Alcohol septal ablation for hypertrophic obstructive cardiomyopathy in a patient with a chronic total occlusion of the right coronary artery: “beware of collateral damage”
Introduction
Alcohol septal ablation (ASA) is an effective semi-invasive alternative to surgical myectomy in selected patients for the management of severely symptomatic and drug-refractory hypertrophic obstructive cardiomyopathy (HOCM) (1). Percutaneous septal reduction with ASA has been proven to reduce left ventricular outflow tract (LVOT) gradient and alleviate symptoms with similar procedural mortality and morbidity as surgical myectomy (2). Downsides of ASA include a more frequent requirement for permanent pacemaker implantation and a significantly higher need for additional septal reduction therapy compared to myectomy (3). Although relatively straightforward in concept, the procedure can lead to serious adverse events due to the high toxicity of ethanol if delivered to a non-target area of the myocardium. To avoid such complication, it is recommended to perform selective septal angiogram and myocardial contrast echocardiography (MCE) prior to ethanol injection in order to delineate the myocardium perfused by the target vessel and identify potential bridging collaterals (4). Slow rate of ethanol injection is also recommended, subsequently preventing alcohol from traversing through the collaterals (5). Despite such preventive measures, remote myocardial infarctions have been documented during ASA, even in patients with normal coronary arteries, supposedly due to a recruitment phenomenon of the septal collaterals (6-9). In that regard, any septal artery providing collateral flow to another territory should be considered unsuitable for ASA due to the risk of creating an extensive myocardial necrosis of the collateralized myocardium. Such contraindication might theoretically be circumvented through prior percutaneous revascularization of the collateralized vessel to restore its antegrade flow and render collaterals ineffective. We report a case illustrating such strategy.
Case presentation
A 70-year-old male was referred to our institution for management of refractory HOCM with increasing dyspnea (NYHA class III) and de novo angina despite optimal medical therapy. Previous medical history included HOCM treated with verapamil 360 mg per day (beta-blockers intolerance) and prior coronary artery bypass graft 15 years earlier, with anastomosis of the left internal mammary artery (LIMA) to the left anterior descending (LAD) and saphenous vein graft to the second diagonal branch.
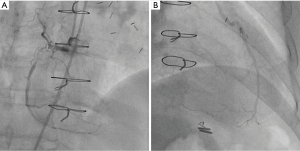
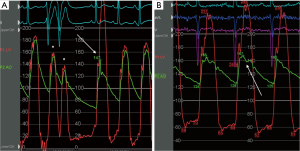
Echocardiography showed a left ventricular ejection fraction of 70%; diffuse left ventricular hypertrophy with asymmetrical septal thickening (basal interventricular septum =21 mm) and LVOT obstruction due to systolic anterior motion (SAM) of the mitral valve with a resting gradient of 35 mmHg and a provoked gradient of 85 mmHg. There was no associated congenital anomaly of the mitral apparatus (e.g., anomalous papillary muscle insertion or elongated mitral leaflets). Left coronary artery (LCA) angiography showed a 50% left main coronary artery stenosis, a 90% stenosis of mid- LAD artery; diffuse non-significant disease of the left circumflex artery (Figure 1). Right coronary artery (RCA) presented a proximal chronic total occlusion (CTO) with the presence of bridging and contralateral collaterals (Rentrop Grade 1) originating from distal septal branches visible on LIMA to LAD graft angiography (Figures 2-4). Bypasses were patent and functional. Concomitant invasive hemodynamic assessment confirmed LVOT obstruction with specific HOCM signs such as spike and dome aspect of the aortic pressure curve and Brockenbrough-Braunwald sign (Figure 5A,B). Nuclear stress test demonstrated viability and moderate ischemia of the left ventricle basal inferior wall.



Interventional options included surgical myectomy plus RCA bypass (redo) versus percutaneous treatment: CTO percutaneous coronary intervention (PCI) of RCA followed by ASA. Treatment options were discussed with the Heart Team, and due to the potential risks of a redo open chest surgery in a patient with functional bypasses, co-morbidities, and patient preference after thorough explanations of benefits and risks of both procedures, we opted for percutaneous management.
Due to the high probability of RCA collaterals originating from the first septal perforator (although only distal septal collaterals were clearly visible) and the risks of injecting ethanol in such setting, we decided to perform CTO PCI of the RCA first in order to render the collaterals ineffective and to treat the documented ischemia. The CTO PCI procedure was performed radially using antegrade approach and wire escalation strategy. The RCA occlusion was successfully crossed from lumen to lumen with a HIGH-TORQUE STANDARD (Abbott Vascular, Santa Clara, CA, USA) 0.014-inch guidewire, followed by progressive balloon pre-dilation. Eventually, a 3 mm × 28 mm everolimus-eluting stent was implanted and post-dilated to a 3.5-mm diameter with optimal angiographic result (Figure 6A). The patient was discharged the next day on dual antiplatelet therapy with clopidogrel and aspirin.
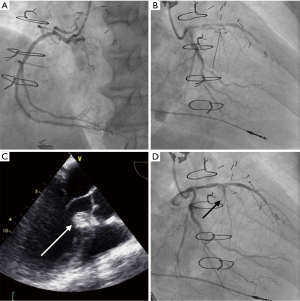
Two months after successful RCA PCI, as dyspnea persisted (NYHA III), ASA was attempted. The procedure was performed using transradial approach, after temporary transvenous pacemaker implantation, under general anaesthesia and using transesophageal echocardiography (TEE) guidance due to poor transthoracic echogenicity. Left main coronary artery was catheterized using an XB 3.5 6F-guiding catheter (Cordis, Fremont, CA, USA) and the most proximal branch of the first septal perforator was wired using a BMW guidewire (Abbott Vascular, Santa Clara, CA, USA) and isolated with a 1.5 mm × 8 mm over-the-wire (OTW) balloon (Figure 6B). We confirmed the absence of residual collateral flow to the RCA, as well as balloon positioning and sealing of the branch with selective septal angiogram using diluted (1:1) contrast medium injection through the balloon central lumen in the target septal branch. We also performed MCE to confirm proper vessel selection and absence of remote myocardial involvement by injecting Definity® echocardiography contrast agent (Lantheus Medical Imaging, Billerica, MA, USA) in the balloon central lumen (Figure 6C). Afterward, slow injection of 2 mL of desiccated ethanol was performed over 2 min through the OTW balloon in the target artery. The patient did not experience high-grade atrioventricular block during the procedure. Ten minutes after ethanol injection, the OTW balloon was deflated and final angiogram confirmed the occlusion of the target septal branch and normal flow in the other coronary arteries (Figure 6D). Echocardiography corroborated that ethanol injection was limited to the target area (Figure 7). Temporary pacemaker was removed 72 h post-procedure and the patient was discharged at day 4. At 1-year follow-up the patient is symptom free (NYHA functional class I) and echocardiographic LVOT gradient has reduced to a resting gradient of 18 mmHg and a provoked gradient of 28 mmHg.

Discussion
ASA is an effective and mini-invasive alternative to surgical myectomy for selected patients presenting symptomatic and drug-refractory HOCM (4). ASA is a relatively straightforward procedure in concept, but can be very challenging in clinical practice and cause serious complications. Those include remote myocardial necrosis in case of ethanol passage through collaterals or spillage into the LAD. Such complications have been previously reported, even in patients with no obvious collaterals originating from septal arteries (10-13) and despite the use of preventive measures such as selective septal angiogram and MCE. Therefore, patients presenting patent collaterals originating from the septal perforators to other territories should usually be considered unsuitable for ASA.
In the present case, one could argue that septal collaterals to RCA were only clearly apparent from distal septal branches and were not confirmed with selective septal angiogram of the first septal branch prior to RCA PCI, but when in doubt, caution is called for before any alcohol injection. Indeed, in the light of potential complications due to ethanol embolization and probability of concomitant recruitable collaterals originating from the first septal branch, it seemed mandatory to perform RCA PCI first in order to minimize the risk of remote myocardial infarction. Wustmann et al. demonstrated that one fifth of patients with normal coronary angiograms have immediately recruitable collaterals able to prevent ischemia during brief coronary occlusions (14). Recruitment of such dormant collaterals between septal arteries has also been observed per procedure (15), and may require balloon occlusion of the collateralized septal branch in order to avoid passage of ethanol into the collateralized vessel (16). Consequently, one must bear in mind the possibility of dynamic recruitment of such collaterals, especially in a case where collaterals are present and functional prior to ASA. In this regard, selective septal angiography and MCE are mandatory and should be carefully analysed in different views to identify the presence of collaterals, but even so remote myocardial infarctions can occur.
It must also be kept in mind that redo surgery in patients with previous sternotomy and patent bypasses may lead to serious complications in almost 10% of the patients (17). Therefore, mini-invasive percutaneous alternative is of great interest in such patients.
Even if the patient experienced an uneventful course, one has to keep in mind that such strategy should not be generalized, and patients requiring septal reduction therapy with concomitant significant coronary disease should be managed surgically. Nevertheless, such strategy can be useful when surgical risk is felt to be too high.
Conclusions
In conclusion, ASA is a useful and effective semi-invasive alternative to surgical myectomy in selected patients with symptomatic drug-refractory HOCM that should theoretically not be performed in patients presenting collateral flow originating from septal perforators to other myocardial territories, as ethanol injection in such conditions might create a non-target remote myocardial infarction.
In selected patients presenting collaterals due to coronary occlusion and contraindications to surgery, a two-step approach using percutaneous coronary revascularization followed with ASA can be effective and safe.
Acknowledgements
Dr. Picard was supported by a grant from the French Federation of Cardiology. Dr. de Hemptinne received an educational grant from Abbott Vascular.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733-79. [Crossref] [PubMed]
- Agarwal S, Tuzcu EM, Desai MY, et al. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol 2010;55:823-34. [Crossref] [PubMed]
- Liebregts M, Vriesendorp PA, Mahmoodi BK, et al. A systematic review and meta-analysis of long-term outcomes after septal reduction therapy in patients with hypertrophic cardiomyopathy. JACC Heart Fail 2015;3:896-905. [Crossref] [PubMed]
- Fifer MA, Sigwart U. Controversies in cardiovascular medicine. Hypertrophic obstructive cardiomyopathy: alcohol septal ablation. Eur Heart J 2011;32:1059-64. [Crossref] [PubMed]
- Holmes DR Jr, Valeti US, Nishimura RA. Alcohol septal ablation for hypertrophic cardiomyopathy: indications and technique. Catheter Cardiovasc Interv 2005;6:375-89. [Crossref] [PubMed]
- de Hemptinne Q, Picard F, L’Allier PL. Selective angiogram of the LCA showing left main stenosis, severe mid-LAD stenosis with competitive flow from LIMA and developed septal branches. Note that collaterals to RCA are not clearly visualized on native LCA angiogram. Asvide 2017;4:008. Available online: http://www.asvide.com/articles/1314
- de Hemptinne Q, Picard F, L’Allier PL. Selective angiogram of RCA showing a chronic total occlusion of its proximal portion with presence of bridging collaterals. Asvide 2017;4:009. Available online: http://www.asvide.com/articles/1315
- de Hemptinne Q, Picard F, L’Allier PL. Selective angiogram of the LIMA anastomosed to the LAD, showing collateralization (Rentrop grade 1) of the RCA postero-lateral branches through septal perforator arteries. Asvide 2017;4:010. Available online: http://www.asvide.com/articles/1316
- de Hemptinne Q, Picard F, L’Allier PL. TEE aspect, in mid-esophageal long axis view (140°), of the interventricular septum after alcohol injection in the target septal perforator artery. Asvide 2017;4:011. Available online: http://www.asvide.com/articles/1317
- Chikkabasavaiah NA, Puttegowda B, Panneerselvam A, et al. Remote infarction following percutaneous transluminal septal myocardial ablation: a report of two cases. Cardiovasc Interv Ther 2011;26:142-6. [Crossref] [PubMed]
- Chowdhary S, Galiwango P, Woo A, et al. Inferior infarction following alcohol septal ablation: a consequence of "collateral damage"? Catheter Cardiovasc Interv 2007;69:236-42. [Crossref] [PubMed]
- Agarwal SC, Purcell IF, Furniss SS. Apical myocardial injury caused by collateralisation of a septal artery during ethanol septal ablation. Heart 2005;91:e2. [Crossref] [PubMed]
- Parham WA, Kern MJ. Apical infarct via septal collateralization complicating transluminal alcohol septal ablation for hypertrophic cardiomyopathy. Catheter Cardiovasc Interv 2003;60:208-11. [Crossref] [PubMed]
- Wustmann K, Zbinden S, Windecker S, et al. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation 2003;107:2213-20. [Crossref] [PubMed]
- Spacek M, Zemanek D, Tomasov P, et al. Early opening of dormant septal collaterals during alcohol septal ablation: a possible hazard of remote necrosis. Can J Cardiol 2013;29:1531.e5-7.
- Rigopoulos A, Sepp R, Palinkas A, et al. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: collateral vessel communication between septal branches. Int J Cardiol 2006;113:e67-9. [Crossref] [PubMed]
- Ellman PI, Smith RL, Girotti ME, et al. Cardiac injury during resternotomy does not affect perioperative mortality. J Am Coll Surg. 2008;206:993-7. [Crossref] [PubMed]


