
Percutaneous closure of postoperative residual ventricular septal defects, including dehiscence of surgical patches
Highlight box
Key findings
• Multiple occluders can safely close postoperative residual ventricular septal defects (VSDs), even in infants weighing ≤5 kg, without compromising their tiny vessels using specific approaches with an acceptable early and midterm result.
What is known, and what is new?
• Residual postoperative VSDs of hemodynamic significance may carry a high risk of morbidity and mortality, especially after complex congenital cardiac surgery.
• Percutaneous closure of postoperative residual VSDs is appropriate for additional muscular defects and those related to surgical patches if catheter closure can be performed without compromising adjacent cardiac valves or the vascular accesses used for catheter closure.
What is the implication, and what should change now?
• Redo surgery for postoperative residual VSD should be reserved for patients whose catheterization closure attempts failed or whose defects are too big to be closed by cardiac catheterization.
Introduction
Residual ventricular septal defect (VSD) is a frequently observed complication after congenital cardiac surgery. Residual VSDs could be related to the surgical patches used for VSD closure or additional muscular VSDs that have been missed or were inaccessible during cardiac surgery. Residual defects with hemodynamic significance can complicate the postoperative course due to significant heart failure and low cardiac output; moreover, patients with residual VSDs, especially those related to surgical patches, have a greater risk of infective endocarditis (1-3).
After surgical closure of VSD, minor residual defects may close spontaneously over time, while larger ones should be closed surgically or percutaneously. Redo surgery for closure of residual defects carries a high risk due to the need for cardiopulmonary bypass (CPB) or the requirement of extracorporeal membrane oxygenation (ECMO). Reoperation can also result in complete heart block, infection, bleeding, phrenic palsy, emotional stress for patients and their families, and a cost associated with hospitalization. Transcatheter closure of residual VSDs may be more advantageous than surgical closure, especially if the catheter closure can be performed without compromising the adjacent cardiac valves. The benefits include avoiding CPB, faster recovery, fewer complications, and shorter hospital stays (1,4-6).
Data about the suitable type of VSD device, the risk, and the outcome of transcatheter closure of postoperative residual VSD, particularly VSD related to the surgical patches utilized for VSD closure, is variable among publications (1-4,7-10). This research aimed to assess the outcome of transcatheter closure of postoperative residual VSDs at two pediatric cardiac centers. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-624/rc).
Methods
This multicenter retrospective cohort study was conducted between March 2012 and March 2022 at the tertiary care facilities of King Faisal Specialist Hospital and King Abdulaziz University Hospital, Saudi Arabia. Every patient who underwent transcatheter closure of postoperative residual VSD was incorporated into the study (none of the patients who underwent postoperative VSD catheter closure was excluded). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of King Abdulaziz University Hospital (No. 462-22). Per the hospital policy, informed consents were obtained prospectively from the patients’ legal guardians on hospital admission regarding the potential to use their data in future research without violating patients’ privacy.
Patient preparation
Twelve leads electrocardiogram (ECG), chest X-ray, and complete transthoracic echocardiography (TTE) were done for all patients. TTE was used to evaluate the VSD size, location, and proximity to tricuspid and aortic valves. Hemodynamically significant VSDs were selected for percutaneous closure. VSD was defined as significant if causing heart failure symptoms, left heart chambers dilatation, VSD diameter in echocardiography >33–66% of the left ventricular outflow tract (LVOT) diameter or the pulmonary to systemic flow (QP/QS) ratio during catheterization ≥1.5 (11).
Patients were subdivided into two groups: Group 1 included patients with residual VSDs at the margin of the surgical patch used for VSD closure. Group 2 included patients with additional residual muscular ventricular septal defects (mVSDs). Procedures are done under general anesthesia, especially in risky patients with significant residual VSDs, infants, and critical patients, or if TEE was planned for better visualization and assessment of the defect during catheter closure. Some patients underwent procedures with conscious sedation.
Femoral access was used for all procedures. According to the institutional policy and as the catheterization is clean and done under complete aseptic conditions, no routine antibiotics were given. After vascular sheath insertion, unfractionated heparin 100 IU/kg was given. In some cases, diagnostic cardiac catheterization was done to evaluate the magnitude of the left-to-right shunt and the pulmonary flow to systemic flow (QP/QS) ratio. Angiography was performed in 4 chamber projections and a left anterior oblique (LAO) to delineate the residual VSD size, location, and proximity to the aortic valve. Based on TTE or TEE and LV angiography and after a complete evaluation of VSD position, size, and geometry, the size and the type of the VSD occluder were chosen according to the suitability and availability of the preferred occluders.
Equipment and techniques
Multiple catheters and wires were used to cross the VSD. Angled Radifocus Guide Wire 0.035/260 cm (Terumo Corporation, Leuven, LE, Belgium) was used for crossing most of the VSDs. It has the advantage of being hydrophilic with a rounded tapered atraumatic tip. The most used catheter for crossing the VSD was the 4 Fr Judkins Right (JR4) INFINITI diagnostic Catheter (Cordis Corporation, Florida, FL, USA) because of its primary and secondary curves, which give the needed bends to cross the VSD. Another catheter used frequently for crossing the VSDs was a 4 Fr Vertebral Radifocus Glidecath (Terumo Corporation, Leuven, LE, Belgium). Most of the occluders were deployed through Amplatzer TorqVue 180° Delivery System (AGA Medical Corporation, Minnesota, MN, USA) because of its flexible material and thin cable that allows tracking of all the curves.
As most of the VSDs in this cohort were related to the surgical patch, and these defects are not in the usual common anatomical areas, we predetermined the best approach for closing residual VSDs depending on many factors as follows:
- The proximity of the residual defect to the tricuspid valve, moderator band, or the right ventricle trabeculations: we used two Amplatzer duct occluders I (ADO I, Abbott Medical, MN, USA) for closing two muscular VSDs using the antegrade approach. The absence of the right disc of ADO I makes it suitable for muscular VSD if excessive trabeculations are present on the right aspect of the VSD. For the tricuspid valve proximity to the VSD, no specific distance was followed if the VSD is a perimembranous or high anterior muscular. If the VSD clearly showed an inlet extension or the VSD was an inlet type, it was not considered for device closure. In cases with an inlet VSD was closed by a surgical patch with a residual VSD related to the patch, this VSD was considered for closure if it was away from the atrioventricular valve by ≥2 mm.
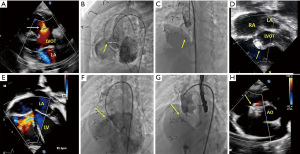
- Proximity of the VSD to the aortic valve: we preferred to use small devices like Amplatzer duct occluder II (ADO II, Abbott Medical, Minnesota, MN, USA), especially in moderate-size VSDs, as it has small discs. Figure 1A-1D and Figure 1E-1H shows successfully closed VSD related to the surgical patch post TOF repair using an MFO occluder.
- The size of the VSD: in cases of large VSD related to the surgical patch, especially after TOF repair or Rastelli operation, using Amplatzer septal occluder (ASO, Abbott Medical, Minnesota, MN, USA) was preferred. ASO has the advantage of being soft, causing less distortion to the surgical patch, and has an excellent left disc that prevents slipping in addition to adequate sealing of the VSD. Although ASO has a sizeable left disc, it is less likely to affect the aortic valve due to sufficient space created by the surgical patch and the far distance between it and the VSD in such cases. Figure 2 and
Video 2 illustrate the successful closure of residual VSD related to the surgical patch post-TOF repair using ASO. Using ADO II to close such significant defects carries a high risk of embolization as it is softer and can dislodge easily across large residual VSDs. - Type of VSD. Softer devices like ADO II and ASO are suitable for VSD related to surgical patches as they cause less distortion to the patch. For single muscular VSD, the preferred device was the Amplatzer muscular occluder (Abbott Medical, Minnesota, MN, USA). In case of multiple small muscular VSDs, we preferred Amplatzer Multi-fenestrated Septal Occluder “Cribriform” (Abbott Medical, Minnesota, MN, USA) by placing its small waist in one of the middle VSDs, and both discs are opposed to the interventricular walls closing the remaining VSDs. Figure 3A-3E and Figure 3F,3G show closure of muscular VSDs using ASD cribriform. We also used Amplatzer vascular plug IV (AVP IV, Abbott Medical, Minnesota, MN, USA) for muscular VSDs occlusion. For Gerbode VSD, being very close to the atrioventricular node, heart block is one of the common complications after device closure. We used ADO II to close one Gerbode VSD, as shown in Figure 4A-4D and
Video 5. ADO II is a low-profile occluder, softer, and associated with a low risk for heart block. - The approach used for VSD closure: we used many approaches to close residual VSDs. The retrograde approach without arteriovenous looping was preferred when two-disc occluders were chosen, e.g., ADO II and AVP IV. The anterograde approach with accessing the VSD from the right ventricle was used when ADO I, ASO, and Amplatzer muscular occluders were preferred. The antegrade approach was also used in small infants ≤5 kg to avoid injury of femoral arteries. Figure 4E-4H,
Videos 6,7 show examples of transcatheter closure of additional muscular VSDs in young infants weighing <5 kg. The retrograde approach with arteriovenous looping was performed in 2 patients. Successful VSD device closure was defined if no or only minor residual (≤3 mm) related to the VSD occluder was present.



Approaches used for VSD closure
The antegrade approach
Typically, a 4 French JR4 catheter was advanced from the femoral vein to the inferior vena cava (IVC), then to the right atrium (RA), and then one of the following procedures was utilized to cross the VSD: first technique is to stay out with the catheter in the RA and go with a Terumo 0.035 J tipped exchange wire and probe the septum posteriorly and toward the aortic valve, then cross over the wire with JR4 catheter and place it in the LV apex, then exchange the Terumo wire with a Teflon 0.035 exchange wire and put it in a curved rounded pattern in the LV apex, then proceed with placing the long sheath after complete detailed angiography and echocardiography to decide about the VSD size and device needed. If this approach does not work, a second technique could be used in which we go in the RV with JR4 in a counterclockwise pattern making the tip of the catheter face posteriorly and using the wire to cross the VSD and continuing the same way by placing the wire in the LV apex and continue the same steps as described earlier. The third technique is to place JR4 up in the right ventricle outflow tract (RVOT), then slide the catheter down with counterclockwise torquing making its tip face posteriorly; then, the catheter will fall into the VSD. Last technique, if there is a Patent foramen ovale (PFO) or atrial septal defect (ASD), the ASD is crossed through a balloon-tipped catheter through the mitral valve, then to the LV apex, crosse with a Terumo wire exchanged with a Teflon wire. The last technique is mainly used if there are multiple apical VSDs or low muscular VSD in small infants. This technique gives us the advantage of placing a balloon-tipped catheter (like Berman’s wedge 4 Fr) in the LV apex, and then the shunt will suck the catheter into the RV through the biggest VSD, allowing us to close the most important muscular apical VSD without wasting time trying to cross the small defects.
The retrograde approach
We use a 4 Fr JR4 or pigtail catheter with a Terumo 0.035 J tipped wire through the femoral artery to the descending and ascending aorta. We usually inject contrast in the ascending aorta to show if there is an aortic insufficiency (AI) or prolapse of the aortic cusp across the VSD. After that, we cross the aortic valve with the Terumo wire, place the catheter into the LV, and do LV angiography in the four chambers and straight lateral projections. After performing angiography and determining the VSD size and shape (by both angiography and echocardiography), we cross the VSD in two ways. First, with the JR4 catheter, place it in the apex, then clockwise rotation and pull it up till it falls in the VSD, then cross the VSD using the Terumo wire or 0.014 coronary wire, then we place it in the apex unless the VSD is opening in the RVOT then we push the wire up to the pulmonary artery (PA). Second, if the device needed is a smaller device and its sheath can go over the Terumo or the coronary wire, we did not cross with the JR4 catheter down to the RV apex or up to pulmonary arteries. In case we need to place a stiffer wire like a Teflon wire, in that case, we cross over the Terumo or coronary wire with JR4 down to the apex, flip the catheter to look superiorly or up to the PA, and then place the Teflon wire. By this, access has been established through the VSD, and after that, the suitable sheath is chosen according to the device size, and the sheath is put in a retrograde pattern through the femoral artery. We only recommend this technique if the sheath size is ≤7 Fr. We often use the 5 Fr ADO II delivery system in the retrograde approach. After placing the sheath, the VSD occluder is loaded in the sheath. If the sheath is in the RV apex, the RV disc is not fully opened to become rounded (grape-like) to avoid entangling any part of the tricuspid valve apparatus. Then we push up until we see it has freed from the LV apex and away from the tricuspid valve. The entire disc of the device is then produced on the right side, and the whole system is pulled back until the right disc matches the interventricular septum with a gentle pull. After that, the sheath is uncovered, allowing the LV disc to form, and then push the sheath and cable down to position the VSD device properly. After that, we usually do an injection through the sheath together with TEE or TTE to ensure the device is in good shape with no significant residual, no encroachment on TV, away from the aortic valve, and no AI than that caused by the delivery system. Finally, we release the device with keeping the sheath in the LV for doing another injection to confirm a good position, then come out and do another injection in ascending aorta to ensure no significant AI.
Arteriovenous loop approach
Both the femoral vein and artery were accessed. We go through the artery and, in the same way, inject contrast in the ascending aorta to make sure there is no AI and no aortic cusp prolapse through the VSD, then cross the aortic valve to do the LV injection. Determine the device size and type that will most likely be needed, then we cross the VSD with the catheter, as mentioned in the retrograde approach. We sometimes cross only with the wire (Terumo 0.0 35 J tipped), keeping the catheter up in the aortic arch or ascending aorta crossing with the wire as if it is the catheter, and then putting the catheter through it if we need to exchange to a Teflon wire or be comfortable using the same wire to put the delivery system sheath on it.
Once the VSD is crossed, the wire is usually placed in the left PA. Following that from the Venus access, we use a Berman wedge or JR4 catheter up to the IVC, RA, RV, and PA, then go with the Snare catheter to the PA then place the snare itself into the PA, usually open it near the mouth of the left PA. Then snare the wire. Take the wire down to the venous access with enough length to place the chosen sheath. We pulled the wire along with the catheter from the left side to keep the intracardiac wire part always covered with a catheter. We believe that leaving bare wires, especially metallic wires like Teflon, may lead to conduction abnormalities. After that, we place the sheath over the wire from the venous side, let the tip of the sheath meet, and kiss the tip of the catheter. Once that is done, we lock the wire from the venous and arterial sides on the hub of the catheter and sheath using arterial forceps. Then we gently pushed the sheath in and pulled from the arterial side but kept a straight path with no redundancy. Once the sheath is across the TV, RV VSD, we place it in the distal ascending aorta; then the wire is retrieved, keeping the catheter in the ascending aorta or the aortic arch; Afterward, we make sure that the sheath is flushed well; with no air or clots. Once the sheath is up there, the device is loaded, placed into the sheath, and pushed to the tip of the sheath. We pulled back the sheath down the descending aorta to form a rounded grape-like structure from the disc of the left side and then pulled back gently till it dropped from the aortic valve to the LVOT, where we formed the entire disc. Pull back that gently against the septum, and confirm the proper position of the device by echocardiography and angiography, ensuring the device is not encroaching on the aortic valve and there is no significant leak.
After that, we keep pulling on the disc on the LV side of the septum, then uncover the sheath to uncover the RV disc of the VSD device, then let the system take its position with no push, no pull, and let it relax. The device will position itself. If we are happy with that, do the echocardiography and angiography. Once there is no significant leak, tricuspid insufficiency, or AI, we release the device, then follow it with an LV angiogram and ascending aorta angiogram. Sometimes we do not do an angiogram if echocardiography is sufficient (12).
After the catheterization procedure, three doses of low molecular weight heparin 1 mg/kg/dose are given. The first dose was given 2 hours after the procedure, while the subsequent two doses were given 12 hours apart. Aspirin 5 mg/kg/day was started after the procedure and continued for six months. TTE and 12 leads ECG was done for all patients on the 2nd day after the procedure, one week after discharge, then at 1, 3, 6 months, then yearly. The patients’ demographic, clinical, echocardiographic, catheterization, and outcome data were collected. As the study is a retrospective cohort study and the hemodynamic evaluation during cardiac catheterization was only done for some patients, some catheterization data, besides some minor data were missing. Concerning missing data, we performed a complete-case analysis assuming that individuals with missing data could be regarded as a random subset of the whole sample and that the missing data were missing completely at random.
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA) and Jamovi software [2021] (Version 2.2) [Computer Software] jamovi. Retrieved from https://www.jamovi.org. We examined the normality of the numeric variables using the Shapiro-Wilk test. As all our numeric data were non-normally distributed, we presented them as a median and 25th–75th percentile as the interquartile range (IQR). Nominal variables were expressed as numbers or numbers and percentages. We tested the comparisons between groups using the non-parametric Mann-Whitney U test for numeric variables and Chi-square & Fisher exact tests for categorical variables. For comparison before and after the procedure, Wilcoxon signed-rank test was used for numeric variables; and we considered statistical significance if the P value was ≤0.05.
Results
Clinical characteristics
This retrospective cohort study included 33 patients. The median age of the patients was 3.3 years, and the IQR ranged between 9 months and 7 years; the minimum age was two months, while the maximum was 56 years. The median weight was 13.1 kg, with an IQR of 5.1 to 16.8 kg. We performed 59.45% of the procedures for the closure of VSDs related to the surgical patch (22/37), while 40.55% of procedures (15/37) were done to close additional mVSDs. Residual VSDs at the surgical patch margins were common (15/22) after the repair of TOF or after the Rastelli operation for patients with transposition of great arteries with ventricular septal defect and pulmonary stenosis (TGA-VSD-PS). Most procedures for closing additional residual muscular VSDs were common after surgical repair of VSD with or without aortic coarctation or aortic arch interruption (9/15). Group 1 patients with residual VSD related to the surgical patch were relatively older and had greater weight than Group 2 (P=0.02, P=0.005, respectively). There were no significant differences between patient groups regarding gender, primary surgery, or follow-up duration. Table 1 illustrates the clinical characteristics of the patient groups.
Table 1
| Parameters | All procedures (n=37) | Group 1: VSD at patch margin (n=22) | Group 2: mVSD (n=15) | P value |
|---|---|---|---|---|
| Age (months) | 40 [9–86] | 66 [13.8–134] | 14 [5–44.5] | 0.028 |
| Weight (kg) | 13.1 [5.1–16.8] | 15.7 [8.8–28] | 8.4 [4.5–13.2] | 0.005 |
| Gender | 0.191 | |||
| Male | 22 (59.5) | 15 (68.2) | 7 (46.7) | |
| Female | 15 (40.5) | 7 (31.8) | 8 (53.3) | |
| Duration between surgery and catheterization (months) | 4.6 [1.27–25] | 6 [2.16–57.9] | 2.37 [1.25–7] | 0.164 |
| Follow-up duration (months) | 22.3 [6.6–59.2] | 22 [2–31.1] | 26.5 [8.5–80] | 0.368 |
| Primary surgery | 0.057 | |||
| VSD closure | 6 (16.2) | 1 (4.5) | 5 (33.3) | |
| TOF repair | 9 (24.3) | 8 (36.4) | 1 (6.7) | |
| TOF/PA repair | 3 (8.1) | 3 (13.6) | 0 | |
| Rastelli | 4 (10.8) | 2 (9.1) | 2 (13.3) | |
| Truncus repair | 2 (5.4) | 0 | 2 (13.3) | |
| VSD-COA repair | 7 (18.9) | 3 (13.6) | 4 (26.7) | |
| Hemi mustard, RV-PA conduit | 1 (2.7) | 1 (4.5) | 0 | |
| AVSD repair | 2 (5.4) | 1 (4.5) | 1 (6.7) | |
| AVSD-TOF repair | 2 (5.4) | 2 (9.1) | 0 | |
| Yasui | 1 (2.7) | 1 (4.5) | 0 | |
| Heart block before catheterization | 2 (5.4) | 1 (4.5) | 1 (6.7) | 0.667 |
Data are shown as median [25th–75th percentile] or n (%). AVSD, atrioventricular septal defect; COA, coarctation of the aorta; mVSD, muscular ventricular septal defect; VSD, ventricular septal defect; PA, pulmonary atresia; RV-PA, right ventricle to pulmonary artery; TOF, tetralogy of Fallot.
Cardiac catheterization and procedures outcome
In this study, thirty-three patients underwent 37 procedures to close residual VSDs (four patients underwent a second procedure to close additional VSDs after the first catheterization). Hemodynamic measurements were performed in 28 procedures. The median pulmonary to systemic flow ratio (QP/QS) was 1.6 with an IQR of 1.5–2.44; the median systolic pulmonary pressure was 46 mmHg with an IQR of 32–54 mmHg, the median procedure duration was 120 minutes with an IQR of 90–160 minutes. For all procedures, there was a sufficient distance from the VSD margin and the aortic valve (median 6 mm, minimum 4 mm). Regarding VSDs related to surgical patches, ADO II was the most frequently used occluder (15 occluders), followed by Amplatzer muscular occluder (6 occluders) and Amplatzer septal occluder (6 occluders). For residual Muscular VSDs, variable occluders were used, like AVP IV (5 occluders), Amplatzer muscular occluder (4 occluders), ADO II (4 occluders), and ASD cribriform (3 occluders). More than one occluder was used during the same catheterization in nine procedures, and ten procedures were done successfully for patients weighing ≤5 kg. Of 37 procedures, nineteen used the retrograde approach, fourteen used the antegrade approach, two used the arteriovenous loop approach, and two used both antegrade and retrograde approaches (if more than one occluder were used). Group 1 patients had a lower systolic and mean PA pressure than Group 2 (P=0.01, P=0.007, respectively). As most of the muscular VSDs are far from the aortic valve, Group 2 patients had a higher subaortic rim than Group 1 (P<0.001). There was no significant difference between patient groups regarding fluoroscopy time, procedure time, contrast volume used for angiography, QP/QS ratio, left ventricle diastolic pressure, intensive care unit stay, hospital stay, or survival. Table 2 illustrates the procedural and outcome data for patient groups.
Table 2
| Parameters | Group 1: VSD at patch margin (n=22) | Group 2: Additional mVSD (n=15) | P value |
|---|---|---|---|
| Procedure time (min) | 106 [80–160] | 132 [102–157] | 0.189 |
| Fluoroscopy time (min) | 30.8 [13–54] | 38.8 [33.8–44] | 0.441 |
| Contrast volume (mL/kg) | 4.5 [2.3–7.4] | 5 [3.5–10] | 0.354 |
| Residual VSD diameter (mm) | 6 [4.8–7] | 5 [4–7] | 0.593 |
| QP/QS | 1.6 [1.5–2.3] | 1.5 [1.5–2.7] | 0.827 |
| Systolic PAP (mmHg) | 35 [30–49] | 52 [46–59] | 0.015 |
| Mean PAP (mmHg) | 22 [20–32] | 32 [29–39] | 0.007 |
| LVEDP (mmHg) | 13 [10–15] | 14 [11–17] | 0.417 |
| Subaortic rim (mm) | 6 [5–9], minimum 4 mm | 18 [15–21] | <0.001 |
| Approach | 0.098 | ||
| Retrograde | 14 (63.6) | 5 (33.3) | |
| Antegrade | 5 (22.7) | 9 (60.0) | |
| Arteriovenous loop | 1 (4.5) | 1 (6.7) | |
| Antegrade + retrograde | 2 (9.1) | – | |
| Type of devices used, (n)* | |||
| ADO I | 0 | 2 | |
| ADO II | 15 | 4 | |
| Amplatzer muscular occluder | 6 | 4 | |
| Amplatzer septal occluder | 6 | 1 | |
| Vascular plug IV | 1 | 5 | |
| ASD cribriform | 0 | 3 | |
| MFO VSD | 1 | 0 | |
| Vascular coil | 1 | 0 | |
| Another procedure during the same catheterization | |||
| MAPCA closure | 1 (4.5) | 1 (6.7) | 0.688 |
| COA balloon dilatation | 1 (4.5) | 0 | |
| Pulmonary valvuloplasty | 1 (4.5) | 0 | |
| Mitral valve balloon dilation | 1 (4.5) | 0 | |
| Bronchial stenting | 1 (4.5) | 0 | |
| ICU stay (days) | 2 [0–27] | 0.5 [0–39] | 0.964 |
| Hospital stay (days) | 3 [2–45] | 4.5 [3–113] | 0.660 |
| Mortality | 2 (9.1) | 1 (6.7) | 0.791 |
Data are shown as median [25th–75th percentile] or n (%). *, the total number of devices used. VSD, ventricular septal defect; mVSD, muscular ventricular septal defect; QP/QS, pulmonary/systemic blood flow ratio; PAP, pulmonary artery pressure; LVEDP, left ventricle end diastolic pressure; ADO, Amplatzer duct occluder; ASD, atrial septal defect; MFO, multifunctional occluder; MAPCA, major aortopulmonary collateral arteries; COA, coarctation of the aorta; ICU, intensive care unit.
After device closure, no residual shunts were reported in 43.2% (16/37) of procedures and minor residuals in 51.4% (19/37), as illustrated in Table 3. VSD closure was successful in 35 procedures and failed in two patients who underwent redo surgery. One of these patients was 23 years old and referred to our center from another country with TGA-VSD-PS and severe cyanosis. Immediately after the Rastelli operation, the patient had severe cardiac and multiorgan dysfunction with multiple residual VSDs related to the VSD patch. After using three occluders with only TEE without intravenous contrast, there was still a big 4th residual VSD. The patient was taken for redo surgery, came on ECMO then expired. The other patient had a significant residual VSD related to the VSD patch after TOF repair; the patient had device embolization to the PA that was successfully snared and retrieved, then redo surgery was done successfully. There were no reported vascular access-related complications, postprocedural heart block, hemolysis, or significant new valvular regurgitation after the procedure. One patient had impaired renal function tests after the procedure, which improved later. There was no procedure-related mortality.
Table 3
| Parameters | Before Cath | After Cath | P value | ||
|---|---|---|---|---|---|
| Within 6 months post-Cath | Early after Cath (total procedures =37) | On follow-up (total procedures =27)* | |||
| Echocardiographic parameters before and after cardiac catheterization | |||||
| LVEDD | 3.6 [2.3–4.2] | 3.3 [2.2–3.9] | 0.103 | ||
| EF | 60 [55–65] | 64 [60–69] | 0.109 | ||
| Residual shunts immediately after catheterization and on follow-up | |||||
| Residual shunt | 0.007 | ||||
| No | 16 (43.2) | 22 (81.5) | |||
| Minor | 19 (51.4) | 5 (18.5) | |||
| Large | 2 (5.4) | – | |||
| Clinical status before and after cardiac catheterization | |||||
| NYAH/Ross score | 0.0001 | ||||
| I | 4 (10.8) | 27 (73.0) | |||
| II | 22 (59.5) | 7 (18.9) | |||
| III | 9 (24.3) | 1 (2.7) | |||
| IV | 2 (5.4) | 2 (5.4) | |||
Data are shown as median [25th–75th percentile] or n (%). *, mortalities, redo surgeries and lost follow-up are excluded. LVEDD, left ventricle end-diastolic dimension; EF, ejection fraction; NYHA, New York Heart Association.
After catheterization procedures, three patients died later on due to severe cardiac dysfunctions and multiorgan system failure. As discussed earlier, the first patient had TGA-VSD-PS and underwent a Rastelli operation. The second patient also had TGA-VSD-PS; after the Rastelli operation, the patient had significant Gerbode VSD with severe biventricular dysfunction. However, after the successful closure of the Gerbode VSD, the patient had a prolonged ICU stay, sepsis, a multiorgan system failure then died. The third patient had trisomy 21 with a complete AVSD. The patient underwent surgical repair, then VSD device closure, and was discharged home. One year later and during follow-up, the patient had severe mitral regurgitation and underwent mechanical mitral valve replacement. After the mechanical valve, the patient had infective endocarditis on the mechanical valve, stroke, sepsis, and multiorgan failure. Table 2 illustrates the outcome of catheterization procedures.
Follow-up after VSD device closure
Although LV dimensions and ejection fraction did not show statistically significant change after cardiac catheterization, the functional heart failure class improved significantly post-catheterization (P<0.001, Table 3). The median follow-up duration was 22.3 months (Table 1). Ten patients had more than 4 years of follow-up, and five lost follow-ups after hospital discharge. After excluding the three mortalities, the two redo surgeries, and the five lost follow-ups, echocardiography showed that 81.5% of procedures (22/27) had no residual VSDs compared to 43.2% in the immediate post-catheterization, as illustrated in Table 3. Figure 5 is a graphical abstract that summarizes the study, and a separate table illustrating data for all patients is included as Supplementary file (Table S1).
Discussion
Residual postoperative VSDs are frequently seen after congenital cardiac surgery; their incidence ranges between 5–25% in literature according to the VSD type. Partial dehiscence of surgical patches, incomplete closure, intentional fenestration of VSD patch in cases with right ventricular hypertension, and infective endocarditis are well-known causes of residual VSD related to the surgical patch used for VSD closure (4,13,14). Most residual VSDs associated with the surgical patch are situated around the posteroinferior and superior edges of the VSD patch, where sutures are prone to avulsion. In this cohort, all residual VSD related to surgical patches were secondary to inadequate surgical closure/partial dehiscence. No intentional fenestrations in the VSD patches or dehiscence secondary to infective endocarditis near the surgical patches were reported.
Acquired Gerbode VSD may develop secondary to endocarditis or be iatrogenic during surgical repair. In this cohort, one Gerbode VSD was reported as probably iatrogenic during surgical repair. Like our report, Kouakou et al. reported the successful closure of one Gerbode VSD using ADO I in their series developed after the double outlet right ventricle repair (2). Residual muscular defects, especially low muscular or apical defects, are also common postoperatively. These defects are usually missed intraoperatively due to inadequate exposure or have difficulty being handled surgically without ventriculotomy, as reported in this cohort (15-18).
In this cohort, the median QP/QS was 1.6, and the maximum was 6.6, indicating a significant left-to-right shunt. Residual VSDs with hemodynamic significance can cause considerable lung plethora resulting in clinical heart failure. Residual VSDs can also increase the risk of pulmonary hypertension and infective endocarditis. In previously published reports, for prevention of the consequences of residual post-operative VSD, the presence of QP/Qs ≥1.5 was an accepted indication for reoperation for a residual VSD (3,13,19).
Redo surgery for residual VSD may be associated with poor outcomes, particularly after complex congenital cardiac surgery, such as those with baffling of the VSD to the aorta with complex RVOT obstruction. Due to this reason, reoperation for residual VSD should be reserved for patients whose catheterization attempts have failed or whose remaining defects are so significant that they cannot be treated by cardiac catheterization (1,4,20).
Group 1 patients in this study were older and had lower PA pressure than Group 2 patients. This finding is because Group 1 consisted primarily of TOF and TGA-VSD-PS patients, who typically have restrictive pulmonary flow and low PA pressure, and whose corrective surgery is not performed in early infancy. In contrast, Group 2 patients consisted primarily of those with VSD, VSD with coarctation of the aorta, or aortic arch interruption; these patients typically have unrestricted pulmonary flow with elevated pulmonary pressure and require corrective surgery in early infancy.
In this cohort, ten procedures were done successfully for patients weighing ≤5 kg. In previous literature, VSD device closure was not recommended in small infants, and most centers exclude patients ≤5 kilograms. In these patients, closing larger VSDs may necessitate larger sheaths, a condition associated with vascular access-related complications. Utilizing the antegrade or retrograde approach with ADO II, which has a delivery system that can pass through 4F or 5F sheaths, may prevent vascular access-related issues in these patients (1-3,7,8,10).
Previous studies reported using variable occluders to close residual postoperative and post-myocardial infarction VSDs, including occluders designed primarily to close patent ductus arteriosus and ASD (21-23). In this study, ADO II was the most frequent device to close residual VSDs as the retrograde approach was the preferred one in most procedures, especially those related to VSD surgical patches. ADO II is a relatively new device primarily used to close patent ductus arteriosus (24). Several recent reports and meta-analysis reported that ADO II could be used for VSD closure with high success rates, less residual shunting, and fewer complications (21,23,25). Because the ADO II has a low profile, it is significantly easier to track through angulations. The delivery system can pass through 4F or 5F vascular sheaths. Therefore, ADO II improved occluder deliverability and decreased the frequency of vascular access-related complications, especially in infants <5 kg. Since ADO II is softer and has a longer central waist, it may lessen the physical compression of the conductive system, lowering the chance of atrioventricular block (AVB), particularly in Gerbode VSD (Figure 4A-4D) (26,27). In their study, Kouakou et al. used ADO I and ADO II successfully to close postoperative residual VSD (2).
Because mVSDs, particularly apical or large ones reaching the ventricular apex, are concealed in the coarse right ventricular trabeculations, finding them via a standard surgical approach through the RA might be difficult. Transcatheter or hybrid closure of mVSDs using Amplatzer muscular Occluders is related to favorable short- and long-term results (28,29). In this report, we used Amplatzer muscular occluder to close residual mVSDs (Figure 3A-3E) and some VSDs related to the surgical patches. Similar publications reported the successful use of Amplatzer muscular occluder to close postoperative residual VSDs, especially the muscular ones (3,7).
Amplatzer Cribriform Multi-fenestrated Septal Occluder is designed to close multi-fenestrated ASDs. We used this device to close multiple muscular defects, especially when the VSDs are many and close to each other, so the thin waist of the device can go through the central defect. Simultaneously, the opposing discs can cover other peripheral defects (Figure 3F,3G). Like our report, Szkutnik et al. and Maiya et al. reported the successful closure of multiple VSDs using Cribriform Septal Occluders (22,30). This cohort also used AVP IV to close additional muscular VSDs, especially if the residual defects were of small size and multiple. Other occluder devices used to close residual VSDs in this retrospective cohort were ASO (Figure 2) and Konar multifunctional occluder (Figure 1E-1H). In contrast to our work, previous research mostly used Amplatzer membranous VSD occluders to close residual VSDs related to the surgical patch and muscular occluders to close residual muscular defects (3,7,8). Data about the optimal device for postoperative residual VSD type is developing, and consensus on the kind of occluder has yet to be established.
Heart block, hemolysis, device embolization, valvular regurgitation, vascular access-related complications, infective endocarditis, arrhythmias, contrast-induced nephropathy, residual defects, stroke, and device embolization are known complications after VSD device closure. In this cohort, one patient had device embolization during the procedure. Improper use of adequate size devices may be associated with a high risk of embolization. In clinical practice, a 1–2 mm larger device is essential to guarantee good closure of the VSD. VSDs related to surgical patches have difficulty estimating the actual size due to the complex geometry of residual VSDs (1,2). In this cohort, none of our patients reported hemolysis or a significant change in urine color or Hb drop following the procedure.
Minor residual shunts are frequently seen after VSD device closure; these tiny residuals usually close over time. In this report, the success rate for transcatheter closure of residual VSDs was 94%. During follow-up, we reported 81.5% complete closure of the minor residual VSDs compared to 43.2% in the immediate post-catheterization.
AVB is a well-known complication after VSD device/surgical closure. It is usually developed after the closure of perimembranous VSD. As the Hiss bundle courses in the posteroinferior margin of perimembranous VSD, it is susceptible to compression by the VSD device causing AVB. In this cohort, no new cases of AVB were reported. This may be explained by the less pressure caused by commonly used low-profile occluders such as ADO II for closure of Group 1 VSDs, particularly Gerbode VSDs, and the fibrosis at the VSD patch margin after surgical closure being VSD device closure performed at a median time of 4.6 months after surgical closure (1,2,26).
In contrast to our findings, Zhang et al. documented hemolysis in one patient (4.8%) following device closure of postoperative residual VSD, although persistent heart block was not recorded in comparative trials (3,4,8,10,31). Preparing children with complete heart block and a permanent epicardial pacemaker for a transvenous pacemaker at an older age is another reason for the closure of residual VSD. Two patients in this cohort with preprocedural complete heart block and permanent epicardial pacemakers had VSD device closure.
Infective endocarditis is a well-known complication after VSD device closure; for this reason, endocarditis prophylaxis is recommended for 6 months after device closure unless there is a residual (32,33). In this cohort, one patient had infective endocarditis that developed one year after VSD device closure and was related to a mechanical mitral valve and not to the VSD occluder.
Although we have three mortalities, they were not related to the procedures. The patient groups had no significant differences regarding outcome parameters like a hospital stay, complications, or survival. While the left ventricle dimensions and ejection fraction were comparable before and after VSD device closure, the functional class of heart failure considerably improved after VSD closure. This finding may be because post-catheterization echocardiogram was not performed for all patients simultaneously; some patients received echocardiography only on the second day after catheterization and subsequently lost follow-up, while others got echocardiography at various periods throughout follow-up.
Strengths and limitations
Compared to previously published reports, this study included a relatively large number of patients. It also demonstrated the closure of residual VSD in infants weighing ≤5 kg. The use of diverse occluders to close different types of residual VSD distinguishes this cohort from others. The study’s limitations include its retrospective design, the diversity of treatment populations in the analysis, the absence of hemodynamic measures for some procedures, and its relatively short follow-up period.
Conclusions
Percutaneous closure of residual postoperative VSDs is a feasible treatment option for additional muscular defects or those related to the surgical patches. Before attempting catheter closure of residual VSD, it is essential to ensure that it can be performed without compromising the cardiac valves close to the residual VSD or the vascular accesses used for closure, especially in young infants with tiny hearts and tiny vessels. Multiple occluders can effectively close postoperative residual VSDs, even in young infants weighing less than 5 kg, without damaging their delicate vascular access. Catheter closure of residual VSDs has favourable short- and midterm outcomes, so redo surgery for residual VSDs should only be considered in patients whose catheterization closure attempts have been ineffective or whose VSDs are too large to be closed via cardiac catheterization.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-624/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-624/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-624/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-624/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of King Abdulaziz University Hospital (No. 462-22). Per hospital policy, informed consents were obtained prospectively from the patients’ legal guardians on hospital admission regarding the potential to use their data in future research without violating patients’ privacy.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou W, Li F, Fu L, et al. Clinical Experience of Transcatheter Closure for Residual Ventricular Septal Defect in Pediatric Patients. Congenit Heart Dis 2016;11:323-31. [Crossref] [PubMed]
- Kouakou NYN, Song J, Huh J, et al. The experience of transcatheter closure of postoperative ventricular septal defect after total correction. J Cardiothorac Surg 2019;14:104. [Crossref] [PubMed]
- Dua JS, Carminati M, Lucente M, et al. Transcatheter closure of postsurgical residual ventricular septal defects: early and mid-term results. Catheter Cardiovasc Interv 2010;75:246-55. [Crossref] [PubMed]
- Knauth AL, Lock JE, Perry SB, et al. Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation 2004;110:501-7. [Crossref] [PubMed]
- Deng X, Huang P, Luo J, et al. Residual Shunts Following Isolated Surgical Ventricular Septal Defect Closure: Risk Factors and Spontaneous Closure. Pediatr Cardiol 2020;41:38-45. [Crossref] [PubMed]
- Giardini A, Donti A, Gargiulo G, et al. Transcatheter residual ventricular septal defect closure after Rastelli operation. Catheter Cardiovasc Interv 2005;64:209-12. [Crossref] [PubMed]
- Walsh MA, Coleman DM, Oslizlok P, et al. Percutaneous closure of postoperative ventricular septal defects with the Amplatzer device. Catheter Cardiovasc Interv 2006;67:445-51; discussion 452. [Crossref] [PubMed]
- Zhang B, Liang J, Zheng X, et al. Transcatheter closure of postoperative residual ventricular septal defects using Amplatzer-type perimembranous VSD occluders. J Invasive Cardiol 2013;25:402-5. [PubMed]
- Pedra CA, Pontes SC Jr, Pedra SR, et al. Percutaneous closure of postoperative and post-traumatic ventricular septal defects. J Invasive Cardiol 2007;19:491-5. [PubMed]
- Gu MB, Bai Y, Zhao XX, et al. Transcatheter closure of postoperative residual perimembranous ventricular septal defects. Ann Thorac Surg 2009;88:1551-5. [Crossref] [PubMed]
- Gokalp S, Guler Eroglu A, Saltik L, et al. Relationships between left heart chamber dilatation on echocardiography and left-to-right ventricle shunting quantified by cardiac catheterization in children with ventricular septal defects. Pediatr Cardiol 2014;35:691-8. [Crossref] [PubMed]
- Jameel AA, Arfi AM, Arif H, et al. Retrograde approach for device closure of muscular ventricular septal defects in children and adolescents, using the Amplatzer muscular ventricular septal defect occluder. Pediatr Cardiol 2006;27:720-8. [Crossref] [PubMed]
- Carminati M, Butera G, Chessa M, et al. Transcatheter closure of congenital ventricular septal defects: results of the European Registry. Eur Heart J 2007;28:2361-8. [Crossref] [PubMed]
- Carminati M, Butera G, Chessa M, et al. Transcatheter closure of congenital ventricular septal defect with Amplatzer septal occluders. Am J Cardiol 2005;96:52L-8L. [Crossref] [PubMed]
- Thomson JD, Gibbs JL, Van Doorn C. Cardiac catheter guided surgical closure of an apical ventricular septal defect. Ann Thorac Surg 2000;70:1402-4. [Crossref] [PubMed]
- Myhre U, Duncan BW, Mee RB, et al. Apical right ventriculotomy for closure of apical ventricular septal defects. Ann Thorac Surg 2004;78:204-8. [Crossref] [PubMed]
- Singh AK, de Leval MR, Stark J. Left ventriculotomy for closure of muscular ventricular septal defects. Treatment of choice. Ann Surg 1977;186:577-80. [Crossref] [PubMed]
- Perez-Negueruela C, Carretero J, Mayol J, et al. Surgical closure of multiple large apical ventricular septal defects: how we do it. Cardiol Young 2017;27:588-91. [Crossref] [PubMed]
- Bol-Raap G, Weerheim J, Kappetein AP, et al. Follow-up after surgical closure of congenital ventricular septal defect. Eur J Cardiothorac Surg 2003;24:511-5. [Crossref] [PubMed]
- Agarwal HS, Hardison DC, Saville BR, et al. Residual lesions in postoperative pediatric cardiac surgery patients receiving extracorporeal membrane oxygenation support. J Thorac Cardiovasc Surg 2014;147:434-41. [Crossref] [PubMed]
- Esmaeili A, Behnke-Hall K, Schrewe R, et al. Percutaneous closure of perimembranous ventricular septal defects utilizing almost ideal Amplatzer Duct Occluder II: Why limitation in sizes? Congenit Heart Dis 2019;14:389-95. [Crossref] [PubMed]
- Szkutnik M, Kusa J, Bialkowski J. The use of two Amplatzer "Cribriform" Septal Occluders to close multiple postinfarction ventricular septal defects. Tex Heart Inst J 2008;35:362-4. [PubMed]
- Cen H, Peng B, Li J, et al. Efficacy and safety of the Amplatzer Duct Occluder II for ventricular septal defect closure: a meta-analysis. Kardiol Pol 2021;79:401-9. [Crossref] [PubMed]
- Saliba Z, El-Rassi I, Abi-Warde MT, et al. The Amplatzer Duct Occluder II: a new device for percutaneous ductus arteriosus closure. J Interv Cardiol 2009;22:496-502. [Crossref] [PubMed]
- Polat TB, Türkmen E. Transcatheter closure of ventricular septal defects using the Amplatzer Duct Occluder II device: a single-center experience. Postepy Kardiol Interwencyjnej 2016;12:340-7. [Crossref] [PubMed]
- Kanaan M, Ewert P, Berger F, et al. Follow-up of patients with interventional closure of ventricular septal defects with Amplatzer Duct Occluder II. Pediatr Cardiol 2015;36:379-85. [Crossref] [PubMed]
- Ghosh S, Sridhar A, Solomon N, et al. Transcatheter closure of ventricular septal defect in aortic valve prolapse and aortic regurgitation. Indian Heart J 2018;70:528-32. [Crossref] [PubMed]
- Hijazi ZM, Hakim F, Al-Fadley F, et al. Transcatheter closure of single muscular ventricular septal defects using the amplatzer muscular VSD occluder: initial results and technical considerations. Catheter Cardiovasc Interv 2000;49:167-72. [Crossref] [PubMed]
- Kang SL, Tometzki A, Caputo M, et al. Longer-term outcome of perventricular device closure of muscular ventricular septal defects in children. Catheter Cardiovasc Interv 2015;85:998-1005. [Crossref] [PubMed]
- Maiya SS, Patel SV, Reddy C, et al. Percutaneous closure of multiple ventricular septal defects: simultaneous use of muscular ventricular septal defect device and Multi-Fenestrated Septal Occluder - "Cribriform" to close residual ventricular septal defects after complex cardiac surgery in a child. Cardiol Young 2017;27:181-3. [Crossref] [PubMed]
- Bu H, Yang Y, Wu Q, et al. Percutaneous Puncture Closure of Postoperative Residual Ventricular Septal Defects Without Radiation. Ann Thorac Surg 2020;109:e457-9. [Crossref] [PubMed]
- Scheuerman O, Bruckheimer E, Marcus N, et al. Endocarditis after closure of ventricular septal defect by transcatheter device. Pediatrics 2006;117:e1256-8. [Crossref] [PubMed]
- Tang C, Zhou K, Hua Y, et al. Very late-onset endocarditis complicated by non-significant aortic regurgitation after device closure of perimembranous ventricular septal defect. Medicine (Baltimore) 2020;99:e20120. [Crossref] [PubMed]



