trainer, increases muscle blood flow in patients with heart failure with reduced ejection fraction (21). In the Fontan circulation, increased systemic venous pressure and chronically decreased output are the cause of pathologic impairment. The increase in pulmonary artery and venous pressure may therefore benefit from targeted training of the skeletal and respiratory muscle pumps (22).
Whether specific IMT in young patients after Fontan surgery can also improve exercise capacity and well-being has not yet been investigated so far.
The aims of the current study were to investigate the effects of a six-month home based inspiratory muscle training, supervised by telephone, (I) on exercise capacity, (II) on lung volumes and (III) on peripheral oxygenation in children and adolescents with Fontan circulation. We present the following article in accordance with the CONSORT reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-308/rc).
Methods
Study cohort
In this nonblinded randomized controlled trial, a total of 40 children and adolescents (12.4±1.9 years, range 8–18) with Fontan circulation were included. The included patients were under regular follow-up by the outpatient clinic of the Department of Congenital Heart Defects and Pediatric Cardiology of the German Heart Centre Munich in Germany and participated in our study from April 2014 to December 2016.
Inclusion criteria were (I) the presence of a Fontan circulation, (II) age of 8 to ≤17 years and (III) written informed consent from the legal guardian(s). Exclusion criteria were (I) refusal of consent and lack of cognitive competence, (II) no physician-certified ability to exercise, (III) change of medication in the past three months, (IV) cardiac catheterization in the past six months, (V) cardiac surgery in the past twelve months, or (VI) an upcoming planned surgery.
All examination methods were established and regularly used for follow-up of these patients. All patients who met the inclusion criteria had a clinical examination, anthropometric measurements, assignment to one of four functional classes (according to Perloff), resting electrocardiogram (ECG), transthoracic echocardiography, laboratory tests, lung function test (LFT) and a cardiopulmonary exercise test (CPET).
J.K. Perloff’s functional classes in congenital heart defects are to be weighed as follows: asymptomatic (Class 1), present symptoms, that do not interfere with normal activities (Class 2), symptoms that interfere with some but not most activities (Class 3) and symptoms that interfere with most if not all activities (Class 4) (23).
After the baseline examination, all patients were assigned to either an intervention group (IG, n=20) or control group (CG, n=20) by stratified randomization in a parallel arm design and computer-generated letter randomization. Patients were assigned to the IG received a training device “Powerbreathe®-Classic” (HAB International Southam, UK). Following the baseline examination, the IG started a daily IMT until a six-month follow-up visit.
For the purpose of the study, patients visited our institution a total of two times. After the initial examination and instruction within May 2014 and May 2015, patients performed the training independently at home. An experienced sports scientist monitored the training by telephone and was available to answer any pending questions and to maintain motivation. The CG continued their usual daily activities without IMT. Within the timeframe November 2014 and November 2015 all study participants were re-examined in the second examination.
Inspiratory muscle training (IMT)
After the baseline examination, all study participants of the IG were introduced to the physiology of the ventilatory system in general and how IMT can support a resistance to fatigue, reduced breathlessness, and exercise capacity. At the beginning, the upright posture was instructed, a certain body awareness and various breathing techniques were taught to all children and adolescents by an experienced physiotherapist. All subjects were then introduced to the inspiratory resistive training device “Powerbreathe®-Classic” (HAB International Southam, UK). Patients were instructed to begin the inhalation phase with abdominal/diaphragmatic breathing and to additionally include flank breathing and the intercostal muscles, for an extensive inspiration. The warm-up phase was used to warm-up the breathing muscles prior to exercise with the goal to improve further exercise performance. Patients were asked to either set the respiratory trainer to the lowest level (Level 1) or one level lower than their present training level. Then they were asked to perform 20 breaths, without much effort. The focus of the warm up phase was on the abdominal and flank breathing, as well as an upright posture while sitting or standing.
The exercises were carried out at our institution, and patients were immediately made aware of any mistakes at the department, so that the home-based training could be performed as well as possible.
With the IMT device, all patients were instructed to aim for the completion of three sets with 30 repetitions in an either standing or upright sitting, relaxed position. If 30 breaths were managed straight away, patients were instructed to turn the tensioner knob to increase the resistance load, measured with a standard medical measurement of pressure [centimeters of water (cmH2O)]. In case the execution was very difficult or even caused an excessive effort, the resistance was immediately reduced. During the inspiratory phase, patients inhaled quickly and forcefully against an adapted resistance, generated by a load calibrated spring and a nose-clip. The exhalation phase was unloaded, slow and passively to prevent dizziness due to hyperventilation.
All children were supervised during the initial implementation and we customized the respiratory training device to their individual level of training in our department. In most cases, a relative was present to whom the exercises and the execution were also shown.
The training load was set as follows: at the first day of training, all patients started at the lowest level (Level 1=10 cmH2O). They were further recommended to set the resistance to a level that was challenging, yet not uncomfortable. In a sitting or standing upright position, patients were instructed to complete 30 breaths at Level 1. If they could complete 30 breaths with ease, patients were instructed to turn the tension knob to increase the resistance by either a half turn (+5 cmH2O) or a full turn (+10 cmH2O) and repeat the maneuver. The device (POWERbreathe medic®) has nine Levels with a total load of 90 cmH2O. Through a process of trial and error, patients were able find the optimal training level with the goal of completing 30 breaths and continue to adjust it, so the training remained challenging and respiratory muscles were steadily built. The completion of 30 breaths with resistance accounted as one training session. Patients were asked to perform 1–2 training sessions every day.
In the six months following the initial examination, all study participants and parents were then contacted weekly via telephone. That was done to address any questions in a timely manner, to motivate the patients, and to jointly increase the resistance of the device, if necessary.
Lung function test and CPET
Lung functional test was performed at least three times and values were normalized by age, sex and height. A test was considered, when the difference of the forced vital capacities (FVC) and the forced expiratory volumes in one second (FEV1) measurements was within 0.2 liters.
CPET was performed with a bicycle ergometer (Corival, Lode, Groningen, The Netherlands) in an upright position. In patients with a responsive pacer the examination was conducted on a treadmill (Care Fusion, LE 200 CE) according to international guidelines (24). All tests were implemented by the same experienced sport scientist. A 3-minute baseline measurement at rest was followed by a 3-minute cycling warm-up with pedaling with 0 watts. Depending on the expected individual exercise capacity by the investigator, and with the aim of an 8–12-minute cycle duration after warm up until exhaustion, load was increased with either 5, 10, 15, 20 or 30 Watts per minute. This was followed by a 3-minute cool down protocol with 5–30 watts and an additional 2-minute monitoring in a quiet sitting position. The following criteria considered the CPET to be of maximal effort: a respiratory exchange ratio (RER) of >1.0, an inability to maintain a cycling pedaling rate of 60 repetitions per minute or a peak heart rate ≥85% was present. Respiratory gas-exchanges were measured by a breath-by-breath analysis using a metabolic chart (Vyasis Healthcare Vmax Encore 29, Hochberg, Germany). The device was calibrated prior each test according to the manufacturer’s examination. Blood pressure was measured every 2 minutes during the examination by an automated, ECG-triggered acoustic device (SunTech Medical Inc.: Tango M2, Morrisville, NC USA). Heart rate and rhythm was monitored with a continuous ECG during the examination. Oxygen saturation (SpO2) was measured continuously with a forehead sensor (Nonin Medical Sensor 7.500, Inc., MN, USA).
Peak oxygen uptake (VO2peak) was defined as the highest moving average 30s interval during the exercise period. Reference values (mL•kg−1•min−1) were calculated according to Cooper and Weiler-Ravell (25). For adolescents 12 years and older, sex-specific reference values (mL/kg/min) were calculated by the following formula:
Female: VO2peak = (22.5 ∙ height (cm) −1837.8)/weight (kg)
Male: VO2peak = (43.6 ∙ height (cm) −4547.1)/weight (kg)
For children (younger than 12 years of age), reference values (mL ∙ kg−1∙ min−1) were calculated from pooled data of both sexes:
VO2peak = (37.1 ∙ height (cm) −3770.6)/weight (kg)
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of the Technical University of Munich (No. 52/14) and informed consent was taken from all individual participants or their legal guardians before the start of documentation. The study was registered on the DRKS.de website (registration ID: DRKS00030340). Guidelines on good epidemiological practice (GPP) and data protection guidelines were followed. Data collection and processing were carried out in compliance with the respective federal and state data protection laws.
Statistical analysis
To characterize the samples, parameters were compared descriptively. After verifying the parametric test requirements using the Kolmogorov-Smirnov test (normal distribution) and Levene’s test (variance homogeneity), the groups could be contrasted using two-tailed t-tests for independent samples. To compare the two groups (CG vs. IG), the changes from the start time to the end examination were first recorded within the groups. Pre-treatment and post-treatment data from the IG were compared to the data from the CG with a Wilcoxon rank sum test (Δ).
The study sample was calculated as follows: In an exercise study of patients with a congenital heart defect (26), an increase in peakVO2 of 2.14±2.83 mL/kg/min was measured in the exercise group and 0.35±4.2 mL/kg/min in the control group. Taking the standard deviation from this study, and calculating an increase of 3 mL/kg/min for the improvements in hemodynamics, a P<0.05 and a power of >80% yields a case number of 16 patients per randomization group. With a drop out rate of 10–20%, the inclusion of 20 patients per group was necessary.
SPSS 22.0.0 (SPSS Inc., IBM Company, Chicago Illinois/USA) was used for all analyses. A significance level less than 0.05 was considered statistically significant. The 95% confidence interval is displayed as (upper-, lower bound).
Results
Study sample, patient characteristics and demographic data
From April 2014 to December 2016, 136 patients were selected and invited to participate. Based on the case number calculation, 40 children and adolescents (29.4%) with Fontan operation (TCPC) were enrolled in the order of their presentation at our institution and if the inclusion criteria were met (Figure 1).
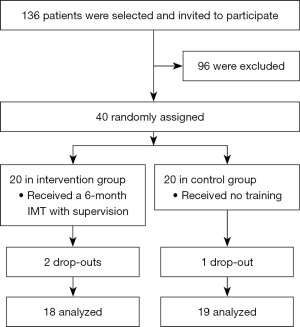
Baseline data and an exact listing of the underlying cardiac defects, type and number of interventions, and additive clinical data of the IG and CG are given in Table 1.
Table 1
| Patients characteristics | Study population (n=40) | Intervention group (n=20) | Control group (n=20) | P value (95% CI) |
|---|---|---|---|---|
| Gender, female, n (%) | 10 (25%) | 4 (20%) | 6 (30%) | 0.478 (−0.18, 0.38) |
| Age (years), mean ± standard deviations [range] | 12.3±2.2 [8.34–17.95] | 12.4±1.9 [10.0–16.4] | 12.6±2.4 [8.9–17.9] | 0.806 (−1.42, 1.47) |
| Age at Fontan surgery (months), mean ± standard deviations [range] | 27.5±9.6 [17–55] | 27.6±10.3 [17–55] | 28.4±9.8 [18–54] | 0.806 (−1.42, 1.47) |
| Body mass index (kg/m2), mean ± standard deviations | 18.2±3.0 | 18.13±3.2 | 18.2±2.8 | 0.927 (−1.84, 2.02) |
| 02 Sat <90%, n (%) | 10 (25%) | 7 (35%) | 3 (15%) | 0.001* (−5.60, −0.68) |
| Diagnosis | ||||
| HLHS | 14 | 6 | 8 | |
| TGA/DORV | 7 | 5 | 2 | |
| UVH | 6 | 3 | 3 | |
| TrA | 6 | 3 | 3 | |
| Heterotaxy-Syndrome | 4 | 1 | 3 | |
| PA + VSD | 3 | 2 | 1 | |
| Ventricular morphology | ||||
| Left | 18 | 9 | 9 | |
| Right | 21 | 10 | 11 | |
| Undetermined | 1 | 1 | - | |
| Type of operation | ||||
| Conduit, extracardiac | 37 | 19 | 18 | |
| Lateral tunnel | 3 | 1 | 2 | |
| Number of cardiovascular operations | ||||
| 2 heart surgeries | 3 | 2 | 1 | |
| 3 heart surgeries | 25 | 14 | 11 | |
| 4 heart surgeries | 8 | 2 | 6 | |
| 5 heart surgeries | 4 | 2 | 2 | |
| Number of interventional treatment | ||||
| Total | 11 | 6 | 5 | |
| 1 intervention | 7 | 3 | 4 | |
| 2 interventions | 3 | 2 | 1 | |
| 3 interventions | 1 | 1 | 0 | |
| Cardiac rhythm | ||||
| Sinus rhythm | 25 | 11 | 14 | |
| Atrial rhythm, ectopic | 7 | 4 | 3 | |
| Junctional rhythm | 2 | 1 | 1 | |
| AV dissociation | 2 | 2 | - | |
| Pacing | 4 | 2 | 2 | |
| Medication | ||||
| Oral anticoagulation | 40 | 19 | 21 | |
| ACE inhibitors/AT blockers | 6 | 2 | 4 | |
| Beta-blockers | 1 | 1 | - | |
| Diuretic | 4 | 2 | 2 | |
| Digitalis | 2 | 1 | 1 | |
| Targeted pulmonary vasoactive medication | 1 | - | 1 | |
| Other medication | 6 | 2 | 4 |
*, significance value was set to ≤0.05. CI, confidence interval; HLHS, hypoplastic left heart syndrome; TGA, transposition of the great arteries; DORV, double outlet right ventricle; UVH, univentricular heart; TrA, tricuspid atresia; PA + VSD, pulmonary atresia with ventricular septal defect; TCPC, total cavopulmonary connection; AV, atrioventricular; ACE, angiotensin-converting enzyme; AT, angiotensin.
Age at the time of TCPC ranged from 17 to 55 months (mean, 27.5±9.6 months). At the time of study inclusion, the mean age of patients was 12.3±2.2 years (range, 8.34–17.95 years). There was a mean interval of 10.0±2.0 years between Fontan completion and study inclusion (range: 6.6–14.5 years).
After the assignment to the CG and IG, no significant differences were found with respect to sex distribution, age at TCPC, age at entry examination, time interval between TCPC and entry examination, and body mass index adjusted for age and sex for children and adolescents.
Patients with resting oxygen saturation below 90% were found in both study groups. Their percentage was significantly higher in the intervention group (IG: n=7 vs. CG: n=3, P=0.001).
Type of congenital heart disease
The 40 patients with UVH after Fontan surgery could be assigned to six main groups as shown in Table 1: Hypoplastic left heart syndrome (n=14); transposition/malposition/double outlet right ventricle (DORV) (n=7) (including: complete transposition (n=4), DORV-TGA (n=3); UVH (n=6) [including: double inlet left ventricle (n=4), single ventricle (n=1), congenitally corrected transposition (n=1)]; tricuspid atresia (n=6); heterotaxy syndrome (n=4), or pulmonary atresia with intact septum (n=3).
Type of surgical treatment
The majority of the 40 patients had been treated with an extracardiac conduit (n=37). The lateral tunnel technique was used in only three cases (Table 1). During surgery, fenestration had been applied in two patients. Four patients (10%) were supplied with a pacemaker.
Additive clinical data
All 40 patients included were in good clinical condition and without signs of cardiac decompensation at the time of enrollment. None of the patients had oedema, effusions, chylothorax, ascites, or manifest protein losing enteropathy. Residua or sequelae not currently requiring treatment included bronchitis plastica (n=1), diaphragmatic paresis (n=2), pulmonary artery stenosis (n=3), and aorto-pulmonary collaterals or fistulae (n=6).
All included patients were on chronic pharmacotherapy with oral anticoagulants (n=40) and cardiovascular agents [ACE inhibitors (n=6), diuretics (n=6), digitalis (n=6), and beta-blockers (n=6)]. A targeted pulmonary vasoactive treatment with a PDE-5 inhibitor (sildenafil) was given to one patient (Table 1).
Lung function
A detailed overview of the lung functional values is given in Table 2. At baseline, there were no significant differences between the IG and the CG. After a six-month IMT, the forced vital capacity (FVC) in the IG increased by 10% from 2.58±0.75 to 2.84±0.75 liters. There was an 11% increase in the CG, from 2.3±0.79 to 2.57±0.87 liters [P=0.946 (−0.16, 0.17)]. Further, the FEV1 and the predicted FEV1 did not change significantly after IMT between the IG and CG [ΔFEV1: CG: 0.14±0.30 vs. TG: 0.17±0.20, P=0.707 (−0.20, 0.14); ΔFEV1 predicted: CG: 0.71%±11.36% vs. IG: 1.18%±6.85%, P=0.884 (−6.91, 5.98)].
Table 2
| Variables | Control group (n=19) | Intervention group (n=18) | P value (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline evaluation | 6-month follow-up | Difference (Δ) | Baseline evaluation | 6-month follow-up | Difference (Δ) | |||
| Lung function | ||||||||
| FVC, L | 2.3±0.79 | 2.57±0.87 | 0.22±0.31 | 2.58±0.75 | 2.84±0.75 | 0.21±0.16 | 0.946 (−0.16, 0.17) | |
| FVC predicted, % | 79.3±14.8 | 82.6±15.40 | 2.71±9.79 | 81.8±15.10 | 83.7±15.80 | 1.55±5.40 | 0.669 (−4.29, 6.60) | |
| FEV1, L | 2.1±0.73 | 2.28±0.78 | 0.14±0.30 | 2.25±0.67 | 2.45±0.71 | 0.17±0.20 | 0.707 (−0.20, 0.14) | |
| FEV1 predicted, % | 82.8±17.10 | 84.1±16.10 | 0.71±11.36 | 81.8±13.5 | 83.2±16.10 | 1.18±6.85 | 0.884 (−6.91, 5.98) | |
| FEV1/FVC ratio, % | 91.36±6.07 | 89.3±7.18 | −1.98±6.20 | 87.26±7.13 | 86.13±9.41 | −0.81±6.29 | 0.576 (−5.41, 3.05) | |
| FEV1/FVC ratio predicted, % | 105±7.40 | 102.82±8.41 | −2.07±7.27 | 100.6 ±8.00 | 99.35±10.75 | −0.92±7.29 | 0.639 (−6.08, 3.78) | |
| Exercise capacity | ||||||||
| VO2 peak, mL/kg/min | 32.98±5.84 | 33.93±6.35 | 0.83±3.91 | 35.74±6.89 | 36.76±7.84 | 1.05±5.76 | 0.893 (−3.52, 3.08) | |
| VO2 peak predicted, % | 80.1±14.30 | 81.88±16.38 | 1.90±7.96 | 79.5±17.10 | 78.97±17.12 | 0.88±13.19 | 0.793 (−6.84, 8.89) | |
| VE/VCO2 slope | 32.4±3.95 | 32.04±2.89 | −0.43±3.35 | 32.05±3.75 | 31.43±3.5 | 0.36±1.74 | 0.936 (−1.91, 1.76) | |
| RER at peak exercise | 1.15±0.06 | 1.14±0.07 | 0.00±0.07 | 1.16±0.09 | 1.13±0.07 | 0.03±0.07 | 0.298 (−0.02, 0.07) | |
| Heart rate at rest, bpm | 82.5±13.17 | 83.37±14.81 | 0.74±10.47 | 84.3±12.98 | 82.59±13.3 | −1.00±7.66 | 0.578 (−4.53, 8.01) | |
| Heart rate max, bpm | 168.05±20.39 | 170.11±15.74 | 2.84±14.47 | 171.95±17.37 | 171±11.83 | 0±6.95 | 0.452 (−4.80, 10.48) | |
| RR rest, mmHg | 110.5±13.23 | 114.63±14.77 | 4.37±13.76 | 114.6±13.09 | 110.53±12 | −2.82±7.76 | 0.066 (−0.49, 14.88) | |
| RR max, mmHg | 152.05±21.29 | 157.21±19.54 | 6.79±27.27 | 163.45±27.07 | 165.29±27.44 | 5.94±14.55 | 0.910 (−14.22, 15.91) | |
| SpO2 at rest, % | 93.7±3.30 | 94.1±2.40 | 0.17±2.92 | 91.5±4.90 | 94.4%±4.00 | 3.31±4.09 | 0.014* (−5.60, −0.68) | |
| SpO2 at peak exercise, % | 87±6.00 | 89.1±6.50 | 1.84±7.96 | 87.1±5.30 | 91.0±3.40 | 4.63±4.54 | 0.225 (−7.35, 1.79) | |
| Breathing reserve | 18.7±16.62 | 15.68±18.43 | −3.21±17.91 | 13.7±16.17 | 16.35±17.12 | 1.76±17.70 | 0.409 (−17.06, 7.11) | |
| Workload, W, max | 111.33±48.23 | 118.5±49.17 | 7.17±13.36 | 123.89±46.36 | 140.44±42.54 | 14.19±11.55 | 0.113 (−15.8, 1.76) | |
Mean differences ± standard deviations; *, significance value was set to ≤0.05. CI, confidence interval; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; VO2peak, oxygen uptake at peak exercise; VE/VCO2slope, slope of the minute ventilation and carbon dioxide production; RER, respiratory exchange ratio; bpm, beats per minute; HR, heart rate; RR, blood pressure (systolic); SpO2, oxygen saturation.
Exercise capacity
Within the observation period, both groups increased their peak VO2 and their predicted VO2. The difference was not statistically significant [ΔVO2 peak: IG: 1.05±5.76 mL/kg/min vs. CG: 0.83±3.91 mL/kg/min, P=0.893 (−3.52, 3.08); ΔVO2 peak predicted: IG: 0.88%±13.19% vs. CG: 1.90%±7.96%, P=0.793 (−6.84, 8.89)]. Further, between the IG and the CG, there were no significant differences within the ventilatory efficiency [ΔVEVCO2 slope: IG: 0.36±1.74 vs. CG: −0.43±3.35, P=0.936 (−1.91, 1.76)]. The maximum watts achieved increased by 14% from 123 to 140 watts in the IG vs. 6.5% from 111 to 119 watts in the CG. However, the difference was also not significant [P=0.113 (−15.8, 1.76)] (Table 2).
Oxygen saturation
At baseline, individual patients had a resting oxygen saturation below 90%. Their proportion was significantly higher in the IG (IG n=7 vs. CG n=3, P=0.001). Further, all included patients in both groups had an oxygen saturation at peak exercise below 90% [IG: 87.1%±5.30% vs.CG: 87%±6.00%, P=977 (−3.73, 3.63)].
After a six-month IMT, there was a significant increase of oxygen saturation at rest in the IG compared to the CG [IG: 3.31%±4.09% vs. CG: 0.17%±2.92%, P=0.014 (−5.60, −0.68)]. Furthermore, it is notable that, if compared to the CG, the mean oxygen saturation at peak exercise no longer dropped below 90% in the IG. This observation is not statistically significant, yet certainly of clinical relevance (Table 2).
Side effects/Drop out within the study period
No change in medication was required during the study period, and no patient had died. All but one patient were clinically stable. In this case, cardiac decompensation occurred, study participation was discontinued, and the patient received an aortic valve replacement and recovered. In total, three patients discontinued the study due to lack of motivation to continue to participate in the study. This concerned one patient in the CG and two patients in the IG.
Discussion
To the best of our knowledge, this randomized controlled trial is the first to address the effects of IMT in children and adolescents with Fontan circulation. The results of the study did not show a significant improvement in lung function or exercise capacity, yet the maximum workload achieved in the IG trended to improve if compared to the CG. Patients in the IG showed a significant increase of oxygen saturation at rest after a daily IMT for six months.
Hence the outcomes of patients with Fontan circulation have improved remarkably, patients suffer from chronic heart disease due to multiple anatomic and functional residua and sequelae. Morbidity in the long-term course after Fontan operation is preferentially determined by limited exercise ability, cardiac arrhythmias, cyanosis, thromboembolism, dysfunction of the systemic ventricle, AV valve insufficiency, hepatic dysfunction, increasing cyanosis due to veno-venous collaterals between the systemic and pulmonary venous circulation, protein-losing enteropathy, and plastic bronchitis (8,9). Clinically, patients may present with typical heart failure symptoms such as an impaired exercise tolerance, dyspnea, and fatigue (27,28).
Apart from cardiac disease, there is sufficient evidence that Fontan patients have restrictive ventilatory function in terms of reduced FVC and FEV1. According to Callegari et al., a total of 59.5% of the included 232 Fontan patients had restrictive ventilatory dysfunction, and none had obstructive ventilatory dysfunction. These findings were associated with diaphragmatic paresis, scoliosis, number of surgical procedures, and low BMI. Exercise capacity and quality of life were severely affected by these pulmonary dysfunctions (29). In contrast, the values obtained in the current study did not indicate a restrictive or obstructive flow pattern in any case in the overall collective of study participants. The included patients showed a comparatively high predicted FVC of 80.5%±14.8% and a predicted FEV1 of 82.3%±15.2% under baseline conditions. Further, a large cohort study highlighted the important role of the lung in exercise response of young Fontan patients, with FVC below the normal range in nearly half of Fontan patients, resulting in impaired exercise capacity and limited ventilatory efficiency (30).
Exercise capacity in children and adolescents with Fontan circulation ranges from 50–60% of their peers (13,14) and decreases disproportionately with increasing age while maintaining the same level of activity and an unrestricted quality of life (31-33). The underlying causes of reduced exercise capacity in Fontan patients are multifactorial and are often related to a reduced stroke volume due to insufficient diastolic and/or systolic function, insufficient preloading conditions as well as chronotropic insufficiency (34-38). Accordingly, the limitation of exercise capacity is attributed to both cardiovascular and pulmonary factors.
Such limitations did not occur in the investigated patient collective. The included patients showed a comparatively high exercise capacity with a mean VO2peak of 34.4±6.6 mL/kg/min at baseline. Further, only a few patients (n=5; 12.5%) showed a reduction of ventricular function in the echocardiography, which may explain the good performance of the study participants. Moreover, all patients were able to adequately increase blood pressure under exercise conditions and did not show an exercise-induced chronotropic insufficiency.
IMT is a simple and low-cost preventive tool, that is well tolerated by Fontan patients (39). Our collective quickly understood the use of the training device and the breathing movements and the instruction could thus be easily integrated into the daily clinic routine. The application and the individual adjustment at home did not cause any difficulties to the participants.
Studies on IMT in Fontan patients have been conducted with varying results (39-41).
Laohachai et al. (40) conducted a home-based program of IMT to measure its benefits on inspiratory muscle strength and ventilatory efficiency in 19 adolescent patients (aged 16±2 years, 48% female) with a nonfenestrated extracardiac conduit. The IMT included the components of endurance training and was done using a Philips Threshold IMT device. The inspiratory load was set to 30% of the participant’s measured maximal inspiratory pressure. After six weeks of IMT, maximal inspiratory pressure, ventilatory efficiency during exercise and cardiac output at rest improved significantly. There were no statistically significant changes in lung function, O2 saturation or O2 pulse (40).
Wu et al. (39) conducted a single-arm pilot intervention trial in eleven adult patients [45% female, 28.8 (25.7, 45.5) years]. The 12-week IMT included the components of endurance training. Subjects practiced daily for 30 minutes with an IMT device (Philips Respironics, Murrysville, PA) set at 40% of measured maximal inspiratory pressure and were supervised weekly by telephone. Subjects were instructed to breathe at a rate of 12–16 breaths per minute and to increase resistance by a set value every two weeks. At follow-up, IMT was associated to a significant improvement in peak workload (116.5±45.0 to 126.8±47.0 W, P=0.019). There was a trend towards an enhancement of ventilatory efficiency during exercise, but compared to Laohachai et al. these findings were not significant. Further, there was no significant change in predicted peak VO2, maximal inspiratory pressure, peak HR or peak O2 pulse.
Our results show similar results concerning exercise capacity values if compared to recent studies. In our patients, the maximum workload trended to improve with an increase of 14% from 123 to 140 watts in the IG vs. 6.5% from 111 to 119 watts in the CG (P=0.113). Further, both groups increased their peak VO2 and their predicted VO2. In accordance to previous results, our differences were also not significant.
The current study did not investigate maximal inspiratory pressure and cardiac output at rest.
Fritz et al. (41) conducted a randomized controlled trial with 42 adult Fontan patients (50% female; 30.5±8.1 years). Their daily IMT consisted of a six-month home-based weekly telephone-supervised strength training with a POWERbreathe (International Ltd., Southam, UK) device. Patients started without an inspiratory load and increased the load individually upon their individually adapted maximum, with the goal to achieve three sets with 10–30 repetitions a day. At the 6-month follow-up examination, the IG had not improved their peak VO2 and predicted VO2peak significantly. Further, there was no significant change concerning ventilatory efficiency during exercise. Regarding lung function, no significant changes could be observed between the study groups concerning FVC, the predicted FVC, FEV1 and the predicted FEV1 after IMT. As in the current study, a significant result was shown with an increase oxygen saturation at rest in the intervention group [ΔSpO2: IG: 1.50% (−0.25%, 3.00%) vs. CG: −0.50% (−1.75%, 0.75%); P=0.017]. This result could also be shown in our patients. All included patients in both groups had an oxygen saturation at peak exercise below 90% at baseline (IG: 87.1%±5.30% vs. CG: 87%±6.00%, P=977). Further, the IG had a significantly higher rate of patients with a resting oxygen saturation below 90% (IG n=7 vs. CG n=3, P=0.001). In six cases, aorto-pulmonary collaterals or fistulas were present, which could be the causative factor. Their presence may explain the clinical relevance, since after a six-month IMT, there was a significant increase of oxygen saturation at rest in the IG compared to the CG (IG: 3.31%±4.09% vs. CG: 0.17%±2.92%, P=0.014).
The improvement in oxygen saturation can be considered as an important outcome of this work. Oxygen saturation in Fontan patients rarely exceeds 95%. Due to numeral reasons, such as a mismatch between ventilation and perfusion and the nonpulsatile pulmonary arterial blood flow it usually ranges between 90–95%. In addition, in some Fontan patients show a remarkably low arterial oxygen saturation (<90%) at rest. This may be caused by the presence of fenestration or the development of veno-venous collaterals with a right-to-left shunt. The results suggest that Fontan patients may benefit from a respiratory training program since a lower oxygen saturation was previously associated with worse exercise performance (22).
Furthermore, even if not statistically significant, the mean oxygen saturation at peak exercise no longer dropped below 90% in the IG. In this patients group, arterial oxygen saturation often falls below 90% during exercise. Cyanosis mechanisms may be exaggerated during exercise and the extent of desaturated lower extremity venous return might increase (22).
Regarding the IMT training design and duration, these studies differ remarkably in the type of intensity and implementation.
Since the above studies incorporated endurance training into IMT showed significant benefits after a shorter period of time, it may indicate that respiratory training with endurance components may be more efficient than strength training in Fontan patients, as previously shown (41).
However, when comparing the studies, numerous components of diagnostic findings must be considered, since patients differ in age, medical care (such as type of surgery, medication), and possibly in the type of medical follow-up. In contrast to other studies, a mostly homogeneous surgical approach had been applied for the surgical treatment in our study population. With the exception of three cases in which the lateral tunnel technique was used, all patients had received an extracardiac conduit. Only in two cases fenestration had been performed intraoperatively.
Data suggest that the timing of surgery has a major impact on prognosis, as there have been major advances in the medical treatment options for these patients. In a recently published study from the Mayo Clinic that examined the long-term outcome of 1,052 patients, the 10-, 20-, and 30-year survival rates after Fontan surgery were 74%, 61%, and 43%, respectively (42). A prospective, 40-year study from Norway shows the survival of patients with UVH operated between 1971 and 2011 in dependence on the timing of surgery. In this study, patients operated on from 2000 had the best prognosis (43). The latter period also includes our included patients. The clinical status of the patients included in the current study was above average, since all were in a defined functional class II, showed a comparatively high exercise capacity and lung functional values. No patient had edema, effusions, a chylothorax, ascites, or a manifest protein wasting syndrome. None had to be classified as “failing Fontan”.
To date, there are hardly any detailed recommendations regarding prevention for Fontan patients. As patients now reach adulthood due to improved surgical techniques and treatment modalities, but suffer from chronic heart disease, secondary and tertiary prevention for Fontan patients is becoming increasingly important. Based on the available data, it appears that the improvement of exercise capacity and cardiopulmonary function can have a positive effect on long-term outcomes. It is of importance that in Fontan patients physical training is individually adapted, controlled and medically supervised (31,44-46).
The current study results suggest that it is reasonable to integrate an additional, targeted IMT into the rehabilitation measures in order to further improve the performance and perhaps also the prognosis of the affected patients. Further studies are certainly needed to be able to further improve the effectiveness of these prophylactic measures and to document a long-term effect.
Limitations
The present study recruited a remarkably large sample of children and adolescents with Fontan circulation and focused in this prospective, randomized controlled study on a targeted IMT in children and adolescents. However, some limitations must be considered when interpreting the current results.
Since complex CHD after Fontan operation is not a common disease, it is a general challenge to obtain a representative sample of patients. Nevertheless, the present study recruited a remarkably sample of patients.
It is suggested that further studies may include further lung capacity parameters such as the FRC or DLCO, two essential examination parameters that could not be collected due to lack of technical equipment in this study. Respiratory muscle function, which is not assessed in the study could be included as an important additional aspect of investigation for further projects.
Lastly, the presented data derive solely from patients living in Germany, Austria and South Tyrol. Generalization of the conclusions and transmission to patients living in other countries or different culture groups is debatable. Further studies are needed in this regard.
Despite these potential limitations, the present study highlights the multiple limitations of patients with Fontan circulation and the need for regular follow-up and advanced treatment, including preventive or rehabilitative measures.
Conclusions
Although not all data obtained were statistically significant, they can still be considered clinically relevant. Therefore, the current study results suggest that IMT might be integrated into the exercise and rehabilitation program to improve the performance and perhaps the prognosis of Fontan patients. Further studies are certainly needed to further improve the effectiveness of these measures and to document the long-term effects.
Acknowledgments
Funding: This work was supported by the Stiftung KinderHerz (ID: DHM130805).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-308/rc
Trial Protocol: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-308/tp
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-308/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (https://cdt.amegroups.com/article/view/10.21037/cdt-22-308/coif). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” was commissioned by the editorial office without any funding or sponsorship. HK served as the unpaid Guest Editor of the series. HK has the following potential conflicts of interest to report: Sponsorship/Honoraria: Actelion/Janssen, Bristol-Myers Squibb, Steering Board: COMPERA International Steering Board and Research grant/support: Patient organizations: Deutsche Herzstiftung and Herzkind e.V. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of the Technical University of Munich (No. 52/14) and informed consent was taken from all individual participants or their legal guardians before the start of documentation. The study was registered on the DRKS.de website (registration ID: DRKS00030340). Guidelines on good epidemiological practice (GPP) and data protection guidelines were followed. Data collection and processing were carried out in compliance with the respective federal and state data protection laws.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kreutzer E, Kreutzer J, Kreutzer G. Univentricular Heart. In: Moller J, Hoffman J, editors. Pediatric cardiovascular Medicine. New York: Churchill Livingstone; 2000. p: 469-98.
- Hager A, Ovroutski S, Cesnjevar R. Leitlinien Pädiatrische Kardiologie: Univentrikuläres Herz. In: Weil J, editor. Leitlinien zur Diagnostik und Therapie in der Pädiatrische Kardiologie. Urban und Fischer: Elsevier; 2016. p: 273-88.
- Lange R, Hörer J. Funktionell singulärer Ventrikel und die Fontan-Operation. Herzchirurgie: Die Eingriffe am Herzen und an den herznahen Gefäßen. Berlin: Springer; 2010. p: 331-63.
- Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971;26:240-8. [Crossref] [PubMed]
- Gewillig M, Brown SC, Eyskens B, et al. The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg 2010;10:428-33. [Crossref] [PubMed]
- Castaneda AR. From Glenn to Fontan. A continuing evolution. Circulation 1992;86:II80-4. [PubMed]
- Marcelletti C, Corno A, Giannico S, et al. Inferior vena cava-pulmonary artery extracardiac conduit. A new form of right heart bypass. J Thorac Cardiovasc Surg 1990;100:228-32. [Crossref] [PubMed]
- Khairy P, Fernandes SM, Mayer JE Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008;117:85-92. [Crossref] [PubMed]
- Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart 2016;102:1081-6. [Crossref] [PubMed]
- Gewillig M. The Fontan circulation. Heart 2005;91:839-46. [Crossref] [PubMed]
- Khairy P, Poirier N, Mercier LA. Univentricular heart. Circulation 2007;115:800-12. [Crossref] [PubMed]
- Piran S, Veldtman G, Siu S, et al. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation 2002;105:1189-94. [Crossref] [PubMed]
- Driscoll DJ, Danielson GK, Puga FJ, et al. Exercise tolerance and cardiorespiratory response to exercise after the Fontan operation for tricuspid atresia or functional single ventricle. J Am Coll Cardiol 1986;7:1087-94. [Crossref] [PubMed]
- Zellers TM, Driscoll DJ, Mottram CD, et al. Exercise tolerance and cardiorespiratory response to exercise before and after the Fontan operation. Mayo Clin Proc 1989;64:1489-97. [Crossref] [PubMed]
- Brassard P, Bédard E, Jobin J, et al. Exercise capacity and impact of exercise training in patients after a Fontan procedure: a review. Can J Cardiol 2006;22:489-95. [Crossref] [PubMed]
- Greutmann M, Le TL, Tobler D, et al. Generalised muscle weakness in young adults with congenital heart disease. Heart 2011;97:1164-8. [Crossref] [PubMed]
- Zentner D, Celermajer DS, Gentles T, et al. Management of People With a Fontan Circulation: a Cardiac Society of Australia and New Zealand Position statement. Heart Lung Circ 2020;29:5-39. [Crossref] [PubMed]
- Cordina RL, O'Meagher S, Karmali A, et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol 2013;168:780-8. [Crossref] [PubMed]
- DeMaso DR, Calderon J, Taylor GA, et al. Psychiatric Disorders in Adolescents With Single Ventricle Congenital Heart Disease. Pediatrics 2017;139:e20162241. [Crossref] [PubMed]
- Lin SJ, McElfresh J, Hall B, et al. Inspiratory muscle training in patients with heart failure: a systematic review. Cardiopulm Phys Ther J 2012;23:29-36. [Crossref] [PubMed]
- Trevizan PF, Antunes-Correa LM, Lobo DML, et al. Effects of inspiratory muscle training combined with aerobic exercise training on neurovascular control in chronic heart failure patients. ESC Heart Fail 2021;8:3845-54. [Crossref] [PubMed]
- Rychik J, Atz AM, Celermajer DS, et al. Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation 2019; Epub ahead of print. [Crossref] [PubMed]
- Perloff JK. Congenital heart disease and pregnancy. Clin Cardiol 1994;17:579-87. [Crossref] [PubMed]
- Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 2002;40:1531-40. [Crossref] [PubMed]
- Cooper DM, Weiler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis 1984;129:S47-8. [Crossref] [PubMed]
- Therrien J, Fredriksen P, Walker M, et al. A pilot study of exercise training in adult patients with repaired tetralogy of Fallot. Can J Cardiol 2003;19:685-9. [PubMed]
- Billett J, Majeed A, Gatzoulis M, et al. Trends in hospital admissions, in-hospital case fatality and population mortality from congenital heart disease in England, 1994 to 2004. Heart 2008;94:342-8. [Crossref] [PubMed]
- Engelfriet P, Boersma E, Oechslin E, et al. The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. The Euro Heart Survey on adult congenital heart disease. Eur Heart J 2005;26:2325-33. [Crossref] [PubMed]
- Callegari A, Neidenbach R, Milanesi O, et al. A restrictive ventilatory pattern is common in patients with univentricular heart after Fontan palliation and associated with a reduced exercise capacity and quality of life. Congenit Heart Dis 2019;14:147-55. [Crossref] [PubMed]
- Opotowsky AR, Landzberg MJ, Earing MG, et al. Abnormal spirometry after the Fontan procedure is common and associated with impaired aerobic capacity. Am J Physiol Heart Circ Physiol 2014;307:H110-7. [Crossref] [PubMed]
- Giardini A, Hager A, Pace Napoleone C, et al. Natural history of exercise capacity after the Fontan operation: a longitudinal study. Ann Thorac Surg 2008;85:818-21. [Crossref] [PubMed]
- Anderson PA, Sleeper LA, Mahony L, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol 2008;52:85-98. [Crossref] [PubMed]
- Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 2008;52:99-107. [Crossref] [PubMed]
- Chatzis AC, Giannopoulos NM, Tsoutsinos AI, et al. Successful surgical correction of congenital heart disease in adults: seven years' experience. Hellenic J Cardiol 2005;46:128-34. [PubMed]
- Zajac A, Tomkiewicz L, Podolec P, et al. Cardiorespiratory response to exercise in children after modified fontan operation. Scand Cardiovasc J 2002;36:80-5. [Crossref] [PubMed]
- Senzaki H, Masutani S, Ishido H, et al. Cardiac rest and reserve function in patients with Fontan circulation. J Am Coll Cardiol 2006;47:2528-35. [Crossref] [PubMed]
- Durongpisitkul K, Driscoll DJ, Mahoney DW, et al. Cardiorespiratory response to exercise after modified Fontan operation: determinants of performance. J Am Coll Cardiol 1997;29:785-90. [Crossref] [PubMed]
- Goldberg DJ, Avitabile CM, McBride MG, et al. Exercise capacity in the Fontan circulation. Cardiol Young 2013;23:824-30. [Crossref] [PubMed]
- Wu FM, Opotowsky AR, Denhoff ER, et al. A Pilot Study of Inspiratory Muscle Training to Improve Exercise Capacity in Patients with Fontan Physiology. Semin Thorac Cardiovasc Surg 2018;30:462-9. [Crossref] [PubMed]
- Laohachai K, Winlaw D, Selvadurai H, et al. Inspiratory Muscle Training Is Associated With Improved Inspiratory Muscle Strength, Resting Cardiac Output, and the Ventilatory Efficiency of Exercise in Patients With a Fontan Circulation. J Am Heart Assoc 2017;6:e005750. [Crossref] [PubMed]
- Fritz C, Müller J, Oberhoffer R, et al. Inspiratory muscle training did not improve exercise capacity and lung function in adult patients with Fontan circulation: A randomized controlled trial. Int J Cardiol 2020;319:69-70. [Crossref] [PubMed]
- Pundi KN, Johnson JN, Dearani JA, et al. 40-Year Follow-Up After the Fontan Operation: Long-Term Outcomes of 1,052 Patients. J Am Coll Cardiol 2015;66:1700-10. [Crossref] [PubMed]
- Erikssen G, Liestøl K, Seem E, et al. Achievements in congenital heart defect surgery: a prospective, 40-year study of 7038 patients. Circulation 2015;131:337-46; discussion 346. [Crossref] [PubMed]
- Takken T, Giardini A, Reybrouck T, et al. Recommendations for physical activity, recreation sport, and exercise training in paediatric patients with congenital heart disease: a report from the Exercise, Basic & Translational Research Section of the European Association of Cardiovascular Prevention and Rehabilitation, the European Congenital Heart and Lung Exercise Group, and the Association for European Paediatric Cardiology. Eur J Prev Cardiol 2012;19:1034-65. [Crossref] [PubMed]
- Müller J, Christov F, Schreiber C, et al. Exercise capacity, quality of life, and daily activity in the long-term follow-up of patients with univentricular heart and total cavopulmonary connection. Eur Heart J 2009;30:2915-20. [Crossref] [PubMed]
- Opocher F, Varnier M, Sanders SP, et al. Effects of aerobic exercise training in children after the Fontan operation. Am J Cardiol 2005;95:150-2. [Crossref] [PubMed]


