
Multimodality imaging evaluation of arteriovenous fistulas and grafts: a clinical practice review
Introduction
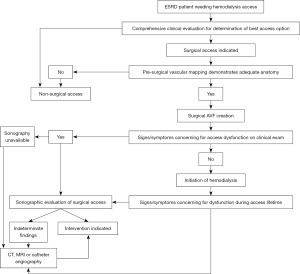
Renal replacement therapy (RRT) is required for the management of end stage renal disease (ESRD) in more than 2 million patients worldwide (1,2). Vascular access sites for hemodialysis include tunneled or non-tunneled central venous catheters (CVCs), arteriovenous fistulas (AVFs) and arteriovenous grafts (AVGs). Overall, AVF is the preferred vascular access type due to lower rate of complications and increased longevity when compared to other access sites. Ultimately, the choice of the vascular access type relies on the individual clinical situation, judgement of the physician and local availability of resources and expertise. Imaging plays an important role both pre-procedurally and following access site creation for evaluation of vessel adequacy and detection of post-procedural complications. Each imaging modality has unique disadvantages that need to be considered when deciding which might be best to serve each particular patient while considering local exam availability.
In this article, we review the role of multimodality imaging in the pre- and post-procedural evaluation of patients with AVF and AVG (Figure 1), explore the individual advantages and disadvantages of each imaging modality, discuss post-procedural complications using illustrative case examples and briefly introduce novel non-invasive imaging modalities that have shown promise in this patient population. While prior imaging reviews of AVF and AVG mostly focus on a single modality, our work to our knowledge is the first aiming to comprehensively introduce the multimodality evaluation of AVF and AVG.
Vascular access types
AVFs have become the preferred hemodialysis vascular access since first described by Brescia et al. in 1966 (3). AVF creation involves the anastomosis of an artery and vein in an end-to-end, side-to-end or side-to-side fashion with the goal of eventual maturation of the venous outflow tract to allow repeated cannulation with the two large bore lines required for hemodialysis (4). Immediate hemodynamic alterations across the fistula such as increased flow, altered pressure and increased shear-stress facilitate the eventual maturation of the fistula (5). AVGs are the second preferred long-term vascular access type, involving the creation of an arteriovenous conduit by anastomosing a vein and artery to a graft, usually made from synthetic material. Less commonly used graft materials include femoral vein allografts and xenografts (6). AVFs are associated with lower risk of infection, thrombosis & steal syndrome, fewer reinterventions, and longer access lifespans than AVGs (7).
The KDOQI (Kidney Disease Outcomes Quality Initiative) guidelines recommend creation of initial AVF in the distal forearm with a snuffbox or distal radiocephalic or transposed radio-basilic fistula (8). This preserves the vascular integrity of the proximal forearm and arm for subsequent AVF creation (Figure 2). After forearm options are exhausted, a brachiobasilic or brachiocephalic fistula may be considered (9). Lower extremity access options are less commonly used, and include femoral artery-femoral vein transposition, superficial femoral artery-great saphenous vein looped transposition and posterior tibial artery-greater saphenous vein AVF as well as AVGs in the upper or midthigh (10). Less commonly, looped grafts connecting an axillary vein with the contralateral axillary artery in a “necklace” configuration have also been described in patients with exhausted conventional access site options (11).
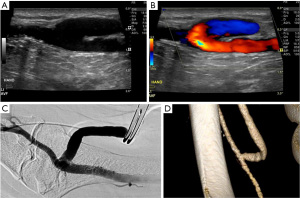
Percutaneous AVF (EndoAVF) is an innovative, minimally invasive technique with high technical success, low re-intervention rates and good usability (12). WavelinQ 4F endoAVF system (Becton, Dickinson and Company, NJ, USA) and the Ellipsys Vascular Access System (Avenu Medical, San Juan Capistrano, CA, USA) are the two percutaneous AVF systems currently available for clinical use. The WavelinQ EndoAVF device involves magnetic attraction of catheters placed in an artery and a vein (usually the ulnar/radial arteries and ulnar/radial veins) followed by creation of a fistula using radiofrequency (RF) energy under fluoroscopic guidance. The WavelinQ device is potentially more cost-effective than a surgical AVF (13). The Ellipsys Vascular Access System uses thermal resistance via a single catheter to create an arteriovenous anastomosis between the proximal radial artery and a perforating vein under sonographic guidance.
CVCs are currently used only when an AVF or AVG is not a viable option. Non-tunneled CVCs are directly inserted in the central veins and are not used longer than 2 weeks due to risk of complications. Tunneled CVCs can be used longer, with the portion of catheter tunneled under the skin prior to venous insertion serving as a potential barrier to infection (8). Long-term use of CVCs may cause thrombosis or stenosis of the accessed central vein which may preclude consideration for AVF/AVG creation in the future (14).
Imaging evaluation of AVFs and AVGs
Multiple imaging modalities can be used in the pre- and post-procedural evaluation of AVF and AVG patients, each with their advantages and disadvantages (Table 1). Ultrasound is the cornerstone imaging modality for comprehensive vascular evaluation, both prior to and following the procedure. Grayscale, color, and spectral techniques allow anatomic and hemodynamic assessment of the vasculature as well as surrounding extravascular structures. Computed tomography (CT), magnetic resonance imaging (MRI) or catheter angiography are considered when sonography is not available or diagnostic and when further characterization of sonographic abnormalities is required.
Table 1
| Imaging modality | Advantages | Disadvantages |
|---|---|---|
| Ultrasound | Non-invasive | Operator dependence |
| Portable | Inter-reader variability | |
| Hemodynamic assessment | Cannot evaluate central vasculature | |
| Guidance for endo-AVF (Ellipsys) | Small field-of-view | |
| No radiation | ||
| Catheter angiography | Ability to both diagnose and intervene in single session | Invasive |
| Creation of endo-AVF (WavelinQ) | Radiation | |
| Cannot assess extra-vascular structures | ||
| Limited evaluation of vascular wall | ||
| Operator dependence | ||
| Computed tomography | Large field of view | Artifacts (motion, mixing) |
| Evaluation of central vasculature | Radiation exposure | |
| Evaluation of extravascular structures | Iodinated contrast use in severe renal dysfunction | |
| Rapid turnaround | ||
| Multiplanar reconstruction | ||
| Not operator dependent | ||
| Magnetic resonance | Large field of view | Prolonged scan time |
| Evaluation of central vasculature | Nephrogenic systemic fibrosis risk (with Gadolinium) | |
| Evaluation of extravascular structures | Artifacts (motion) | |
| Hemodynamic assessment | ||
| Multiplanar acquisition |
AVFs, arteriovenous fistulas; AVGs, arteriovenous grafts; endo-AVF, endo-arteriovenous fistula.
Pre-procedural imaging
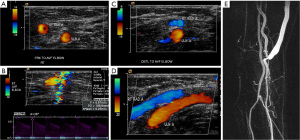
Detailed sonographic anatomic evaluation and vascular mapping is important for procedural planning (Figure 3). Pre-procedural arterial evaluation includes documentation of vessel diameter, stenosis, vessel course, wall thickness and wall alterations (e.g., atherosclerosis) (4). For the upper extremity, the subclavian, axillary, brachial, radial and ulnar arteries are evaluated. The distance from the brachial artery bifurcation to the antecubital fossa is reported. Venous evaluation includes mapping of the entire course and branching of the superficial and deep systems including the internal jugular, brachiocephalic, subclavian, axillary, and cephalic veins. Diameter measurements (measured inner-wall to inner wall) are reported for the cephalic and basilic veins at the upper/mid/lower humerus, antecubital fossa, upper/mid/lower forearm and wrist. Vessel patency and wall characteristics (e.g., thickening or post-thrombotic webbing) are documented (4). For endoAVF procedures, additional parameters evaluated include the presence of perforator vein, distance between the target artery & vein and diameter of access vessels, with minimal diameter of 2 mm required for the last two parameters (Table 2). Reactive hyperemia is measured by resistive index (RI), which is derived from spectral Doppler evaluation at ischemic state induced by forming a fist for 2 minutes followed by hand re-opening. RI is calculated as (PSV-EDV)/EDV with PSV = peak systolic velocity and EDV = end diastolic velocity. The greater the degree of reactive hyperemia, the lower the RI and the higher the ability of the vessel to dilate. The absence of reactive hyperemia (RI >0.7 on hand re-opening) has been shown to be predictive of postoperative AVF failure (15). Venous distensibility can be evaluated by measurement of vessel diameter before and at 2 minutes after placement of a torniquet. Vein distensibility is also a predictor of post-operative outcomes, with a mean dilatation of 48% in successful AVFs versus 11% in AVF failure (15). The presence and diameter of accessory veins including their distance from the anastomosis following AVF creation have also been associated with maturation ability (4).
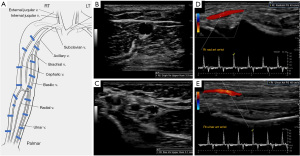
Table 2
| Technical standards | Arterial evaluation | Venous evaluation |
|---|---|---|
| Linear probe with frequency at least 7 MHz for grayscale imaging and 5 MHz for hemodynamic assessment (4) | Doppler and grayscale evaluation of the subclavian, axillary, brachial, radial, and ulnar arteries | Doppler and grayscale evaluation of the deep and superficial venous system |
| Morphological assessment including vessel diameter, wall thickness, wall alterations, vessel course, stenosis/occlusions | Tourniquet used to facilitate evaluation | |
| Hemodynamic assessment including blood flow and vasodilatory ability | Vessel patency and diameter | |
| Calcifications | Number & diameter of accessory veins | |
| >2 mm arterial diameter for AVF | >2 mm venous diameter for AVF creation without tourniquet (>2.5 mm with tourniquet) (4) | |
| Reactive hyperemia assessment | Basilic vein adequate in size for at least 4 cm | |
| EndoAVF—diameter of >2 mm (12) | ||
| Ellipsys endoAVF—perforator <1.5 mm from the radial artery (Medtronic) | ||
| Venous distensibility assessment |
AVF, arteriovenous fistula.
Post-procedural imaging
Following the procedure, the ability of AVF/AVG to sustain hemodialysis is initially assessed by clinical examination. If there are no concerning signs or symptoms, hemodialysis can be attempted. Routine surveillance imaging in the asymptomatic patient is not recommended (16). If any clinical concern arises or if the physical examination is inconclusive, further evaluation with ultrasound may be warranted. CT and MRI can play a complementary role to ultrasound in the comprehensive evaluation of intra-and extra-vascular structures.
Maturation of AVF/AVG
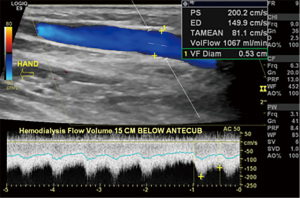
Following AVF creation, sonographic calculation of blood flow through the fistula is a valuable benchmark for assessing fistula adequacy to sustain hemodialysis (Table 3). Blood flow (in milliliters per minute) through the fistula is derived from the velocity and diameter of the blood vessel (18). Although direct measurement of flow within the venous outflow tract is ideal, the outflow tract is susceptible to probe compression which leads to distortion of vessel diameter and potentially inaccurate flow measurements, especially with an inexperienced operator. Arterial flow measurements can be used as a surrogate for fistula flow due to lower measurement errors secondary to its thicker wall, lower compressibility and longer straight segments of similar diameter (18). At our institution, flow measurements are obtained three times and averaged at a straight segment of outflow vein near the anastomosis, where arterialized laminar blood flow (waveform with definite peak systolic and end diastolic velocities) is noted (Figure 4). A sample volume at the center of the vessel that includes 50–70% of the vessel lumen and an alpha angle of ≤60 degrees is essential for accurate measurement of flow (4). The two most important parameters of a mature fistula include a long-segment of venous outflow tract (at least 5–6 cm) to allow space for adequate cannulation and a time-averaged fistula flow of at least 500 mL/min (7,17). Additional parameters of a matured AVF include fistula depth of <5–6 mm and a venous outflow tract diameter of 4 mm or more (7,18).
Table 3
| Technical standards | Anatomic and hemodynamic parameters | Helpful points for interpretation |
|---|---|---|
| Grayscale, Doppler, spectral evaluation | Fistula diameter | Significant stenosis ≥50% luminal narrowing compared to normal upstream vascular segment (artery or vein) |
| Linear probe | PSV at anastomosis | PSV ratio (anastomosis/artery 2 cm upstream) >3 may represent ≥50% stenosis |
| Frequency ≥7 MHz | PSV in feeding artery 2 cm upstream from anastomosis | PSV >375 cm/s at the anastomosis or draining vein may represent ≥50% stenosis |
| Lower frequency for central vessels | Visible narrowing or color aliasing of draining vein with velocity measurements by spectral Doppler | PSV ratio (narrowed draining vein/vein 2 cm caudally) >2:1 may represent ≥50% stenosis |
| Higher frequency (9–15 MHz) for flow rate | If vein narrowing is noted, compared PSV of vein 2 cm caudally | Parvus and tardus arterial waveforms are post-stenotic in the inflow artery |
| Gain & scale adjusted as needed | Angulation of venous origin at anastomosis on grayscale imaging (may elevate PSV if sharp) | Outflow vein diameter equal to or greater than 0.4–0.6 cm and blood flow ≥500–600 mL/min associated with high likelihood of successful use |
| Appropriate wall filter for evaluated vessel | Ipsilateral spectral Doppler evaluation of internal jugular and subclavian veins | Straight segment of outflow vein measuring at least 5–6 cm |
| Doppler angle ≤60 degrees | Comprehensive evaluation of the inflow artery | |
| Flow direction in artery distal (caudal) to anastomosis | ||
| Blood flow at midportion of a straight segment of draining vein with laminar flow typically 10 cm cranial to anastomosis | ||
| Blood flow sample volume alignment perpendicular to venous walls | ||
| Three separate blood flow measurements | ||
| Ensure future blood flow measurements in same location | ||
| Outflow vein diameter and length of straight segment | ||
| Size and distance from anastomosis of outflow vein branches (accessory veins) |
AVF, arteriovenous fistula; ACR, American College of Radiology; AIUM, American Institute of Ultrasound in Medicine; SRU, Society of Radiologists in Ultrasound; PSV, peak systolic velocity.
Non-maturation of fistula
AVF non-maturation is defined as the inability to use a fistula within 3 months of creation despite appropriate interventions (19). Non-maturation can be either due to inflow or outflow problems. Inflow problems can be due to native arterial disease, anastomotic stenosis, or juxta-anastomotic stenosis within 4 cm from the anastomotic site (Figure 5) (7,18). Outflow problems can be due to proximal venous stenosis or presence of collateral/accessory veins (Figure 6) (7). The most common cause of fistula non-maturation is proximal venous stenosis (accounting for 60% of failures), followed by anastomotic stenosis and presence of collateral veins (7). Decreased effective blood flow through the outflow vein, either by dissipation of flow with collateral veins or increased vascular resistance with steno-occlusive lesions prevents the hemodynamic stress needed in the outflow vein for maturation. Low flow volumes are predictive of thrombosis and failure for both AVFs and AVGs (4). Demographic risk factors of non-maturation include age >65 years old, white race, female sex, peripheral vascular disease and coronary artery disease (7). Common causes of AVG dysfunction are venous anastomotic stenosis and central stenosis. AVGs can also develop intragraft stenosis secondary to cumulative graft material disruption from routine use (18).
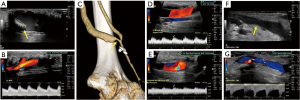
Complications of AVF/AVG
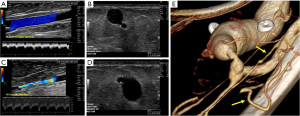
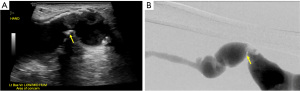
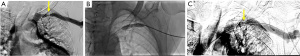
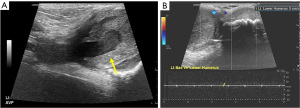
Chronic complications of AVF/AVG include aneurysm, pseudoaneurysm, thrombus, stenosis, steal phenomena or occlusion. Although the outflow vein is expected to dilate normally due to increased flow after AVF, focal dilatation of the outflow vein greater than two times the normal caliber of the adjacent fistula vein segment is considered aneurysmal (20). Pseudoaneurysm occurs at the site of vein puncture and may communicate with the outflow vein via a thin neck, demonstrating to-and-fro flow (Figures 7,8). Aneurysm and pseudoaneurysm can be treated surgically or endovascularly, although a stent would limit future needle access. Small saccular pseudoaneurysms can be treated with thrombin injection or compression (21-23). High resistance Doppler waveform or focally elevated velocity is suggestive of a hemodynamically significant stenosis (Figures 9,10). Balloon dilatation, stent implantation or surgical revision may be necessary when significant stenosis or occlusion develops (Figure 10). Central venous or outflow vein stenosis can result in venous hypertension which may present as chronic extremity swelling and skin changes related to venous stasis. Thrombus is seen in ultrasound as a non-occlusive or occlusive filling defect, typically in the outflow vessel (Figures 11,12).
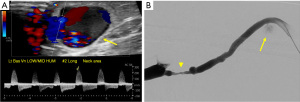
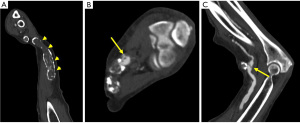
Steal syndrome can result from reduced blood flow distal to the AVF, leading to ischemia or necrosis. Arterial steal symptoms are more common with upper arm accesses with brachial artery anastomosis. In the arm, steal syndrome presents with reduced movement, coldness, color change, and pain at rest or exercise. Ultrasound shows reversed flow in the artery distal to the anastomosis (Figure 13). Although rare, symptomatic myocardial ischemia from coronary artery steal has been reported in AVF patients with an ipsilateral internal mammary artery (IMA) coronary artery bypass graft (CABG) (24). Hence, the use of the contralateral IMA is recommended in AVF patients being considered for CABG (25). If harvesting of the ipsilateral IMA is required, pre-operative hemodynamic assessment of the IMA should be performed as reversed diastolic flow through the vessel carries a higher risk of coronary steal upon access creation (26). If the patient already has an IMA bypass graft, the contralateral arm should be considered for vascular access first.
Shunting of blood through the vascular access can lead to development or worsening of heart failure due to chronically increased venous return to the right heart, especially if AVF/AVG flow is markedly elevated and/or in those with underlying cardiac conditions. One study of 113 renal transplant patients showed development of high flow rate (>2 L/min) and heart failure in 25.7% of patients, that warranted AVF closure to decrease heart failure symptoms and brain natriuretic peptide (BNP) levels (27). Volume overload can cause or worsen pulmonary hypertension in these patients. The prevalence of pulmonary hypertension increases with declining renal function and is an independent predictor for all-cause mortality and cardiovascular events (28). In a study of 215 pre-transplant ESRD patients, elevated right-ventricular systolic pressure (RVSP) increased with longer duration on dialysis. In patients not on dialysis, RVSP was elevated in 25%, whereas in those on dialysis for >2 years, RVSP was elevated in 58% (29).
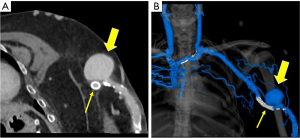
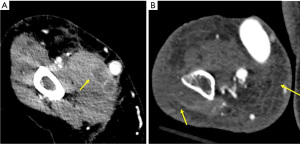
Infection and bleeding of the vascular access site are other possible complications (Figure 14). The risk of infection is inherently higher in those with advanced renal disease. James et al. demonstrated a direct relationship between renal function and risk of a bloodstream infection in chronic kidney disease patients not undergoing dialysis (30). In a meta-analysis including over 70 studies, the rate of AVF site infection was of 3.7% (31). Erythema, swelling, fluid collections or phlegmon about the vascular access in a patient with constitutional symptoms is concerning for infection. Bleeding at the access site may be due to issues with the anastomosis, uremia-associated platelet dysfunction, vessel wall compromise from chronic use or a ruptured aneurysm/pseudoaneurysm. Angiosarcoma is an exceptionally rare complication at the access site. A systematic review by Oskrochi et al. showed 22 patients with angiosarcomas at AVF/AVG, seen on an average of 10 years after creation, with most being immunosuppressed patients (32).
Invasive angiography
Digital subtraction angiography (DSA) is an invasive imaging option which has been historically used in the pre-operative setting for better evaluation of the central venous vasculature when compared to ultrasound. Although other less invasive imaging modalities such as CT and MRI are now able to evaluate the central vasculature with exquisite detail, the ability to both diagnose and treat in a single session is a unique advantage of DSA over other imaging modalities making this a valuable option in this population if indicated. In patients with ESRD, the amount of iodinated contrast used when performing DSA needs to be carefully considered as this may contribute to worsening renal function and may precipitate the need for a hemodialysis vascular access site. Alternatively, the use of CO2 as a contrast agent has been described as a viable alternative to iodinated contrast with the main advantage of preservation of renal function, a special consideration in those with already tenuous renal function. CO2 venography has been described as having a high sensitivity (97%) and specificity (85%) in the evaluation of upper extremity and central venous patency and stenosis (33). An intimate understanding of fistula hemodynamics is required by the interventionalist in order to decrease the risk of CO2 embolism to the brain in the case of arterial reflux of the contrast agent into the aorta. Given the invasive nature of DSA, its use should be limited in patients with suspected deep or superficial AVF/AVG infection due to the theoretical increased risk of septicemia (34).
CT
CT is a valuable non-invasive imaging option in AVF/AVG due to improved assessment of central vasculature and extravascular structures, which are limitations of ultrasound and DSA respectively. CT may be most valuable for further evaluation of a non-diagnostic AVF or AVG segment on DSA or if local resources and expertise with sonography are limited (35). CT can detect and characterize soft tissue infection, fluid collections and/or thrombosed aneurysms associated with the vascular access site (11). CT protocol adaptations for AVF/AVG include contrast injection in the arm opposite to that which has the AVF/AVG, neutral positioning of the arm with the AVF/AVG to prevent compression and elevation of the opposite arm above the head to decrease artifact (36). In addition to standard axial images, maximum intensity projection (MIP) and volume-rendered (VR) images are particularly useful in interpretation (11). In a study of 36 patients, Ko et al. demonstrated that 4-slice multidetector CT had a high sensitivity (98.7%), specificity (97.5%) and negative (97.2%) and positive (98.8%) predictive values in detection of stenosis, thrombosis or aneurysm compared to catheter angiography or surgery (37). Heye et al. showed similar results in a study of 36 patients on 64-slice multidetector CT, with CT sensitivity (90.2%), specificity (92.8%), negative (95.4%) and positive (85.2%) predictive values for detection of significant (≥50%) stenosis in comparison to catheter angiography (35). Low tube voltage (kV) CT techniques allow the use of lower radiation and contrast doses. Dynamic CT angiography can provide high temporal resolution hemodynamic information, at relatively lower radiation dose than older generation scanners. In a study of 35 patients, Meyer et al. showed that dynamic CT angiography provided additional information and improved diagnostic confidence that changed more management options than sonography alone (38).
MRI
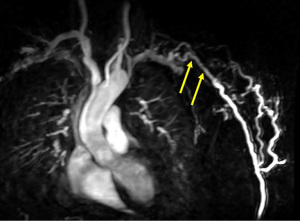
Magnetic resonance angiography (MRA) can also provide a comprehensive evaluation of the entire vasculature of interest. MRA is typically used if nondiagnostic segments are noted at DSA (39,40). Contrast enhanced MRA has excellent specificity (99%), sensitivity (97%) and positive (96%) and negative (99%) predictive values for the evaluation of significant stenoses in both AVFs and AVGs (Figure 15) (39). Despite the improved risk profile of the new-generation gadolinium contrast agents, a theorical possibility of developing nephrogenic systemic fibrosis remains in patients with ESRD. Due to this, careful consideration should be placed when deciding which MRI contrast agent to use in this patient population. Ferumoxytol (Feraheme, AMAG Pharmaceuticals, Waltham, MA, USA) is an iron-oxide nanoparticle compound that is US Food and Drug Administration (FDA)-approved for the treatment of iron deficiency anemia in adults with chronic kidney disease, that is increasingly used off-label as a non-gadolinum based MRI contrast agent. Ferumoxytol holds multiple advantages over standard gadolinium-based contrast agents including safe use in ESRD, prolonged blood pool phase allowing for simplified image acquisition, feasibility of multiple acquisitions due to persistence for 24–48 hours and elimination of risk of gadolinium accumulation (41). Ferumoxytol has a very low serious adverse event rate of 0.2% and anaphylactoid rate of 0.02% when used as an iron-replacement agent (42). In MRI, adverse reactions linked to ferumoxytol are extremely rare with Vasanawala et al. reporting a single case of anaphylactoid reaction in a group of 2,000 patients (43). Slow infusion of ferumoxytol is associated with less adverse reactions than a bolus infusion. A potential disadvantage of slow infusion is arterial & venous contamination and absence of dynamic information, which however are not concerns in hemodialysis access evaluation. 4D flow is another option available with MRI, which provides the ability to assess flow, pressure, and wall shear stress. Suqin et al. showed that 4D flow MRI velocities and flow volumes have moderate agreement with ultrasound values and specific hemodynamic parameters are associated with AVF maturation and complications (44).
Limitations
This study has multiple limitations worth noting. To our knowledge, there are no randomized controlled trials comparing the different imaging modalities in the context of AVF and AVG evaluation. This makes it difficult to discuss superiority of one modality over another. Second, the differences in cost for the individual patient and overall healthcare system between the different imaging modalities were not discussed. For this, the authors strongly recommend use of provider clinical judgement considering the individual patient, available local resources and awareness of the advantages and disadvantages of each modality when deciding how to best evaluate a particular patient.
Conclusions
Hemodialysis is a lifeline for millions of patients with ESRD. Multimodality imaging evaluation plays a key role in assessment of pre-procedural vascular adequacy, as well as post-procedural assessment of access maturation and complications. Familiarity with the full gamut of vascular imaging modalities is important in order to provide optimal patient-specific care in this growing population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sasan Partovi and Lee Kirksey) for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-439/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. PSR serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2021 to August 2023, and received royalties from Elsevier. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011;80:1258-70. [Crossref] [PubMed]
- Eggers PW. Has the incidence of end-stage renal disease in the USA and other countries stabilized? Curr Opin Nephrol Hypertens 2011;20:241-5. [Crossref] [PubMed]
- Brescia MJ, Cimino JE, Appel K, et al. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med 1966;275:1089-92. [Crossref] [PubMed]
- Zamboli P, Fiorini F, D'Amelio A, et al. Color Doppler ultrasound and arteriovenous fistulas for hemodialysis. J Ultrasound 2014;17:253-63. [Crossref] [PubMed]
- Dixon BS. Why don't fistulas mature? Kidney Int 2006;70:1413-22. [Crossref] [PubMed]
- Thomas M, Nesbitt C, Ghouri M, et al. Maintenance of Hemodialysis Vascular Access and Prevention of Access Dysfunction: A Review. Ann Vasc Surg 2017;43:318-27. [Crossref] [PubMed]
- Sheth RA, Freed R, Tavri S, et al. Nonmaturing Fistulae: Epidemiology, Possible Interventions, and Outcomes. Tech Vasc Interv Radiol 2017;20:31-7. [Crossref] [PubMed]
- Lok CE, Huber TS, Lee T, et al. 2019 Update. Am J Kidney Dis 2020;75:S1-164. Erratum in: Am J Kidney Dis 2021;77:551. [Crossref] [PubMed]
- Quencer KB, Arici M. Arteriovenous Fistulas and Their Characteristic Sites of Stenosis. AJR Am J Roentgenol 2015;205:726-34. [Crossref] [PubMed]
- Parekh VB, Niyyar VD, Vachharajani TJ. Lower Extremity Permanent Dialysis Vascular Access. Clin J Am Soc Nephrol 2016;11:1693-702. [Crossref] [PubMed]
- Ahmed S, Raman SP, Fishman EK. Three-dimensional MDCT angiography for the assessment of arteriovenous grafts and fistulas in hemodialysis access. Diagn Interv Imaging 2016;97:297-306. [Crossref] [PubMed]
- Jones RG, Morgan RA. A Review of the Current Status of Percutaneous Endovascular Arteriovenous Fistula Creation for Haemodialysis Access. Cardiovasc Intervent Radiol 2019;42:1-9. [Crossref] [PubMed]
- Rognoni C, Tozzi M, Tarricone R. Endovascular versus surgical creation of arteriovenous fistula in hemodialysis patients: Cost-effectiveness and budget impact analyses. J Vasc Access 2021;22:48-57.
- Rose DA, Sonaike E, Hughes K. Hemodialysis access. Surg Clin North Am 2013;93:997-1012. x. [Crossref] [PubMed]
- Malovrh M. Native arteriovenous fistula: preoperative evaluation. Am J Kidney Dis 2002;39:1218-25. [Crossref] [PubMed]
- Gornik HL, Gerhard-Herman MD, Misra S, et al. ACCF/ACR/AIUM/ASE/IAC/SCAI/SCVS/SIR/SVM/SVS/SVU 2013 appropriate use criteria for peripheral vascular ultrasound and physiological testing part II: testing for venous disease and evaluation of hemodialysis access: a report of the american college of cardiology foundation appropriate use criteria task force. J Am Coll Cardiol 2013;62:649-65. [Crossref] [PubMed]
- American College of Radiology. ACR–AIUM–SRU Practice Parameter for the Performance of Vascular Ultrasound for Postoperative Assessment of Hemodialysis Access. 2019. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/PostOpDialysis.pdf?la=en
- Shenoy S, Darcy M. Ultrasound as a tool for preoperative planning, monitoring, and interventions in dialysis arteriovenous access. AJR Am J Roentgenol 2013;201:W539-43. [Crossref] [PubMed]
- Beathard GA, Arnold P, Jackson J, et al. Aggressive treatment of early fistula failure. Kidney Int 2003;64:1487-94. [Crossref] [PubMed]
- Mudoni A, Cornacchiari M, Gallieni M, et al. Aneurysms and pseudoaneurysms in dialysis access. Clin Kidney J 2015;8:363-7. [Crossref] [PubMed]
- Clark TW, Abraham RJ. Thrombin injection for treatment of brachial artery pseudoaneurysm at the site of a hemodialysis fistula: report of two patients. Cardiovasc Intervent Radiol 2000;23:396-400. [Crossref] [PubMed]
- O'Neill JM, Jenkins DA, Ireland HM. Recurrence of dialysis shunt pseudoaneurysm following percutaneous thrombin embolization. Cardiovasc Intervent Radiol 2001;24:441-2. [Crossref] [PubMed]
- Witz M, Werner M, Bernheim J, et al. Ultrasound-guided compression repair of pseudoaneurysms complicating a forearm dialysis arteriovenous fistula. Nephrol Dial Transplant 2000;15:1453-4. [Crossref] [PubMed]
- Crowley SD, Butterly DW, Peter RH, et al. Coronary steal from a left internal mammary artery coronary bypass graft by a left upper extremity arteriovenous hemodialysis fistula. Am J Kidney Dis 2002;40:852-5. [Crossref] [PubMed]
- Cuthbert GA, Kirmani BH, Muir AD. Should dialysis-dependent patients with upper limb arterio-venous fistulae undergoing coronary artery bypass grafting avoid having ipsilateral in situ mammary artery grafts? Interact Cardiovasc Thorac Surg 2014;18:655-60. [Crossref] [PubMed]
- Kato H, Ikawa S, Hayashi A, et al. Internal mammary artery steal in a dialysis patient. Ann Thorac Surg 2003;75:270-1. [Crossref] [PubMed]
- Schier T, Göbel G, Bösmüller C, et al. Incidence of arteriovenous fistula closure due to high-output cardiac failure in kidney-transplanted patients. Clin Transplant 2013;27:858-65. [Crossref] [PubMed]
- Reque J, Garcia-Prieto A, Linares T, et al. Pulmonary Hypertension Is Associated with Mortality and Cardiovascular Events in Chronic Kidney Disease Patients. Am J Nephrol 2017;45:107-14. [Crossref] [PubMed]
- Issa N, Krowka MJ, Griffin MD, et al. Pulmonary hypertension is associated with reduced patient survival after kidney transplantation. Transplantation 2008;86:1384-8. [Crossref] [PubMed]
- James MT, Laupland KB, Tonelli M, et al. Risk of bloodstream infection in patients with chronic kidney disease not treated with dialysis. Arch Intern Med 2008;168:2333-9. [Crossref] [PubMed]
- Bylsma LC, Gage SM, Reichert H, et al. Arteriovenous Fistulae for Haemodialysis: A Systematic Review and Meta-analysis of Efficacy and Safety Outcomes. Eur J Vasc Endovasc Surg 2017;54:513-22. [Crossref] [PubMed]
- Oskrochi Y, Razi K, Stebbing J, et al. Angiosarcoma and Dialysis-related Arteriovenous Fistulae: A Comprehensive Review. Eur J Vasc Endovasc Surg 2016;51:127-33. [Crossref] [PubMed]
- Heye S, Maleux G, Marchal GJ. Upper-extremity venography: CO2 versus iodinated contrast material. Radiology 2006;241:291-7. [Crossref] [PubMed]
- Beathard GA. Angiography of Hemodialysis Fistulas and Grafts. Semin Dial 2017;30:326-37. [Crossref] [PubMed]
- Heye S, Maleux G, Claes K, et al. Stenosis detection in native hemodialysis fistulas with MDCT angiography. AJR Am J Roentgenol 2009;192:1079-84. [Crossref] [PubMed]
- Chen MC, Tsai WL, Tsai IC, et al. Arteriovenous fistula and graft evaluation in hemodialysis patients using MDCT: a primer. AJR Am J Roentgenol 2010;194:838-47. [Crossref] [PubMed]
- Ko SF, Huang CC, Ng SH, et al. MDCT angiography for evaluation of the complete vascular tree of hemodialysis fistulas. AJR Am J Roentgenol 2005;185:1268-74. [Crossref] [PubMed]
- Meyer M, Geiger N, Benck U, et al. Imaging of Patients with Complex Hemodialysis Arterio-Venous Fistulas using Time-Resolved Dynamic CT Angiography: Comparison with Duplex Ultrasound. Sci Rep 2017;7:12563. [Crossref] [PubMed]
- Froger CL, Duijm LE, Liem YS, et al. Stenosis detection with MR angiography and digital subtraction angiography in dysfunctional hemodialysis access fistulas and grafts. Radiology 2005;234:284-91. [Crossref] [PubMed]
- Doelman C, Duijm LE, Liem YS, et al. Stenosis detection in failing hemodialysis access fistulas and grafts: comparison of color Doppler ultrasonography, contrast-enhanced magnetic resonance angiography, and digital subtraction angiography. J Vasc Surg 2005;42:739-46. [Crossref] [PubMed]
- Toth GB, Varallyay CG, Horvath A, et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int 2017;92:47-66. [Crossref] [PubMed]
- Schiller B, Bhat P, Sharma A. Safety and effectiveness of ferumoxytol in hemodialysis patients at 3 dialysis chains in the United States over a 12-month period. Clin Ther 2014;36:70-83. [Crossref] [PubMed]
- Vasanawala SS, Nguyen KL, Hope MD, et al. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med 2016;75:2107-11. [Crossref] [PubMed]
- Suqin L, Mingli Z, Shiteng S, et al. Assessment of the Hemodynamics of Autogenous Arteriovenous Fistulas With 4D Phase Contrast-Based Flow Quantification MRI in Dialysis Patients. J Magn Reson Imaging 2020;51:1272-80. [Crossref] [PubMed]


