
Sarsasapogenin blocks ox-LDL-stimulated vascular smooth muscle cell proliferation, migration, and invasion through suppressing STIM1 expression
Highlight box
Key findings
• Sarsasapogenin (Sar) suppresses stromal interaction molecule 1 (STIM1) expression to block oxidized low-density lipoprotein (ox-LDL)-triggered vascular smooth muscle cells (VSMCs) proliferation, migration, and invasion.
What is known and what is new?
• The potential protective role of the source of Sar, Anemarrhena asphodeloide, has been revealed in AS.
• The role and regulatory mechanism of Sar in AS have remained elusive.
What is the implication, and what should change now?
• Sar has potential in protecting against VSMCs proliferation, migration, and invasion in response to ox-LDL and may be used as a new therapeutic agent in AS.
Introduction
Cardiovascular and cerebrovascular diseases remain the primary cause of death all over the world and are accompanied with severe complications (1,2). As a pathological basis of cardiovascular and cerebrovascular diseases, atherosclerosis (AS) is viewed as a chronic and progressive disease of the arterial wall (3). Emerging evidence has expounded that the prevalence of AS is escalating annually, and increasingly affecting younger people (4). At present, statins are widely applied to prevent the progression of AS and reduce cardiovascular events (5). However, the adverse effects that the long-term application of statins cause may pose another great threat to the health of AS patients (6). AS demonstrates an increased rate of vascular smooth muscle cells (VSMCs) plasticity characterized by switching from the differentiated contractile phenotype to a de-differentiated synthetic state (7). Imbalances in the phenotypic switching of VSMCs can result in a variety of cardiovascular diseases, including AS, aortic aneurysms, and vascular calcification (8). MMPs is a family of endopeptidases that value metal ions (Ca2+ and Zn2+) as cofactors that has been reported to mediate the progress of various cardiovascular diseases (9). Alternations in MMPs expression are also associated with the progression of AS (10). Thence, effective therapeutic remedies for alleviating VSMCs malignant transformation upon stimulation are in great demand.
Timosaponin AIII, a steroidal saponin, is an active ingredient of the traditional Chinese herb Anemarrhena asphodeloide (Zhi Mu), which has been reported to elicit protective activities on cancers, neuronal disorders, and inflammation (11). The pivotal role of sarsasapogenin (Sar) (Figure 1A) as a secondary metabolite from Timosaponin AIII has also been extensively covered in human diseases. For instance, Sar potentiates podocyte autophagy and modulates GSK3β signaling in diabetic nephropathy (12). Sar alleviates neurotoxicity in Alzheimer's disease (13). Lim et al. proposed that Sar inactivates NF-κB and MAPK and mediates Th17/Treg cell balance to ameliorate colitis (14). All these findings have highlighted the potential protective role of Sar. Moreover, previous literature has supported that Anemarrhena asphodeloide suppresses vascular smooth muscle cell (VSMC) growth (15). Nonetheless, whether Sar participates in the process of AS remains unclear.

Store-operated calcium channel (SOCC) has been revealed to be expressed in immune cells, VSMCs, and endothelial cells and participate in immunologic functions, vascular contraction, cell proliferation, and migration (16,17). A large number of studies have specified the pivotal role of SOCC in cardiovascular diseases (18), such as hypertension (19) and AS (20). Stromal interaction molecule 1 (STIM1) located in the endoplasmic reticulum membrane and Orai are pivotal components of canonical store-operated calcium entry (SOCE) (21). Emerging evidence has supported that STIM1 and Orai both play significant roles in vascular disorders (22,23). Emerging evidence has shown that STIM1 expression is raised in oxidized low-density lipoprotein (ox-LDL)-challenged VSMCs and accelerates the malignant alternations in VSMCs phenotypes (24-26).
This study was conducted with the aim of illuminating the role of Sar and identifying the regulatory relationship between Sar and STIM1 in AS. We present this article in accordance with the MDAR reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-111/rc).
Methods
Cell culture and treatment protocol
Dulbecco’s modified Eagle medium (DMEM; KeyGEN Biotech, Nanjing, China) with 10% fetal bovine serum (FBS; KeyGEN Biotech) was placed in an incubator at 37 ℃ with 5% CO2 to culture human VSMCs (iCell Bioscience Inc., Shanghai, China). The VSMCs of passage 4 were used in the following experiments. To construct an in vitro cell model of AS, VMSCs were treated with 50 mg/L oxidized low-density lipoprotein (ox-LDL; Biosynthesis Biotechnology Company, Beijing, China) for 24 hours. To evaluate the impacts of Sar on ox-LDL-treated VSMCs, VSMCs were pretreated by Sar (1, 2, or 4 µM; Aladdin, Shanghai, China) for 24 hours prior to incubation with 50 mg/L ox-LDL.
Cell Counting Kit-8 assay
Human VSMCs inoculated in 96-well plates overnight with 5×103 cells/well received treatment with ascending concentrations of Sar (1, 2, 4, and 8 µM) alone for 48 hours. Prior to the capture of OD450 nm value using a microplate reader (Beijing Potenov Technology Co., Ltd., Beijing, China), 10 µL Cell Counting Kit-8 (CCK-8) solution (ABclonal, Woburn, MA, USA) was supplemented for extra 2 hours at 37 ℃ strictly referring to the manual from the manufacturer.
Western blot
The proteins collected from human VSMCs were prepared in radioimmunoprecipitation assay (RIPA) buffer (Bestbio, Shanghai, China), subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and bound to polyvinylidene fluoride (PVDF) membranes which were then immersed in 5% non-fat milk. The membranes were subsequently successively hybridized with primary antibodies overnight at 4 ℃ and goat anti-rabbit HRP antibody (cat. no. ab205718; 1/2000; Abcam, Cambridge, MA, USA) for 1 hour. The blots were prepared with the aid of the enhanced chemiluminescence (ECL) reagent (Bestbio) and the gray analysis was carried out with ImageLab4.0 software (Bio-Rad, Hercules, CA, USA). Proliferating cell nuclear antigen (PCNA; cat. No. ab92552; 1/1000; Abcam), Ki67 (cat. No. ab92742; 1/5000; Abcam), matrix metallopeptidase 9 (MMP9; cat. No. ab76003; 1/1000; Abcam), matrix metallopeptidase 2 (MMP2; cat. No. ab23981; 1/1000; Abcam), STIM1 (cat. No. ab108994; 1/1000; Abcam), Orai (cat. No. ab111960; 1/1000; Abcam), and GAPDH (cat. No. ab9485; 1/2500; Abcam) primary antibodies were utilized in this study.
5-Ethynyl-2’-deoxyuridine staining
The proliferation of VSMCs was measured via the employment of kFlour555 Click-iT EdU kit (KeyGEN Biotech, Nanjing, China). Briefly, VSMCs (1×104 cells/well) were resuspended in DMEM composed of 5-Ethynyl-2’-deoxyuridine staining (EDU; 50 µM per well; Ribobio, Guangzhou, China) for 2 hours at 37 ℃ as per the manufacturer’s instructions. Subsequently, 4% paraformaldehyde and 1% Triton X-100 were successively added for immobilization for 30 minutes and permeabilization for 10 minutes, respectively, at room temperature. Cells were labeled with 0.1 µg/mL 4’,6-diamidino-2-phenylindole (DAPI) for 20 minutes at room temperature. Images were prepared for observation under a fluorescence microscope (Shanghai Batuo Instrument Co., Ltd., Shanghai, China).
Wound healing assay
A single scratch was formed across the monolayer with the aid of a 200-µL pipette tip when VSMCs inoculated in 6-well plates (4×105 cells/well) reached 70–80% confluency. The suspended cells were then rinsed with serum-free DMEM medium. A light microscope (Shanghai Batuo Instrument Co., Ltd.) was used to observe the wound area at 0 and 24 hours.
Transwell assay
Matrigel [Becton, Dickinson, and Co. (BD) Biosciences, Franklin Lakes, NJ, USA] pre-coating was performed for 30 minutes at 37 ℃. In short, the upper sides of Matrigel-coated transwell inserts (Beijing Unique Biotech Co., Ltd., Beijing, China) were loaded with VSMCs (5×104) in serum-starved medium whereas the undersides were supplied with 500 µL DMEM medium decorated by 10% FBS as a chemoattractant. Then VSMCs might invade to the lower chamber with high nutritional content and the number of invaded cells might reflect the invasive ability of VSMCs. After 24 hours, non-invaded cells were discarded, and crystal violet staining of the invaded cells was conducted and observed under a fluorescence microscope (Shanghai Batuo Instrument Co., Ltd.).
Reverse transcription-quantitative polymerase chain reaction
Following the preparation of total RNA from human VSMCs using the Trizol reagent (Genenode, Beijing, China), complementary DNA (cDNA) was generated as per the user guide of First Strand cDNA Synthesis Kit (Genenode). Following the implementation of polymerase chain reaction (PCR) analysis with the employment of the SYBR Green I (Genenode), the alternations in messenger RNA (mRNA) levels were reflected in compliance with the 2-ΔΔCt method (27), with GAPDH as the housekeeping gene. The primer sequences used were as follows: STIM1, forward, 5'-AGTCACAGTGAGAAGGCGAC-3' and reverse, 5'-CAATTCGGCAAAACTCTGCTG-3'; GAPDH, forward, 5'-AATGGGCAGCCGTTAGGAAA-3' and reverse, 5'-GCGCCCAATACGACCAAATC-3'.
Plasmid transfection
STIM1 overexpression vector (OV-STIM1) and the empty vector OV-NC were provided by EK-Bioscience (Shanghai, China). Human VSMCs were subjected to plasmid transduction adopting Lipofectamine®2000 (Hengfei Biotechnology, Shanghai, China) as per the manufacturer’s recommendation. Subsequent experiments were carried out 24 hours post-transfection.
Statistical analyses
All experiments were independently repeated in triplicate and all experimental data were biologically repeated in triplicate. The values were given as mean ± standard deviation (SD) employing GraphPad Prism 8 software (GraphPad Software, Inc., San Diego, CA, USA). The differences were deemed statistically significant at P<0.05 adopting one-way analysis of variance (ANOVA) followed by Turkey’s test.
Results
Sar concentration-dependently suppressed ox-LDL-stimulated VSMCs proliferation
To elucidate the effects of Sar on AS, VSMCs viability was appraised following treatment with ascending concentrations (1, 2, 4, or 8 µM) of Sar and the results of CCK-8 assay revealed no apparent alternations in VSMCs viability upon exposure to 1, 2, and 4 µM Sar. In particular, 8 µM Sar significantly reduced the viability of VSMCs (Figure 1B). Hence, 1, 2, and 4 µM of Sar that were nontoxic to VSMCs were chosen for the ensuing assays. Further, EdU staining also corroborated that the fortified VSMCs proliferation imposed by ox-LDL was notably impeded by Sar (Figure 2A). Also, western blot revealed that the augmented Ki67 and PCNA expression in the ox-LDL-treated VSMCs were both lessened by Sar (Figure 2B). Overall, Sar protected against ox-LDL-triggered VSMCs proliferation.

Sar prevented ox-LDL-induced VSMCs migration and invasion
Further, through transwell assay, it was noticed that ox-LDL challenging markedly potentiated the invasive abilities of VSMCs and Sar treatment concentration-dependently obstructed ox-LDL-provoked VSMCs invasion (Figure 3A). Similarly, wound healing assays presented that the exacerbated VSMCs migration in response to ox-LDL was inhibited following the administration with Sar (Figure 3B). To verify this finding, western blot was used to test the expression of metastasis-associated proteins MMP9 and MMP2 and the results showed that ox-LDL-elevated MMP9 and MMP2 expression were both diminished by Sar (Figure 3C). To sum up, Sar functioned as a suppressor in ox-LDL-evoked VSMCs migration and invasion.
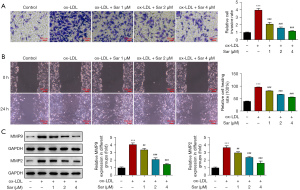
Sar declined STIM1 and Orai expression in ox-LDL-challenged VSMCs
As predicted by the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database, STIM1 might serve as a potential target of Sar. Moreover, STIM1 activates the Orai channels. Interestingly, STIM1 and Orai mRNA and protein expression were discovered to be augmented by ox-LDL in VSMCs and be depleted by Sar in ox-LDL-exposed VSMCs. Due to its prominent effect, 4 µM Sar was chosen applied to the ensuing experiments (Figure 4A,4B).

STIM1 elevation reversed the impacts of Sar on the proliferation of ox-LDL-exposed VSMCs
To validate that Sar functioned in AS via STIM1-dependent mechanism, STIM1 was overexpressed by transduction of OV-STIM1 plasmids and the transfection efficacy was verified by reverse transcription-quantitative PCR (RT-qPCR) and western blot (Figure 5A,5B). As shown in Figure 5C, Sar overtly suppressed ox-LDL-stimulated VSMCs proliferation and STIM1 up-regulation partially exacerbated VSMCs proliferation again. Additionally, the ascending Ki67 and PCNA expression in ox-LDL-treated VSMCs were diminished by Sar, which were then further elevated by STIM1 (Figure 5D). Accordingly, Sar blocked the proliferation of VSMCs exposed to ox-LDL through cutting down STIM1 expression.

STIM1 overexpression reversed the impacts of Sar on the migration and invasion of ox-LDL-exposed VSMCs
Meanwhile, ox-LDL-induced VSMCs invasion was diminished by Sar and the impacts of Sar were partially abrogated by STIM1 elevation (Figure 6A). As expected, STIM1 overexpression reversed the suppressive role of Sar in the migration of ox-LDL-challenged VSMCs (Figure 6B). This finding was accompanied with the results that the upregulated MMP9 and MMP2 expression in ox-LDL-challenged VSMCs were both reduced by Sar and further raised again after STIM1 was overexpressed (Figure 6C). Collectively, Sar obstructed ox-LDL-induced VSMCs migration and invasion via inactivating STIM1.

Discussion
AS represents an underlying driver of cardiovascular events associated with unstable plaques and poor prognosis (28). VSMCs are major constituents of the normal tissue structure of vascular walls and can maintain vascular tension (29). Under normal physiological conditions, VSMCs exist in a quiescent, non-proliferative and non-migratory state in healthy vessels (29). Phenotypic conversion of VSMCs including dedifferentiation, migration, and transdifferentiation into other cell types is responsible for structural remodeling and further leads to the occurrence of vascular remodeling diseases, such as hypertension, AS, vascular restenosis, and so on (30,31). The increased endothelium permeability to low density lipoprotein molecules, which accumulate in the intima and are oxidized by vascular cells, forming ox-LDL, is a dominant contributor to the formation of atherosclerotic plaques (32). Concurrently, ox-LDL accumulation has been identified as a regulatory factor involved in VSMCs calcification which is closely linked to multiple cardiovascular diseases (33). In addition to AS, chronic kidney disease, diabetes, hypertension and aging may be also induced (34). Therefore, this paper utilized ox-LDL to induce human VSMCs to stimulate an in vitro cell model of AS.
Anemarrhena asphodeloide, a traditional Chinese medical component, has been supported to suppress VSMCs growth (15). As a major effective component from Anemarrhena asphodeloide, Sar has been identified as a potential molecule for future drug development due to its properties in inflammatory response, cancers, diabetes, as well as neurological disorders (35). At the same time, Sar suppresses mesangial cell proliferation in diabetes (36) and Sar derivatives halt breast cancer cell MCF-7 proliferation (37). In this study, the experimental data elaborated that the ascending concentrations of Sar elicited no significant activities on human VSMCs viability. Following exposure to ox-LDL, the aberrant increase of VSMCs viability and proliferation occurred and administration with Sar remarkably impeded the viability and proliferation of ox-LDL-challenged VSMCs. In addition, the fortified expression of proliferation-associated proteins Ki67 and PCNA in ox-LDL-stimulated VSMCs were both downregulated by Sar in a concentration-dependent manner.
Dysregulation of VSMCs migration and invasion has been well documented as a pivotal event in the process of AS (38). Our investigations revealed that ox-LDL treatment markedly strengthened the migratory and invasive abilities of VSMCs, which were then further diminished by Sar. Active MMPs present in the atherosclerotic lesions may contribute to plaque destabilization by degrading ECM components (39). Atherosclerotic human vessels display increased MMPs expression as compared with healthy human vessels (40). MMP9 and MMP2 have been understood to be indispensable for VSMCs migration (41). Here, we discovered that the augmented MMP2 and MMP9 expression in ox-LDL-challenged VSMCs were both depleted by Sar.
STIM1 and Orai, the molecular basis for SOCC, has been reported to mediate calcium release-activated calcium signaling and regulate calcium homeostasis due to the initial binding between the SOAR domain of STIM1 and the C terminus of Orai docks STIM1 onto the N terminus of Orai (42). STIM1 is a multidomain protein that clusters and dimerizes in response to Ca (2+) store depletion leading to activation of Orai (43). Through the STRING database, it has been predicted that Sar might target STIM1 and STIM2. Abundant evidence has corroborated that STIM1 participates in AS via regulating the malignant transformation including proliferation and migration of VSMCs in response to ox-LDL (24,26,44,45). Also, STIM1 participates in stable peripheral coupling in contractile VSMCs which is responsible for modulating blood flow and pressure in cardiovascular diseases (46). The existing experimental results expounded that ox-LDL elevated STIM1 and Orai expression in VSMCs, which were then further abolished by Sar. Besides, after STIM1 was overexpressed, the suppressed proliferation, migration, and invasion of VSMCs challenged with ox-LDL on account of Sar treatment were all counteracted.
Conclusions
To sum up, Sar might concentration-dependently prevent ox-LDL-triggered VSMCs proliferation, migration, and invasion to protect against AS via the inhibition of STIM1 expression. This study hinted at the protective role of Sar in AS and further figured out the potential regulatory mechanism involving STIM1/Orai signaling, which introduced the potential application of Sar to the therapy for AS. Nevertheless, the impacts of Sar on AS need to be further verified in animal experiments in the future. In addition, STIM1 needs to be silenced in further experiments to assess the relative effects of Sar on ox-LDL-induced VSMCs migration/proliferation that are independent of STIM1. Orai also needs to be overexpressed to verify the involvement of STIM1/Orai signaling in the effects of Sar on the proliferation, migration, invasion of ox-LDL-treated VSMCs. As predicted by STRING, STIM2 was also a target of Sar. Hence, STIM2 also needs to be investigated in the future. Concurrently, in diabetic nephropathy, Sar has been mentioned to modulate GSK3β signaling which is an important regulator of VSMCs phenotype. Thereafter, whether Sar also protects against ox-LDL-induced VSMCs injury via GSK3β signalling also needs to be explored in the future.
Acknowledgments
Funding: The study was supported by National Health Commission Key Laboratory of Pulmonary Immune-related Diseases (No. 2019PT320003) and Project of Traditional Chinese Medicine Bureau of Guangdong Province (Nos. 20215004, 20223011).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-111/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-111/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-111/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-111/coif). All authors report that the study was supported by National Health Commission Key Laboratory of Pulmonary Immune-related Diseases (No. 2019PT320003). The authors have no other conflicts of interest to declare
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mensah GA, Roth GA, Fuster V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J Am Coll Cardiol 2019;74:2529-32. [Crossref] [PubMed]
- Andjelkovic AV, Keep RF, Wang MM. Molecular Mechanisms of Cerebrovascular Diseases. Int J Mol Sci 2022;23:7161. [Crossref] [PubMed]
- Engelen SE, Robinson AJB, Zurke YX, et al. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat Rev Cardiol 2022;19:522-42. [Crossref] [PubMed]
- Tralhão A, Sousa PJ, Ferreira AM, et al. Cardiovascular risk profile of young adults: changes over time. Rev Port Cardiol 2014;33:147-54. [PubMed]
- Wasim R, Ansari TM, Ahsan F, et al. Pleiotropic Benefits of Statins in Cardiovascular Diseases. Drug Res (Stuttg) 2022;72:477-86. [Crossref] [PubMed]
- Patel KK, Sehgal VS, Kashfi K. Molecular targets of statins and their potential side effects: Not all the glitter is gold. Eur J Pharmacol 2022;922:174906. [Crossref] [PubMed]
- Biros E, Reznik JE, Moran CS. Role of inflammatory cytokines in genesis and treatment of atherosclerosis. Trends Cardiovasc Med 2022;32:138-42. [Crossref] [PubMed]
- Tang HY, Chen AQ, Zhang H, et al. Vascular Smooth Muscle Cells Phenotypic Switching in Cardiovascular Diseases. Cells 2022;11:4060. [Crossref] [PubMed]
- Azevedo A, Prado AF, Antonio RC, et al. Matrix metalloproteinases are involved in cardiovascular diseases. Basic Clin Pharmacol Toxicol 2014;115:301-14. [Crossref] [PubMed]
- Brown BA, Williams H, George SJ. Evidence for the Involvement of Matrix-Degrading Metalloproteinases (MMPs) in Atherosclerosis. Prog Mol Biol Transl Sci 2017;147:197-237. [Crossref] [PubMed]
- Lin Y, Zhao WR, Shi WT, et al. Pharmacological Activity, Pharmacokinetics, and Toxicity of Timosaponin AIII, a Natural Product Isolated From Anemarrhena asphodeloides Bunge: A Review. Front Pharmacol 2020;11:764. [Crossref] [PubMed]
- Li XZ, Jiang H, Xu L, et al. Sarsasapogenin restores podocyte autophagy in diabetic nephropathy by targeting GSK3β signaling pathway. Biochem Pharmacol 2021;192:114675. [Crossref] [PubMed]
- Kashyap P, Muthusamy K, Niranjan M, et al. Sarsasapogenin: A steroidal saponin from Asparagus racemosus as multi target directed ligand in Alzheimer's disease. Steroids 2020;153:108529. [Crossref] [PubMed]
- Lim SM, Jeong JJ, Kang GD, et al. Timosaponin AIII and its metabolite sarsasapogenin ameliorate colitis in mice by inhibiting NF-κB and MAPK activation and restoring Th17/Treg cell balance. Int Immunopharmacol 2015;25:493-503. [Crossref] [PubMed]
- Xiao SZ, Xu ME, Ge YK, et al. Inhibitory effects of saponins from Anemarrhena asphodeloides Bunge on the growth of vascular smooth muscle cells. Biomed Environ Sci 2006;19:185-91. [PubMed]
- Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol Rev 2015;95:1383-436. [Crossref] [PubMed]
- Liu X, Pan Z. Store-Operated Calcium Entry in the Cardiovascular System. Adv Exp Med Biol 2021;1349:303-33. [Crossref] [PubMed]
- Lu T, Zhang Y, Su Y, et al. Role of store-operated Ca2+ entry in cardiovascular disease. Cell Commun Signal 2022;20:33. [Crossref] [PubMed]
- Bhullar SK, Shah AK, Dhalla NS. Store-operated calcium channels: Potential target for the therapy of hypertension. Rev Cardiovasc Med 2019;20:139-51. [Crossref] [PubMed]
- Liang SJ, Zeng DY, Mai XY, et al. Inhibition of Orai1 Store-Operated Calcium Channel Prevents Foam Cell Formation and Atherosclerosis. Arterioscler Thromb Vasc Biol 2016;36:618-28. [Crossref] [PubMed]
- Protasi F, Girolami B, Roccabianca S, et al. Store-operated calcium entry: From physiology to tubular aggregate myopathy. Curr Opin Pharmacol 2023;68:102347. [Crossref] [PubMed]
- Tanwar J, Trebak M, Motiani RK. Cardiovascular and Hemostatic Disorders: Role of STIM and Orai Proteins in Vascular Disorders. Adv Exp Med Biol 2017;993:425-52. [Crossref] [PubMed]
- Simo-Cheyou ER, Tan JJ, Grygorczyk R, et al. STIM-1 and ORAI-1 channel mediate angiotensin-II-induced expression of Egr-1 in vascular smooth muscle cells. J Cell Physiol 2017;232:3496-509. [Crossref] [PubMed]
- Lv Z, Yi D, Zhang C, et al. miR 541 3p inhibits the viability and migration of vascular smooth muscle cells via targeting STIM1. Mol Med Rep 2021;23:312. [Crossref] [PubMed]
- Fang M, Li Y, Wu Y, et al. miR-185 silencing promotes the progression of atherosclerosis via targeting stromal interaction molecule 1. Cell Cycle 2019;18:682-95. [Crossref] [PubMed]
- Huang Z, Li P, Wu L, et al. Hsa_circ_0029589 knockdown inhibits the proliferation, migration and invasion of vascular smooth muscle cells via regulating miR-214-3p and STIM1. Life Sci 2020;259:118251. [Crossref] [PubMed]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101-8. [Crossref] [PubMed]
- Wang J, Kang Z, Liu Y, et al. Identification of immune cell infiltration and diagnostic biomarkers in unstable atherosclerotic plaques by integrated bioinformatics analysis and machine learning. Front Immunol 2022;13:956078. [Crossref] [PubMed]
- Méndez-Barbero N, Gutiérrez-Muñoz C, Blanco-Colio LM. Cellular Crosstalk between Endothelial and Smooth Muscle Cells in Vascular Wall Remodeling. Int J Mol Sci 2021;22:7284. [Crossref] [PubMed]
- Cao G, Xuan X, Hu J, et al. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun Signal 2022;20:180. [Crossref] [PubMed]
- Zhang F, Guo X, Xia Y, et al. An update on the phenotypic switching of vascular smooth muscle cells in the pathogenesis of atherosclerosis. Cell Mol Life Sci 2021;79:6. [Crossref] [PubMed]
- Mundi S, Massaro M, Scoditti E, et al. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res 2018;114:35-52. [Crossref] [PubMed]
- Han R, Luo J, Wang L, et al. miR-33a-5p Suppresses ox-LDL-Stimulated Calcification of Vascular Smooth Muscle Cells by Targeting METTL3. Cardiovasc Toxicol 2021;21:737-46. [Crossref] [PubMed]
- Zhou X, Xu SN, Yuan ST, et al. Multiple functions of autophagy in vascular calcification. Cell Biosci 2021;11:159. [Crossref] [PubMed]
- Mustafa NH, Sekar M, Fuloria S, et al. Chemistry, Biosynthesis and Pharmacology of Sarsasapogenin: A Potential Natural Steroid Molecule for New Drug Design, Development and Therapy. Molecules 2022;27:2032. [Crossref] [PubMed]
- Tang ZZ, Zhang YM, Zheng T, et al. Sarsasapogenin alleviates diabetic nephropathy through suppression of chronic inflammation by down-regulating PAR-1: In vivo and in vitro study. Phytomedicine 2020;78:153314. [Crossref] [PubMed]
- Wang W, Zhang Y, Yao G, et al. Synthesis of new sarsasapogenin derivatives with antiproliferative and apoptotic effects in MCF-7 cells. Steroids 2018;131:23-31. [Crossref] [PubMed]
- Hou X, Dai H, Zheng Y. Circular RNA hsa_circ_0008896 accelerates atherosclerosis by promoting the proliferation, migration and invasion of vascular smooth muscle cells via hsa-miR-633/CDC20B (cell division cycle 20B) axis. Bioengineered 2022;13:5987-98. [Crossref] [PubMed]
- Liu R, Chen B, Chen J, et al. Leptin upregulates smooth muscle cell expression of MMP-9 to promote plaque destabilization by activating AP-1 via the leptin receptor/MAPK/ERK signaling pathways. Exp Ther Med 2018;16:5327-33. [Crossref] [PubMed]
- Lombardi M, Mantione ME, Baccellieri D, et al. P2X7 receptor antagonism modulates IL-1β and MMP9 in human atherosclerotic vessels. Sci Rep 2017;7:4872. [Crossref] [PubMed]
- Fang ZM, Zhang SM, Luo H, et al. Methyltransferase-like 3 suppresses phenotypic switching of vascular smooth muscle cells by activating autophagosome formation. Cell Prolif 2023;56:e13386. [Crossref] [PubMed]
- Nwokonko RM, Cai X, Loktionova NA, et al. The STIM-Orai Pathway: Conformational Coupling Between STIM and Orai in the Activation of Store-Operated Ca(2+) Entry. Adv Exp Med Biol 2017;993:83-98. [Crossref] [PubMed]
- Choi S, Maleth J, Jha A, et al. The TRPCs-STIM1-Orai interaction. Handb Exp Pharmacol 2014;223:1035-54. [Crossref] [PubMed]
- Ma G, Bi S, Zhang P. Long non-coding RNA MIAT regulates ox-LDL-induced cell proliferation, migration and invasion by miR-641/STIM1 axis in human vascular smooth muscle cells. BMC Cardiovasc Disord 2021;21:248. [Crossref] [PubMed]
- Mao YY, Wang JQ, Guo XX, et al. Circ-SATB2 upregulates STIM1 expression and regulates vascular smooth muscle cell proliferation and differentiation through miR-939. Biochem Biophys Res Commun 2018;505:119-25. [Crossref] [PubMed]
- Krishnan V, Ali S, Gonzales AL, et al. STIM1-dependent peripheral coupling governs the contractility of vascular smooth muscle cells. Elife 2022;11:e70278. [Crossref] [PubMed]
(English Language Editor: J. Jones)


