
Transesophageal echocardiography-guided percutaneous closure of the patent foramen ovale only uses the sheath
Highlight box
Key findings
• PFO closure with a TEE-guided approach and where only the sheath is used to pass through the PFO during procedure is feasible and safe.
What is known and what is new?
• Percutaneous closure of PFO is primarily guided by fluoroscopy.
• The percutaneous closure procedure is guided only by TEE, and it is feasible to only pass the sheath through the PFO during the operation.
What is the implication, and what should change now?
• This technique is suitable for hospitals without a catheter room and reduces the risk of surgery.
Introduction
Recent studies have shown that the patent foramen ovale (PFO) is closely related to migraine and cryptogenic stroke (1-3). For patients with PFO who are not responding to systemic medical therapy and have been diagnosed by echocardiography, closure of the PFO may be a better treatment (4). During a PFO closure procedure, transesophageal echocardiography (TEE) is used for diagnosis and only in combination with fluoroscopy. Compared with plain fluoroscopy in the catheter room, the advantages of the TEE-guided closure procedure include low cost, no contrast agent effects on the kidneys, and avoidance of otherwise inevitable radiation damage to patients and operators. It has been shown that complete TEE-guided closure of atrial septal defects is a safe and effective treatment (5,6). Due to the anatomical specificity of PFO, fully TEE-guided closure remains challenging. Guidewires often cause pericardial effusions. In this present study, we aimed to assess the feasibility, safety, and efficacy of transcatheter PFO closure involving only a sheath rather than a sheath and a guidewire passing through the PFO and entering the left atrium to treat patients with migraine or cryptogenic stroke, with the entire procedure being guided by TEE without fluoroscopy. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-513/rc).
Methods
This was a single-center retrospective observational study. Patients who underwent minimally invasive PFO occluder device closure, guided by TEE but without fluoroscopy, at our hospital between December 2018 and December 2021 were selected for the analyses. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The retrospective observation study was approved by the Research Ethics Committee of Nanfang Hospital, Southern Medical University (No. NFEC-2018-046) and written informed consent was obtained from all subjects. A total of 117 patients, who were 18 years or older and without severe comorbidity, were enrolled in this study. Perioperative clinical and operative procedural data were collected and independently analyzed by a retrospective reviewer. All participants in this study are regular employees of our hospital, with relevant occupational certificates, and are fixed as designated participants.
Patient demographics
The patient age distribution was 42.3±7.8 years, and 93 of the patients were female. Before their procedures, all patients were confirmed by contrast-enhanced transcranial Doppler (c-TCD) and TEE in the Department of Neurology. A diagnosis of migraine was made by a neurologist on the basis of the International Classification of Headache Disorders III-beta, and all migraine patients underwent the Headache Impact Test-6 (HIT-6) (7). The diagnosis of cryptogenic stroke was based on each patient’s medical history and magnetic resonance imaging (MRI) by a neurologist. The indications for PFO device closure for migraine in this trial were as follows: (I) age 18 years or older, (II) HIT-6 score >55 and subsequent medical therapy failure; (III) right-to-left shunt with microbubbles of grade 2 or larger revealed by c-TCD; and (IV) PFO was detected by transthoracic echocardiography (TTE) or TEE. The exclusion criteria were as follows: (I) right-to-left shunt due to atrial septal defect; (II) migraine due to other reasons; and (III) c-TCD negativity.
The PFO shunt size was determined by counting the number of bubbles in the left atrium within three cardiac cycles post right atrial opacification with agitated saline. In this trial, the presence of more than 10 bubbles indicated a grade of 2+.
Devices and delivery system
The PFO occluder (Cardio-O-Fix, Starway Medical Technology Inc., Beijing, China) and its delivery system have been described in detail previously (8). Briefly, the occluder is self-expandable, similar to the AMPLATZERTM PFO Occluder (Abbott Laboratories, Chicago, IL, USA), and it is a double-disc device made from a nickel and titanium wire mesh. The two discs are linked together by a small and short connecting waist. The size of the occluder and delivery system was selected based on the left atrium size and the distance between the PFO and aortic valve rim. The device has radiopaque marker bands at the distal and proximal ends of the device. The device contains an end screw on the proximal end to facilitate delivery and deployment.
Surgical procedure
All procedures were carried out in a routine operating room under the guidance of TEE (Vivid E9; GE company, Horten, Norway). All procedures were performed by the same cardiac surgeon under TEE guidance in the operating room. Patients were placed under monitored anesthesia care in the supine position. Intraoperative TEE was mandatory to enable the reevaluation of the PFO, with particular attention to the anatomy, including the distances between the PFO and circumferential margins adjacent to the superior vena cava (SVC), inferior vena cava, and aortic valve rim. Then the distance between the right femoral venous puncture site and the right third intercostal parasternal space of the patient, hereinafter referred to as the ‘safe distance’, was measured and marked on the catheter and delivery sheath as described before (9).
Vascular access was obtained through the right femoral vein. Heparin at a dose of 100 IU/kg and antibiotic prophylaxis were given routinely. First, after determining the position of the right femoral vein, lidocaine was used for local anesthesia and the 5F IntroducerIITM (Terumo China Holdings, Beijing, China) was used to puncture and implant using the Seldinger’s technique. Second, a 180 cm long and 0.035 inch wide pigtail guidewire (Terumo, Somerset, NJ, USA) and a 5-Fr multipurpose angiographic (MPA) catheter (Cordis, Hialeah, FL, USA) was fed through the right atrium into the SVC atrium under TEE and monitored using a bicaval view plane. The MPA catheter was withdrawn progressively, while keeping the guidewire in the SVC. Afterward, the sheath was pushed into the middle of the right atrium through the guidewire after marking the approximate implantation depth. Next, the guidewire was withdrawn, while ensuring all air in the sheath was fully extracted.
The sheath was gently rotated, such that its tip could be identified in the esophageal ultrasound image, and then tip of the sheath was turned towards the fossa ovalis. The distance between the tip and the fossa ovalis was judged by injecting a small amount of saline into the sheath, and gently rotating to the left and right while softly pushing the sheath forward. As the delivery sheath angle was approximately 45°, it was easy to enter the left atrium through the PFO. The entire rotation and sheath pushing process was performed under esophageal ultrasound monitoring. A sudden lack of resistance indicated that the sheath had entered the left atrium through the PFO. The sheath was slowly pushed in a further 0.5 to 1 cm to ensure that its tip was in the left atrium, and saline was injected through the sheath once again. If the esophageal ultrasound suddenly showed a large number of bubbles in the left atrium, this confirmed that the tip of the sheath was located in the left atrium (Figure 1). The Cardio-O-Fix PFO occluder device was inserted into the left atrium through the sheath. After the left disc was released, the conveyor sheath was withdrawn until it was snugly adjacent to the atrial septum. The right disc was then released by rotating the delivery cable. The ‘push and pull’ test was used to ensure the occluder was securely fastened.
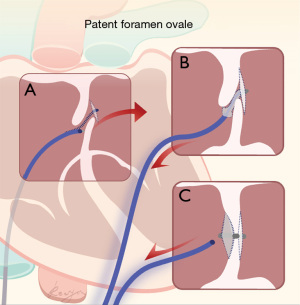
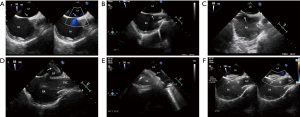
Before the occluder was fully released, esophageal ultrasound was used to verify the occluder position and ensure that there were no significant left-to-right shunts (Figure 2). The procedure was concluded by withdrawing the sheath from the femoral vein and pressing the puncture orifice for at least 20 minutes. Patients were subsequently awakened in the operating room and returned to the ward.
Follow-up
One day after the procedure, oral aspirin (3 mg/kg d) was routinely administered for 6 months. Postoperative TTE was performed in all patients before discharge, checking for device position and any residual shunt or pericardial effusion. Clinical examination was conducted in the case of palpitations, chest pain, or other symptoms, the c-TCDs were scheduled for 1, 3, and 6 months after discharge, and there were no more than grade 2 microbubbles revealed by c-TCDs during following up. HIT-6 scores were used to evaluate migraine symptomatology and compare the pre- and postprocedural headache severities of patients with migraine.
Statistical analysis
All data were analyzed with commercial software (SPSS 21.0; IBM Corp., Armonk, NY, USA). Normally distributed data are presented as mean (standard deviation) and non-normal data as median [interquartile range (IQR)]. Categorical variables are presented as frequency (percentage of the total). The pre- and postoperative HIT-6 scores were compared using the paired t-test. A P value of less than 0.05 represents the statistical significance threshold.
Results
All 117 patients underwent successful PFO occlusion closures under TEE guidance without X-ray or contrast agents (Table 1). Operative variables and intraoperative findings are summarized in Table 2. A total of 74 patients required 25/18 size occluder devices, 25 patients required 18/18 size occluder devices, and the rest required 30/30 size occluder devices. Three patients displayed nausea, transient bradycardia, sweating, or other vagus nerve reflex conditions during surgery. The symptoms disappeared after the use of atropine and metoclopramide. Transient ventricular arrhythmias presented in two patients during the operation and disappeared after adjusting the position of the sheath. There were no residual shunts or peripheral vascular injuries during the procedure. There was no fatal arrhythmia, pericardial effusion, or occlusion into the heart chambers during the perioperative period. The hospital stay after the procedure was approximately 2.15±0.56 days.
Table 1
| Variables | Data |
|---|---|
| Age (years), mean (standard deviation) | 42.3 (7.8) |
| Female gender, n (%) | 93 (79.49) |
| BSA (m2), median (IQR) | 1.7 (1.4–2.1) |
| Hypertension, n (%) | 7 (5.98) |
| Course (years), median (IQR) | 6.0 (3.0–10.0) |
| Migraine | n=89 |
| Threatened migraines, n (%) | 49 (55.06) |
| Rate (time/months), median (IQR) | 9.2 (4.0–20.0) |
| Time (hours/second), median (IQR) | 12.1 (0.5–16) |
| Cryptogenic stroke, n (%) | 28 (23.93) |
BSA, body surface area; IQR, interquartile range.
Table 2
| Parameter | Data |
|---|---|
| Operative time (minutes), median [IQR] | 32 [14–55] |
| Postoperative hospital stay (day), mean (standard deviation) | 2.15 (0.56) |
| Complications, n (%) | |
| Vagus nerve reflex | 3 (2.56) |
| Transient ventricular arrhythmia | 2 (1.71) |
IQR, interquartile range.
The initial follow-up examinations included TTE and c-TCD at 1, 3, and 6 months after the procedure. No small right-to-left shunting, occlusion device position, or cardiac structural abnormalities were revealed by TTE. All 89 patients affected by migraine reported significant relief or resolution during follow-up. The pre- and postprocedural HIT-6 scores for patients with migraine are shown in Table 3. The HIT-6 scores at 1, 3, and 6 months after closure were all significantly lower than those before the operation (P<0.05).
Table 3
| Time | HIT-6, mean (SD) | t | P |
|---|---|---|---|
| Preoperative | 62.51 (4.52) | ||
| 1 month | 56.05 (4.05) | 9.012* | 0.01 |
| 3 months | 52.07 (5.38) | 10.168* | 0.01 |
| 6 months | 46.69 (7.13) | 9.9448* | <0.001 |
*, statistically significant. HIT-6, Headache Impact Test-6; SD, standard deviation.
Discussion
Although PFO is a common malformation, because it does not cause major changes in the structure and function of the heart, patients can remain asymptomatic for a long time and often are only diagnosed during physical examinations and autopsy (10,11). Surgical intervention is still controversial, mainly due to questions about the timing and method of surgery (12,13). Current thinking is that when the patient has an abnormal embolism due to the PFO opening, the PFO opening should be closed to prevent the occurrence of another cerebral infarction. The belief that closing the PFO can improve the onset of migraine is controversial and still lacks the support of a large number of randomized double-blind clinical studies. Researchers believe that patients with migraines should first be treated by a neurologist to eliminate other organic diseases to avoid unnecessarily invasive treatments. The c-TCD is also necessary for further confirmation. Only after excluding pulmonary malformations should the surgeon consider surgically closing the PFO opening (12,13). In the present study, the preoperative HIT-6 score of patients with migraine was greater than 60, the migraine symptoms of the patients after surgery were significantly reduced or disappeared, and the HIT-6 score gradually decreased with the passage of time. PFO closure may be an effective treatment for patients with PFO-related migraine who do not respond to systemic medical treatment. However, it is still controversial whether the decision regarding surgery should be based on the bubble experiment or the closed inner diameter of the PFO (14). This study follows China’s national guidelines on the PFO (15). All patients provided informed consent before surgery and were fully informed about the risks and surgical effects.
At present, percutaneous PFO closure under digital subtraction angiography (DSA) has the advantages of being minimally invasive, and the surgical results indicate that it is the best treatment option (16). Due to the special anatomical structure of the PFO, TEE can help doctors to better ascertain the characteristics of PFO blood flow during routine percutaneous closure operations. Previous reports have reported promising results in blocking atrial septal defects and ventricular septal defects under the guidance of TTE or TEE alone, enabling patients to successfully avoid radiation exposure to the body and contrast media damage to the kidneys (17,18). Although there have been several reports of PFO closure by TEE-only guidance, reports are still relatively rare (5,19). We believe that this is mainly limited by the following factors: (I) The form of the PFO is special, with a tunnel-like structure. The diameter of the oval opening is generally small, and the difficulty of TTE exposure is significantly higher than that of atrial septal defects. (II) The guide wire and catheter currently used to block PFOs are mainly designed for visibility under radioactive imaging, and less experienced surgeons cannot clearly discern the relationship between the catheter and the position of the intracardiac structure with the assistance of TTE. This can potentially lead to surgical damage to the heart, and risk to the patient is significantly increased. (III) The range of surgical action for TEE-assisted PFO closure is usually within 1 cm, which requires a high degree of tacit understanding between the surgeon and the ultrasonic surgeon, and both parties must be familiar with each other’s operating habits to successfully complete the operation.
The method to pass the sheath through the PFO used in this study is based on, and improves upon, the traditional interventional surgical technique and the TEE-guided atrial septal defect closing technology. The operating time and the perioperative complication rate in this group were comparable with the traditional radiation-guided procedure in the catheter room. The traditional transcatheter operating procedure involves a hardened guide wire, a right coronary catheter or MPA catheter, and a sheath. Since all of the interventional devices are very tough for the sake of durability, it is very easy to damage the heart and cause pericardial effusion. In this study, the guide wire used was a highly pliable pig tail guide wire, which can be removed after the sheath reaches the right atrium. After the sheath core is removed from the sheath tube, it will appear as a dual rail track in the TTE image, which makes it easy to determine its position and shape. It is convenient to adjust the depth and angle according to ultrasonic imaging.
The hardness of the tip end of the sheath is relatively soft, and as such it will not perforate or scratch myocardial tissue. This gives the surgeon greater confidence to make as many attempts as necessary to pass through the small tunnel of the PFO. The inherent curvature of the sheath tube along with rapid injections of heparin water into the right atrium to achieve good visibility help to identify the shunt location. However, it is important to note that the right atrial pressure is increased during injections, and the flow against the atrial septum can induce severe vagus nerve reflex manifestations in some patients. Thus, it is important to prepare emergency medications, though the issue is transient even without intervention in most patients.
The occluders used for this group of patients were all made by the same manufacturer. The occlusion size is selected based on the size of the left atrium and the distance from the open edge of the oval foramen to the aortic valve (20). Due to concerns that an oversized occluder may affect the aortic valve and pulmonary vein opening in the future, smaller occluder size is given greater preference. We have found that the 18/25 mm occluder is appropriate for most PFO. It is a functional requirement that it be structurally closed, but the occluder should be no larger than is necessary. This group of patients has no residual shunt after release due to undersized occluders. Replacing the occluder with a different size is always an option for the surgeon.
The treatment after the release of the occluder in all patients was the same as that reported previously (21). All patients were given antiplatelet therapy and followed closely. All patients reported migraine relief after surgery, and it was found that there was no clear correlation between the degree of migraine relief and the diameter of the foramen ovale. There was also no correlation between the degree of migraine relief and the intensity of preoperative pain, which is similar to the results of other studies (22,23). Therefore, it remains to be studied further whether percutaneous foramen ovale occlusion is the best treatment for patients with migraine with a positive bubble test.
Limitations
This study was a retrospective observational study and did not include comparisons with other percutaneous closed PFO methods. Therefore, its superiority cannot be demonstrated, and only the feasibility of this method is described. Moreover, the number of cases included in this study was small, and there was a lack of long-term follow-up results.
Conclusions
The percutaneous transcatheter closure of PFO performed was TEE-guided only, with avoidance of fluoroscopy and contrast agents. Only a sheath was passed through the PFO during surgery to avoid intracardiac structures such as pericardial effusion. The technology employed provided a safe, feasible, and effective method for the closure of PFO.
Acknowledgments
The authors sincerely acknowledge the assistance of Reeyan Jiang, Medical illustrator, for her work in the design of the illustration.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81600321).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-513/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-513/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-513/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The retrospective observation study was approved by the Research Ethics Committee of Nanfang Hospital, Southern Medical University (No. NFEC-2018-046) and written informed consent was obtained from all subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Favilla CG, Messé SR. Patent foramen ovale and stroke: current evidence and treatment options. Curr Opin Neurol 2020;33:10-6. [Crossref] [PubMed]
- Sitwala P, Khalid MF, Khattak F, et al. Percutaneous Closure of Patent Foramen Ovale in Patients with Cryptogenic Stroke - An Updated Comprehensive Meta-Analysis. Cardiovasc Revasc Med 2019;20:687-94. [Crossref] [PubMed]
- Yan C, Li H. Preliminary Investigation of In situ Thrombus Within Patent Foramen Ovale in Patients With and Without Stroke. JAMA 2021;325:2116-8. [Crossref] [PubMed]
- Xu L, Pan X, Zhou C, et al. Long-term efficacy after closure of patent foramen ovale for ischemic neurological events in young adults: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e18675. [Crossref] [PubMed]
- Pan XB, Ou-Yang WB, Pang KJ, et al. Percutaneous Closure of Atrial Septal Defects Under Transthoracic Echocardiography Guidance Without Fluoroscopy or Intubation in Children. J Interv Cardiol 2015;28:390-5. [Crossref] [PubMed]
- Zhu P, Qiang H, Liu F, et al. Clinical evaluation of percutaneous and intra-operative device closure of atrial septal defects under transesophageal echocardiographic guidance: one center experience and mid-term follow-up. J Cardiothorac Surg 2020;15:20. [Crossref] [PubMed]
- The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629-808.
- Chen Z, Yu Z, Su L, et al. Transcatheter closure of muscular ventricular septal defects using the Cardi-O-Fix plug. Cardiol Young 2021;31:1472-5. [Crossref] [PubMed]
- Yang T, Butera G, Ou-Yang WB, et al. Percutaneous closure of patent foramen ovale under transthoracic echocardiography guidance-midterm results. J Thorac Dis 2019;11:2297-304. [Crossref] [PubMed]
- Rigatelli G, Zuin M. Patent Foramen Ovale and Risk of Perioperative Stroke. JAMA 2018;319:2557. [Crossref] [PubMed]
- Giblett JP, Abdul-Samad O, Shapiro LM, et al. Patent Foramen Ovale Closure in 2019. Interv Cardiol 2019;14:34-41. [Crossref] [PubMed]
- He YD, Yan XL, Qin C, et al. Transcatheter Patent Foramen Ovale Closure Is Effective in Alleviating Migraine in a 5-Year Follow-Up. Front Neurol 2019;10:1224. [Crossref] [PubMed]
- Hildick-Smith D, Turner M, Shaw L, et al. Evaluating the cost-effectiveness of percutaneous closure of a patent foramen ovale versus medical management in patients with a cryptogenic stroke: from the UK payer perspective. J Med Econ 2019;22:131-9. [Crossref] [PubMed]
- Kheiri B, Abdalla A, Osman M, et al. Patent foramen ovale closure versus medical therapy after cryptogenic stroke: An updated meta-analysis of all randomized clinical trials. Cardiol J 2019;26:47-55. [Crossref] [PubMed]
- Moon J, Kim M, Oh PC, et al. Residual Shunt after Patent Foramen Ovale Device Closure in Patients With Cryptogenic Stroke: Serial Bubble Contrast Transesophageal Echocardiography Data. J Stroke Cerebrovasc Dis 2019;28:347-53. [Crossref] [PubMed]
- Yang X, Wang H, Wei Y, et al. Diagnosis of Patent Foramen Ovale: The Combination of Contrast Transcranial Doppler, Contrast Transthoracic Echocardiography, and Contrast Transesophageal Echocardiography. Biomed Res Int 2020;2020:8701759. [Crossref] [PubMed]
- Suligoj NC, Rojko M, Suligoj B, et al. Long-term transesophageal echocardiography after patent foramen ovale closure by BioSTAR and Amplatzer patent foramen ovale occluders. Catheter Cardiovasc Interv 2020;95:349-54. [Crossref] [PubMed]
- Scaglione M, Ebrille E, Caponi D, et al. Zero-fluoroscopy atrial fibrillation ablation in the presence of a patent foramen ovale: a multicentre experience. J Cardiovasc Med (Hagerstown) 2020;21:292-8. [Crossref] [PubMed]
- Sauza-Sosa JC, Millan-Iturbe O. Patent Foramen Ovale "High-Risk" Anatomy: Contemporary Echocardiography Evaluation. Mayo Clin Proc 2020;95:415-6. [Crossref] [PubMed]
- Saver JL, Mattle HP, Thaler D. Patent Foramen Ovale Closure Versus Medical Therapy for Cryptogenic Ischemic Stroke: A Topical Review. Stroke 2018;49:1541-8. [Crossref] [PubMed]
- Mir H, Siemieniuk RAC, Ge L, et al. Patent foramen ovale closure, antiplatelet therapy or anticoagulation in patients with patent foramen ovale and cryptogenic stroke: a systematic review and network meta-analysis incorporating complementary external evidence. BMJ Open 2018;8:e023761. [Crossref] [PubMed]
- Michel P, Villablanca PA, Ranka S, et al. Patent Foramen Ovale and Risk of Cryptogenic Stroke - Analysis of Outcomes and Perioperative Implications. J Cardiothorac Vasc Anesth 2020;34:819-26. [Crossref] [PubMed]
- Messerli FH, Meier B. Patent Foramen Ovale Closure-When Number Needed to Treat and Number Needed to Harm Do Not Tell the Whole Story. Am J Cardiol 2018;121:1447. [Crossref] [PubMed]


