Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study
Introduction
Since Osler’s first description in 1885 (1), the clinical features of infective endocarditis (IE) have significantly changed (2,3). Nowadays, the population at risk is different due to several reasons: a decrease in rheumatic heart disease, an increasing percentage of elderly people (4), comorbidities, nosocomial exposures (5), prosthetic valves (6), intracardiac devices (7), intravenous drug addicted, and hemodialysis patients (8). A microbiological shift from streptococci to staphylococci as the most frequent pathogens has been reported (9,10). New prophylaxis guidelines (11,12) have been published, new antibiotics are available and, finally, bacterial resistances are increasing (13).
Most epidemiological studies on IE involve Hospital case-series of referral centers suffering from selection bias (14). Population-based investigations are more accurate but well conducted prospective studies are few (15), study designs are often inaccurate, undersized or lacking in important clinical data, criteria for definite diagnosis are not always clear and follow up data sometimes incomplete, as remarked by recent meta-analysis (16,17). These authors conclude with a call for “urgent well conducted prospective case-series population studies”. Sampling from different populations at risk, diverse diagnostic criteria, inclusion of cases with diagnosis of “possible” IE and referral bias may explain the wide range of incidence rates reported, even in the same country, from 1 to 15 cases per 100,000 per year. A wide range of global mortality is reported too, from 4% to 48% and temporal trend analyses describe an increasing incidence of IE, without a decrease in mortality rate, but these data need confirmation (18). Among population-based studies, discordant data are also present in the percentage of surgically treated patients that ranges from 12.8% to 49% (13).
Aim of our study was to evaluate the contemporary epidemiological trends, over a 17-year period in a definite region of Tuscany, Italy, and to analyze the clinical outcomes and associated prognostic factors.
Methods
Population
A prospective case-series population study was conducted among residents in the province of Grosseto, Italy. The Cardiological Department at Misericordia Hospital is the referral center for three first-level hospitals covering the entire province. From 1 January 1998 to 31 December 2014, all patients referred to our center for a suspected IE were prospectively entered in a data-base if criteria for a definite diagnosis were fulfilled (19,20). The average annual population of the province was 217,778 (99.8% caucasian, average age 45.7 years).
Data collection
- Epidemiological data included clinical risk factors: underlying heart disease, heart failure at admission, renal insufficiency, diabetes, embolic events, septic shock, chronic obstructive pulmonary disease, peripheral vasculopathy, immunocompromised state, intravenous drug abuse;
- Microbiological features: blood cultures, serology and valve/tissue cultures. Blood cultures were performed on at least three samples before initiation of antibiotics. Additional cultures were analyzed in case of valve surgery or pacemaker device/leads extraction. In case of persistent negative blood cultures, and clinical suspicion, serological tests for antibodies anti Mycoplasma species, Coxiella and Aspergillus were performed;
- Transthoracic and multiplane transesophageal echocardiographic exams were carried out as soon as IE was suspected, within 24–48 hours. An Acuson Sequoia 512 (Siemens Healthcare Ultrasound System-Erlangen Germany) was used from 1998 to 2001 and a vivid-7 ultrasound system model (GE-Healthcare-Milan, Italy) from 2002 to 2014. The largest vegetation length was measured. In absence of vegetations, echographic criteria included new valvular insufficiency, like flail or prosthesis dehiscence, abscess, pseudoaneurysm, fistula or new valvular perforation. All definitions were consistent with ESC guidelines (21). Registered parameters included ejection fraction, presence and quantitative analysis of valvular dysfunction, presence of perivalvular extension defined as the presence of an abscess, pseudoaneurysm, fistulae, periprosthetic leaks;
- The Health-Care system data-base was interrogated to capture IE patients who could have been discharged by other institutions and therefore missed by our institutional data-base. Hospital Discharge Records (HDRs) with a primary or secondary International Classification of Diseases (ICDs), 9th Revision (ICD9) diagnosis codes of IE were extracted starting from 1 January 1998. Hospital records have been revised to verify the correctness of the IE codes according to the modified Li-Duke criteria for definite IE. Cases of misclassification have been excluded;
- Uni/multivariate analysis was performed on several clinical parameters: causative microorganism, persistent bacteremia, diabetes, renal insufficiency, heart failure, embolic events, neurological complications, septic shock, age, sex, chronic obstructive pulmonary disease, peripheral vasculopathy, presence of predisposing factors, community or health-acquired pathogenesis, valve involvement, prosthesis, native or catheter related IE, indications for surgery.
- After discharge, a follow up evaluation was performed at 1 month, 6 months and 1 year, including a visit and a transthoracic echocardiography. Death certificates, physician notes, and hospital notes were reviewed in the event of a death.
Data analysis
The incidence rate per 100,000 inhabitants per year was calculated. The temporal trend was statistically examined using the Cochran-Amitage test. Presence of gender differences on incidence rate, surgery and mortality were evaluated. Hospital mortality was defined as death occurring at any time during the diagnostic hospitalization. Categorical variables were expressed as percentages and were evaluated with the χ2 test. Continuous variables were expressed as mean ± standard deviation (SD) and were assessed by independent sample Student’s t-test. Those variables with univariable significance at the 0.1 level were tested for multivariable significance by logistic regression analysis.
Actuarial survival analysis was performed using the Kaplan-Meier method, with the day of diagnosis as the starting point; in-hospital and one-year survival were estimated. Survival curves were compared using the log-rank test. Factors that affected survival at the 0.1 level were analyzed with the Cox proportional hazard model.
P values less than 0.05 were considered statistically significant.
Data were analyzed by Statview statistical analysis software (SAS Institute Inc., Cary, NC, USA), with the exception of the Cochran-Armitage test performed using SPSS statistical software (SPSS 13.0 Inc., Chicago, Illinois, USA) (XLSTAT, Addinsoft, New York, NY, USA).
Results
Epidemiology
Among 1,807 consecutive patients referred for suspected IE between January 1998 and December 2014, 167 had a definite diagnosis of IE and were entered the institutional prospective data-base. The interrogation of the Diagnosis-Related Group (DRG) data-base revealed 194 patients discharged from the Regional Hospitals with IE codes. After revision of medical records, diagnosis of definite IE was confirmed in 143. A total of 140 patients were present in both data-bases and 3 additional cases have been added. Merging the two data-bases the total population study was of 170 cases. Incidence rate of IE was 4.6/100,000/y with 2.8 cases per 100,000/y in men and 1.8 in women. In the cohort of hospitalized patients an incidence of 1.27/1,000 admissions was calculated. According to age class we found 4 cases in the group 0–19 (4.7/100,000/y), 20–39: 12 cases (2.2/100,000/y), 40–59: 27 (4.2/100,000/y), 60–79: 103 (19.6/100,000/y), and >80: 24 (18.6/100,000/y), Figure 1A. In the population over 65 years incidence rate was 11.7/100,000/y. Mean age was 65.7±16 (sd), range: 4 months–88 years. In the 17 years there was a significant ageing of the study population (P=0,033), Figure 1B, and of the general province population too, from 44.9 to 47.1 years, P<0.001. However, the increase in the mean age of the patients affected by IE did not fit the increasing mean age of the whole province population.
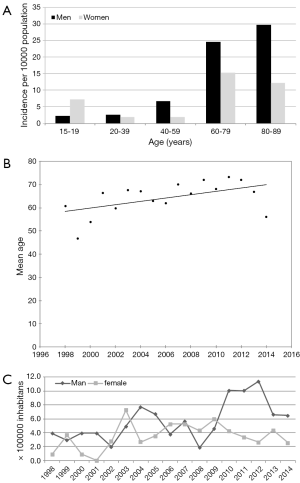
Over the 17 years there was a significant linear increase of the incidence rate that was confirmed even when native and prosthetic IE trends were separately analyzed (P=0.03 and 0.04 respectively). One hundred three patients (60.6%; 95% CI: 53–68) were males with male/female ratio of 1.54:1. No significant gender differences were present at the temporal trend analysis, Figure 1C.
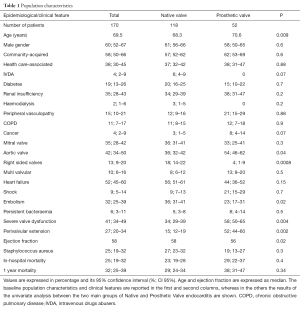
Community acquired IE were 99 (58%; 95% CI: 50–66), healthcare-associated 64 (38%, 95 CI: 30–45) and intravenous drug abuse-associated 7 (4%, 95% CI: 1.8–4.6). A major increase in the incidence of healthcare-associated IE was found, P=0.016. A univariate analysis has been performed in the two main groups of native or prosthetic valve IE as shown in Table 1. Prosthetic valve endocarditis was found in 52 cases (30.6%; 95% CI: 24–38), 22 biological and 30 mechanical, including 17 mitral, 28 aortic and multivalvular in 7. An early prosthetic IE was diagnosed in 14 cases (27%; 95% CI: 16–41). Native valve IE was present in 111 patients (65%; 95% CI: 57–72): the mitral valve was affected in 42 patients (38%, 95% CI: 29–48), the aortic in 43 (39%; 95% CI: 30–48), the tricuspid in 10 (9%; 95% CI: 5–16) and the pulmonary valve in 1 case. Multivalvular involvement was found in 18 (16%; 95% CI: 10–25). Right-sided IE was present in 23 (21%; 95% CI: 14–30) including 7 cases (6%; 95% CI: 3–13) of permanent pace maker (PM) and implantable cardioverter defibrillators leads IE. In the study period, a total number of 3,451 PM were implanted (203 per year) and 57 PM per year were substituted. The estimated incidence of IE among the new PM implantation was 2/1,000. Over the 17 years, the temporal trend analysis showed a significant increase in PM implantation and replacement.
Full table
Risk factors and underlying heart disease
An underlying heart disease was found in 114/170 (67%; 95% CI: 59–74): presence of a prosthetic valve in 51 (45%; 95% CI: 35–54, 30 mechanical and 21 biological), a degenerative valve disease in 35 (32%; 95% CI: 23–41, of native IE), a mitral valve prolapse in 20 (18%; 95% CI: 12–27 of native IE), a bicuspid aortic valve in 9 (8%; 95% CI: 4–15, of native IE), a permanent PM in 7 (6%; 95% CI: 3–13), a congenital heart disease in 5 (4.5%; 95% CI: 1.7–10.7 of NVE: 3 cases of ventricular septal defect, 1 atrioventricular septal defect, 1 congenitally corrected transposition), one case of hypertrophic cardiomyopathy and one of right ventricular arrhythmogenic cardiomyopathy. More than one risk factor was present in 16 cases. A condition of acquired immunodeficiency was present in 9 (5.3%; 95% CI: 2.6–10.1): immunosuppressive treatment in 7 and 2 cases of myelodysplastic disease. A previous history of IE was present in 3 cases and antibiotic prophylaxis was prescribed accordingly to current guidelines recommendations.
Microbiology
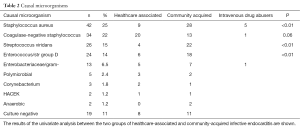
The most common infective organism, isolated in 42 patients (25%; 95% CI: 19–32), was Staphylococcus aureus, followed by coagulase-negative staphylococci in 38 patients (22%; 95% CI: 16–30), as shown in Table 2. Staphylococcus aureus was the causative microorganism in 71% of intravenous drug addicted and 47% of patients with septic shock. Both in community-acquired and healthcare-associated IE, S. aureus was the most frequent causative agent. Streptococcus viridans and enterococcal IE were mainly community-acquired, whereas enterobacteriaceae/gram negative forms were nearly equally distributed between community and nosocomial origin. Incidence of staphylococcus aureus IE did not increase in the study period, P=0.95, not confirming previous studies results (10,22,23). Culture negative IE’s were 11%, a lower prevalence than recently reported in European countries (24,25).
Full table
Echocardiographic data
A transthoracic echo was performed in all patients while a transesophageal study in 166 (98%; 95% CI: 94–99) patients. Valvular vegetations were present in 153 (90%; 95% CI: 84–94). In 17 cases of non vegetation endocarditis a new partial dehiscence of a prosthetic valve was found in 6, perivalvular abscess in 6, acute severe mitral insufficiency due to a cordal rupture (de novo flail) in 4, a newly discovered mitral valve perforation in 1. A perivalvular extension was found in 45 patients (26%; 95% CI: 20–34), a prosthesis leak in 18 (11%; 95% CI: 7‒16), an abscess in 24 (14%; 95% CI: 9‒20), a pseudoaneurysm in 10 (5.9%; 95% CI: 3‒11) and a fistula in 3; in ten cases a perivalvular leak was associated with pseudoaneurysm or abscess. Incidence rate of these perivalvular complications was 1/100,000/y.
Surgical treatment
At least 1-year follow up data were available for all patients. At 1 year follow up 79 patients (46.5%; 95% CI: 39‒54) were operated on, urgent in 48 (61%; 95% CI: 49‒71), emergency in 6 (8%; 95% CI: 3‒16) and elective surgery in 25 (32%, 95% CI: 22‒43%).
The most common indication for surgery was heart failure in 52 (66%; 95% CI: 54‒76) followed by severe native valvular dysfunction in 29, perivalvular extension in 23, prosthesis obstruction in 2. High embolic risk was present in 51. Perioperative mortality was 22.8% (95% CI: 14‒34%). 23 patients (29%; 95% CI: 20‒41) were operated on within the first 10 days after the diagnosis of IE. In permanent PM lead infections, percutaneous lead extraction was performed in 5 patients and surgical removal in one. Medical treatment alone was effective in another case.
Course and predictors of mortality
Forty-two patients died in hospital with a global in-hospital mortality of 24%: 22.8% (18/79) among surgically treated and 26.4% (24/91) in the medically treated group, P=0.5. A total number of 54 pts died within 12 months with a global 1-year mortality rate of 31.7%: 33% among the medically treated (30/91) and 30.4% among the surgically treated patients (24/79), P=0.6. In-hospital mortality was 23% (27/118) in native and 29% (15/52) in prosthetic IE, P=0.4. 1-year mortality was 29% (34/118) in native and 38.5% (20/52) in prosthetic IE, P=0.2. No differences in survival rate were present even after exclusion of PM leads IE from the population of native IE, P=0.34. Over the 17 years a trend towards an increase in mortality rate was found without reaching statistical significance, P=0.055. No gender differences were present in surgery procedures, P=0.9 or in mortality, P=0.2.
At least one complication was present in 131 (77%): heart failure 52.3%, embolism 32%, shock 8.8%, severe valvular dysfunction in 41%, locally uncontrolled infection in 26.5%, prosthesis leak in 10.6% and prosthesis obstruction in 1.8%. Healthcare-associated IE did not result a risk factor for mortality, survival rate was similar to that of community-acquired forms, P=0.5.
Logistic multivariate analysis identified the following as independent predictors of mortality: older age, S. aureus infection, heart failure, septic shock and persistent bacteremia (Table 3).

Full table
Discussion
The study revealed an IE incidence rate of 4.6/100,000/y. The temporal trend analysis showed an increase in the incidence rate and an increasing number of patients with higher age and with healthcare-associated IE; on the contrary no improvement in survival over the period was observed and no temporal trend in the causative microorganisms was found. Similar results were reported by other surveys (18). Methodology is the main peculiarity of our study: the final dataset derived from the results of 2 data-bases, the Institutional prospective collection of consecutive cases and the Health Care System discharge codes. Epidemiology of IE should derive from population-based surveys but they represent only 17% of current literature (16,17). Most studies suffer from referral biases and this explain why even in the United States the reported incidence varies from 1.7 up to 15 cases per 100,000 as reported by in a recent retrospective study. Pant found a higher incidence compared to previous literature and to our study, ranging from 11 to 15 per 100,000 population from 2000 till 2011 (22). This was a retrospective, not controlled study, in which the incidence was probably overestimated as medical records were not checked to verify the correctness of the diagnosis; moreover, it was impossible to determine whether a patient had been hospitalized more than once.
In our study, the check of the hospital records of the 194 patients found in the ICD codes data-base, revealed that in 25 (13%) criteria for definite IE had not been fulfilled and in 28 cases (14%) the same patient had been repeated due to hospital readmissions (in most cases surgical patients). Therefore, 53 (28%) false positive cases of IE were discovered by revising of the discharge sheets. On the other hand, the discharge codes data-base missed 27 cases (14%) which were not in our case series. The lack of proper validation studies for the ICD codes for IE is a limitation. Our data confirm that epidemiological studies, performed only on discharge sheet ICD codes, are not reliable. There are coding issues and different practices among doctors and hospitals; a better standardization is needed. Merging the two data-bases allowed a quality control and greater accuracy; this may explain why the incidence rate was higher than previously reported by other prospective European studies (13,23). Hoen estimated an incidence of 3,1/100.000 per year but an under-reporting may have been due to the absence of an “active search” of cases by investigators (13). In the Euroheart Survey, 159 patients were affected by IE (accounting for 3.2% of the total population). However, the study design did not allow for a precise assessment of the prevalence of the disease (24). Incidence of IE affecting cardiac implantable electronic devices is a raising problem not yet explored in prospective studies. Among the new PM implantations we found an incidence of 2/1,000. A frequency of 1,14/1.000/y was retrospectively reported by Uslan (25). The incidence of definite IE over 65 years was 11.7/100,000/y, significantly higher than in the total study population. Our data confirm that elderly forms, over 65 years, are associated with poor prognosis and higher than 1-year mortality rate even in the multivariate analysis (P=0.0127). In accordance to other experiences, we can confirm an increasing mortality trend, although with a borderline significance (18). Ageing population and healthcare-associated infections may explain the rise of IE incidence (26-28). The new guidelines raised some concerns about the appropriateness of suspending the antibiotic prophylaxis for prevention of IE (29), and an increase of IE has been reported by the AEPEI investigators (30) and recently by Dayer in the United Kingdom (31). Whether the restriction to antibiotic prophylaxis recommended by the recent guidelines, may have contributed to the temporal trend increase is difficult to ascertain by our results and larger studies are needed (12). Increasingly attention to the correctness of the DGR codes attribution over the years may also contribute to the increasing trend result.
The temporal trend analysis did not show gender differences in the incidence rate. No gender differences were present even in the surgery procedures, P=0.9 or in mortality, P=0.2. Since the 80s no significant increase in survival has been reported and nowadays the mortality rate remains high (32) in spite of recent medical and surgical advances. On the contrary, a trend towards an increased mortality has been reported and in recent years (18,32). The widespread of diagnostic modalities with subsequent greater ease of discovery, population ageing and increase invasiveness of modern medicine may justify the absence of survival improvements and our data seem to confirm this trend. A limit of the study may be that 170 cases is not a big number but, given the accurate methodology, our results may reflect the current epidemiological trends in Western countries. Few epidemiological case-series studies on the topic have been recently published, and our data are in line with a similar study performed in Italy in Veneto Region which reported an incidence of 4.9/100.000/year (18).
Conclusions
Our study confirms an increasing mortality trend in IE, although with a borderline significance. Elderly forms are associated with poor prognosis and higher than 1-year mortality rate even in the multivariate analysis. Ageing population, increase in healthcare-associated and staphylococcal infections, may explain the rise of IE incidence and of the mortality trend.
Acknowledgements
A special thank Dr. Paolo Nardini and Cinzia Mori on behalf of the Epidemiological Team of the local Sanitary Service for the DRG data collection and Dr. Alessia Andreini of the Province Statistical Service who elaborated the ISTAT (National Institute of Statistics) data.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional Ethics Committee (Azienda Sanitaria Toscana). The work was performed at Misericordia Hospital Grosseto, Italy and written informed consent was obtained from all patients.
References
- Osler W. Gulstonian lectures on malignant endocarditis. Lancet 1885;1:415-8. [Crossref]
- Prendergast BD. The changing face of infective endocarditis. Heart 2006;92:879-85. [Crossref] [PubMed]
- Hill EE, Herijgers P, Claus P, et al. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J 2007;28:196-203. [Crossref] [PubMed]
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008;168:2095-103. [Crossref] [PubMed]
- Benito N, Miró JM, de Lazzari E, et al. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann Intern Med 2009;150:586-94. [Crossref] [PubMed]
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004;350:1422-9. [Crossref] [PubMed]
- Cabell CH, Heidenreich PA, Chu VH, et al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990-1999. Am Heart J 2004;147:582-6. [Crossref] [PubMed]
- McCarthy JT, Steckelberg JM. Infective endocarditis in patients receiving long-term hemodialysis. Mayo Clin Proc 2000;75:1008-14. [Crossref] [PubMed]
- Moreillon P, Que YA. Infective endocarditis. Lancet 2004;363:139-49. [Crossref] [PubMed]
- Fowler VG Jr, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005;293:3012-21. [Crossref] [PubMed]
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Nishimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118:887-96. [Crossref] [PubMed]
- Hoen B, Alla F, Selton-Suty C, et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 2002;288:75-81. [Crossref] [PubMed]
- Kanafani ZA, Kanj SS, Cabell CH, et al. Revisiting the effect of referral bias on the clinical spectrum of infective endocarditis in adults. Eur J Clin Microbiol Infect Dis 2010;29:1203-10. [Crossref] [PubMed]
- Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463-73. [Crossref] [PubMed]
- Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest 2007;132:1025-35. [Crossref] [PubMed]
- Bin Abdulhak AA, Baddour LM, Erwin PJ, et al. Global and regional burden of infective endocarditis, 1990-2010: a systematic review of the literature. Glob Heart 2014;9:131-43. [Crossref] [PubMed]
- Fedeli U, Schievano E, Buonfrate D, et al. Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC Infect Dis 2011;11:48. [Crossref] [PubMed]
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994;96:200-9. [Crossref] [PubMed]
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633-8. [Crossref] [PubMed]
- Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 2009;30:2369-413. [Crossref] [PubMed]
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015;65:2070-6. [Crossref] [PubMed]
- Ferraris L, Milazzo L, Ricaboni D, et al. Profile of infective endocarditis observed from 2003 - 2010 in a single center in Italy. BMC Infect Dis 2013;13:545. [Crossref] [PubMed]
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005;91:571-5. [Crossref] [PubMed]
- Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007;167:669-75. [Crossref] [PubMed]
- Selton-Suty C, Hoen B, Grentzinger A, et al. Clinical and bacteriological characteristics of infective endocarditis in the elderly. Heart 1997;77:260-3. [Crossref] [PubMed]
- Sy RW, Kritharides L. Health care exposure and age in infective endocarditis: results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J 2010;31:1890-7. [Crossref] [PubMed]
- Baddour LM, Cha YM, Wilson WR. Clinical practice. Infections of cardiovascular implantable electronic devices. N Engl J Med 2012;367:842-9. [Crossref] [PubMed]
- Ashrafian H, Bogle RG. Antimicrobial prophylaxis for endocarditis: emotion or science? Heart 2007;93:5-6. [Crossref] [PubMed]
- Duval X, Delahaye F, Alla F, et al. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol 2012;59:1968-76. [Crossref] [PubMed]
- Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet 2015;385:1219-28. [Crossref] [PubMed]
- Thuny F, Giorgi R, Habachi R, et al. Excess mortality and morbidity in patients surviving infective endocarditis. Am Heart J 2012;164:94-101. [Crossref] [PubMed]


