
Case report: a potential modulation of coronary atheroma by lowering triglyceride-rich lipoproteins with pemafibrate: insights from serial near-infrared spectroscopy imaging
Highlight box
Key findings
• In a statin-treated diabetic patient with coronary artery disease (CAD), a reduction of NIRS-derived MaxLCBI4mm at non-culprit segment was observed after the commencement of pemafbrate.
What is known and what is new?
• Hypertriglyceridemia has been considered as a residual risk associated with atherosclerotic cardiovascular disease in statin-treated patients.
• We observed that adding 0.2 mg pemafibrate to high-intensity statin therapy lowered levels of fasting triglyceride and triglyceride-rich lipoproteins, accompanied by a reduction of lipidic plaque materials in a diabetic patient with CAD.
What is the implication, and what should change now?
• This case suggests a potential benefit of lowering triglyceride with pemafibrate to modulate vulnerable form of disease in statin-treated patients. On-going study (PEMA-CORE) will elucidate how coronary atheroma responds to pemafibrate therapy under stain use.
Introduction
Despite a large body of evidence showing anti-atherosclerotic benefit of statin therapy, atherosclerotic cardiovascular disease (ASCVD) still occurs, which suggests the need to modulate additional atherogenic targets. Triglyceride-rich lipoproteins have been considered as residual risks associated with ASCVD (1). While observational and genetic studies have reported the association of triglyceride-rich lipoproteins with ASCVD (1), clinical benefit to modify these lipoproteins is not fully established yet. Pemafibrate is a potent selective peroxisome proliferator-activated receptor α modulator (2). This agent has been shown to decrease triglyceride level by 30–40%, accompanied by a favourable reduction of apolipoprotein CIII, remnant cholesterol and non-HDL-C. We present the current case which enabled to evaluate serial changes of coronary atherosclerosis under pemafibrate use in type 2 diabetic patients who already received a high-intensity statin. This is the first case report to monitor how coronary atherosclerosis changes under pemafibrate use. We present the following case in accordance with the CARE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-401/rc).
Case presentation
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
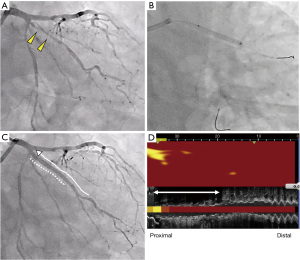
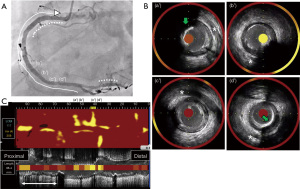
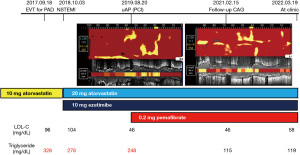
A 75-year-old man with type 2 diabetes mellitus received endovascular treatment on September 18th, 2017. Following the completion of endovascular treatment, coronary angiography was concomitantly conducted and it showed multiple moderate stenotic lesions. Given the absence of significant myocardial ischemia on myocardial perfusion scintigraphy, coronary revascularization was not conducted. He has already received 10 mg atorvastatin since February 5th, 2016. On-treatment low-density lipoprotein cholesterol (LDL-C) and fasting triglyceride levels were 96 and 328 mg/dL, respectively (Figure 1). HbA1c was 6.9% under several glucose lowering agents. One year later (October 3rd, 2018), he presented non-ST-elevation myocardial infarction (NSTEMI) (troponin T level =0.016 ng/mL). Two severe stenosis in his right coronary artery were treated by two bioresorbable polymer sirolimus-eluting stents (Ultimaster®, Terumo, Tokyo, Japan, 4.0 mm × 28 mm, 2.25 mm × 21 mm). Moderate stenosis in his left circumflex artery was medically treated with 2.5 mg bisoprolol, 2.5 mg enalapril, 100 mg aspirin and 3.75 mg prasugrel. Due to poorly controlled LDL-C level (104 mg/dL), dose escalation of atorvastatin to 20 mg with the commencement of 10 mg ezetimibe was conducted, which lowered LDL-C to 50 mg/dL at two months after PCI (December 8th, 2018), but hypertriglyceridemia (fasting triglyceride =278 mg/dL) still existed (Figure 1). One year later after primary PCI (August 20th, 2019), he was hospitalized again due to unstable angina pectoris. Due to typical anginal chest symptom, invasive coronary angiography was performed. There was a mild atherosclerotic disease in the distal segment of his right coronary artery (Video 1), whereas there was a progressed lesion in his left circumflex artery, which was considered as target lesion requiring PCI (Figure 2). One drug-eluting stent (Xience®, 2.25 mm × 23 mm, Abbott Vascular, Santa Clara, USA) was successfully implanted with the use of near-infrared spectroscopy and intravascular imaging (NIRS/IVUS, DualPro®, Nipro Tokyo, Japan) (Figure 2). NIRS/IVUS imaging after PCI showed very small yellow signal at untreated segment of his obtuse marginal branch [Figure 2, maximum 4-mm lipid-core burden index (MaxLCBI4mm) =29]. NIRS/IVUS imaging of right coronary artery elucidated ultrasonic signal attenuation at the mild atherosclerotic disease in the distal segment of his right coronary artery (Figure 3 and Video 2). Furthermore, an extensive lipidic yellow signal was visualized at the corresponding segment, reflected by MaxLCBI4mm at 482 (Figure 3). Since he still continued to exhibit hypertriglyceridemia (fasting triglyceride =248 mg/dL) with very low LDL-C level (46 mg/dL), we added pemafibrate 0.2 mg.
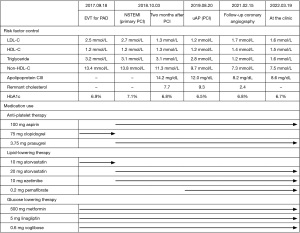
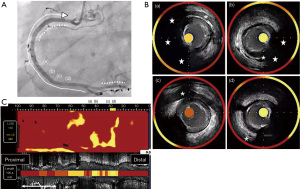
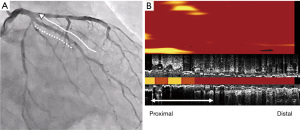
Eighteen months later after his last PCI (February 15th, 2021), evaluation of his coronary arteries was conducted again due to chest pain at rest. Given that computed tomography is not capable to clearly evaluate stented segments due to its smaller size of stent diameter, invasive coronary angiography was undertaken. In-stent restenosis and progression of coronary stenosis were not observed (Figures 4,5, and Video 3). Further evaluation of obtuse marginal branch and right coronary artery was conducted by NIRS/IVUS imaging. Newly formation of lipidic plaque was not observed in his obtuse marginal branch (Figure 4, MaxLCBI4mm =20). With regard to his right coronary artery (Video 4), a reduction of attenuated ultrasonic signals was observed, accompanied by plaque calcification. In addition, the amount of yellow signal was lowered, and its MaxLCBI4mm was 358 (Figure 5). Serial changes in percent atheroma volume and minimum lumen area at the non-culprit middle and distal segments of RCA (analyzed longitudinal length of matched segment =56 mm) were shown in Table S1. This case does not experience any cardiovascular events since August 21st, 2019. His LDL-C and triglyceride-rich lipoprotein levels are favourably controlled (Figure 1 and Figure 6).
Discussion
Several genetic studies have shown that triglyceride-rich lipoproteins and remnant cholesterol causally associate with ASCVD (1), which indicates these lipids as additional therapeutic targets to further reduce a risk of ASCVD under statin use. Pemafibrate is a potent selective peroxisome proliferator-activated receptor α modulator, which lowers triglyceride level by 30–40% with a reduction of apolipoprotein CIII, remnant cholesterol and non-HDL-C (2). In the current case, serial NIRS/IVUS imaging elucidated a reduction of MaxLCBI4mm, accompanied by lowering triglyceride-rich lipoproteins with pemafibrate use. Mechanistically, apolipoprotein CIII promotes atherosclerosis via stimulating the production of adhesion molecules and inflammatory cytokines (3). Remnant cholesterol contains a large amount of cholesterol per particle and more avidly crosses the endothelial barrier (3), which potentially causes lipid-rich plaque formation. Modulating these triglyceride-rich lipoproteins with pemafibrate may induce the delipidation of coronary atheroma. MaxLCBI4mm >400 at non-culprit segment has been reported to predict future cardiac events (4). A reduction of MaxLCBI4mm in our case suggests plaque stabilization effect of pemafibrate which ultimately results in the prevention of future coronary events.
Plaque calcification under pemafibrate use is another intriguing observation. While statin promotes plaque calcification (5), it remains unknown whether plaque calcification was driven by statin and/or pemafibrate. However, several mechanistic studies have reported an increased bone mineral density with the peroxisome proliferator activated receptor alpha agonist (6,7). The effect of pemafibrate on calcification requires further investigation.
The distance between NIRS imaging catheter and surface of plaque could affect MaxLCBI4mm. We conducted serial NIRS imaging in this case. However, NIRS imaging catheter was not necessarily positioned at the exactly same location relative to evaluated plaques. Further refinement of this imaging technique will be required for serial evaluation of MaxLCBI4mm.
The strength of this case report is that serial NIRS/IVUS imaging allows to reveal anti-atherosclerotic effects of pemafibrate in vivo. Currently, the PEMA-CORE study, a prospective randomized study using NIRS/IVUS and optical coherence tomography (OCT) is conducted to investigate the efficacy of pemafibrate on coronary atherosclerosis in 300 patients with coronary artery disease who already received a statin (jRCTs031210067). This study is expected to further elucidate the efficacy of lowering triglyceride-rich lipoproteins with pemafibrate on coronary atheroma progression and instability. The limitation of this study is that we did not conduct three-vessel NIRS/IVUS imaging in this case. It remains unknown how much anti-diabetic agents modulated coronary atherosclerosis.
Conclusions
In conclusion, a delipidation of coronary atheroma, accompanied by greater plaque calcification was observed after the commencement of pemafibrate. This finding highlights potential anti-atherosclerotic benefit of pemafibrate use in patients receiving a statin.
Acknowledgments
Funding: This work has been supported by Health, Labour and Welfare Sciences Research Grant for Research on Rare and Intractable Diseases (No. 21-FC-1009).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-401/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-401/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-401/coif). YK has received research support from Kowa, Nipro and Abbott, and honoraria from Nipro, Abbott, Kowa, Amgen, Sanofi, Astellas, Takeda and Daiichi-Sankyo. YK serves as an unpaid Editorial Board Member of Cardiovascular Diagnosis and Therapy from September 2021 to August 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nordestgaard BG. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ Res 2016;118:547-63. [Crossref] [PubMed]
- Fruchart JC. Pemafibrate (K-877), a novel selective peroxisome proliferator-activated receptor alpha modulator for management of atherogenic dyslipidaemia. Cardiovasc Diabetol 2017;16:124. [Crossref] [PubMed]
- Rosenson RS, Shaik A, Song W. New Therapies for Lowering Triglyceride-Rich Lipoproteins: JACC Focus Seminar 3/4. J Am Coll Cardiol 2021;78:1817-30. [Crossref] [PubMed]
- Waksman R, Di Mario C, Torguson R, et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet 2019;394:1629-37. [Crossref] [PubMed]
- Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 2015;65:1273-82. [Crossref] [PubMed]
- Syversen U, Stunes AK, Gustafsson BI, et al. Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC Endocr Disord 2009;9:10. [Crossref] [PubMed]
- Stunes AK, Westbroek I, Gustafsson BI, et al. The peroxisome proliferator-activated receptor (PPAR) alpha agonist fenofibrate maintains bone mass, while the PPAR gamma agonist pioglitazone exaggerates bone loss, in ovariectomized rats. BMC Endocr Disord 2011;11:11. [Crossref] [PubMed]


