
Management of the left subclavian artery during aortic arch replacement using a frozen elephant trunk approach: a review
Overview
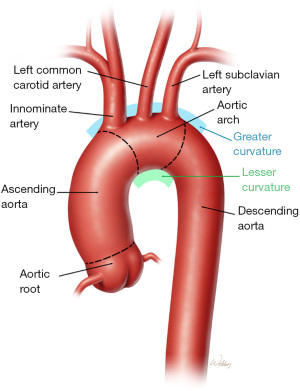
The surgical treatment of aortic arch disease remains a physiological and technical challenge for patients. The technical management of an aortic arch replacement necessitates the development of a reconstructive plan for the brachiocephalic arteries, including the left subclavian artery (LSCA; Figure 1). Total arch replacement (TAR) is typically performed through a median sternotomy, with the LSCA remaining deep in the operative field. Traditional methods of TAR commonly rely on creating the distal anastomosis just beyond the LSCA; however, its position deep in the chest makes establishing hemostasis difficult, especially if a large discrepancy exists between the diameter of the graft and the residual native aorta. Additionally, the LSCA may be diseased in the following ways: aneurysmal, atherosclerotic, calcified, dissected, or fragile. Furthermore, there may be a need to manage anatomical variation regarding how the brachiocephalic arteries arise from the greater curvature of the aortic arch. Thus, the ideal strategy for LSCA management in contemporary TAR requires careful consideration.
Borst and colleagues (1) described staged repair of extensive thoracic disease using an approach Borst called the “elephant trunk” technique. In the first stage of repair, TAR was performed, along with the extension of a short segment of “soft” graft into the proximal portion of the descending thoracic aorta. In the second stage of repair, this free-floating trunk was used to facilitate repair of the distal aorta, allowing a secure graft-to-graft anastomosis as the descending thoracic or thoracoabdominal aorta was replaced.
Kato and coauthors (2) introduced an approach later recognized as the “frozen elephant trunk” in the 1990s. The frozen elephant trunk (FET) was created using a hybrid approach that combined open and endovascular strategies; in this case, the soft elephant trunk extension was pressurized by placing a stent-graft inside of it and making it stiff or “frozen.” After decades of iterations, this approach eventually led to the development of one-piece specialty devices such as the E-Vita (Artivion, Baden-Württemberg, Germany) and the Thoraflex Hybrid device (Terumo Aortic, Sunrise, FL, USA) that have been available in Europe and other countries outside the USA for many years. In April 2022, the FDA approved the Thoraflex Hybrid device to be used in the USA. The FET approach is increasingly being used in modern practice for total arch replacement—in cases with and without acute aortic dissection.
Additionally, the majority of FET approaches incorporate the use of anastomotic collar, which was introduced into aortic arch repair in the early 2000s (3). The collar compensates for any graft-to-aorta discrepancy in diameter and facilitates a more proximal anastomosis [i.e., just distal to left common carotid artery (LCCA) instead of distal to the LSCA]; in this approach, the LSCA may be bypassed or left in its native state without revascularization. Importantly, in traditional unpressurized elephant trunk (ET) repair, there will often be some measure of retrograde flow into the LSCA. In FET repair, pressurization prevents retrograde blood flow to the LSCA. As a result, when developing a patient-specific surgical plan for TAR with FET, appropriately managing the LSCA is critical.
With the advent of thoracic endovascular aortic repair (TEVAR), controversy developed regarding the management of the LSCA when repair encroached upon this artery to ensure an adequate seal. Initially, some clinicians believed the origins of the LSCA could safely be obstructed by a stent-great without revascularization (4). However, such coverage was associated with left arm claudication, stroke, and spinal cord deficit (5). The 2010 Society for Vascular Surgery (SVS) Practice Guidelines (6) for LSCA revascularization after TEVAR are largely based on a Mayo Clinic Knowledge and Encounter Research Unit analysis of 51 studies. These studies discovered a 48-fold increase in left arm ischemia, an 11-fold increase in vertebrobasilar ischemia, a 2.7-fold increase in spinal cord ischemia and 2.6-fold increase for anterior circulation stroke when the LSCA was covered without revascularization. Despite a lack of definitive evidence, the SVS guidelines reviewed TEVAR coverage of the LSCA in three major scenarios. The first recommendation suggests preoperative revascularization should be routine (Grade 2, Level C). The second recommendation is centered on anatomical scenarios that limit perfusion to key organs, including the presence of a patent left internal mammary artery to coronary artery circulation, a patent left arm arteriovenous (AV) shunt for dialysis, termination of the left vertebral artery into the posterior inferior cerebellar artery, an absent, atretic, or occluded right vertebral artery, prior infrarenal abdominal aortic procedure, and planned extensive endovascular coverage of the descending thoracic aorta; here, routine preoperative revascularization is strongly recommended (Grade 1, Level C). The third recommendation is aimed at emergency repair and suggests individualized revascularization based on anatomy, the urgency of repair, and the experience of the surgeon (Grade 2, Level C). Clinicians should consider applying these recommendations to the LSCA in total aortic arch replacement with a FET approach.
Another important but little-discussed scenario is the management of the left vertebral artery that originates from the aorta. With a prevalence of 4% to 7%, this is the second most common anatomical variation of the aortic arch (7,8). It is associated with a longer prevertebral course before entering the cervical vertebrae and has been linked to vertebral artery dissection. In addition, the direct origin of the artery from the aorta is associated with increased diameter of the artery and direct flow of blood into the brainstem as a terminal artery on the posterior cerebellar artery. This variation can predispose to posterior circulation ischemic events and should be identified on computed tomographic angiogram (CTA) or magnetic resonance angiogram (MRA) as part of the plan for arch reconstruction.
When the aortic arch is replaced with an elephant trunk, there are primarily three clinical scenarios in which the LSCA may be addressed. The first is when the primary operation is performed on an elective basis. The second is during an emergency situation involving acute dissection or contained ruptured aneurysm. The third involves postoperative management in the event of ligation of the artery.
Preoperative
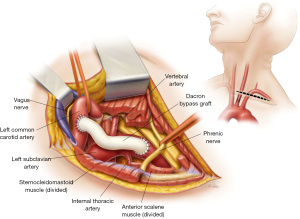
During an elective total arch replacement, there is an opportunity to reduce the branching brachiocephalic arteries from three to only two during repair, simplifying the overall procedure. In general, there are two common techniques: one is the transposition of the LSCA to the LCCA, and the other is a LCCA-LSCA bypass. Both procedures are typically performed through a left supraclavicular incision. Exposure for the transposition involves entering the carotid sheath separating the common carotid artery from the internal jugular vein and exposing the LSCA between the transverse aortic arch and the vertebral artery. This approach also entails roughly dividing the LSCA, thereby eliminating the possibility of handling the artery at that level later. If possible, transposition is preferred because it requires only one anastomosis and requires no additional prosthetic material. However, the length of the artery between the arch and the left vertebral artery must be adequate, and if aortic dissection extends into the LSCA or the LCCA, a bypass procedure may be preferrable. A LCCA-LSCA bypass is typically performed using a short section of an 8- or 10-mm Dacron graft (Figure 2). Exposure of the LSCA is generally distal to the left vertebral and internal mammary artery, sometimes requiring the ligation of the thyrocervical trunk.
Intraoperative
When preoperative management of the LSCA is not feasible, the LSCA revascularization should be addressed intraoperatively. The three-vessel en bloc island reattachment to the Dacron graft was historically the most common technique for managing the LSCA during total aortic arch replacement. Occasionally, the LSCA may be separately reimplanted directly to the graft (i.e., a 2-vessel patch with a separate LSCA reimplantation). Graft replacement of the brachiocephalic arteries, using either anatomically based grafts or trifurcated extra-anatomic Y-graft approaches, are additional management options (9-11). In other cases, simple ligation or division and oversewing the LSCA is a possible option in order to avoid any additional dissection deep into the chest that is typically necessary to isolate this artery. If a dominant left vertebral artery circulation with an origin directly from the aortic arch, it should be mobilized and re-implanted on the bypass graft or native artery if a transposition was performed.
With the approval of the Thoraflex Hybrid Device (Terumo Aortic) by the FDA in the USA in 2022, contemporary FET strategies include branched graft or a Y-graft approach using a straight tube graft and “straight tube” graft options for incorporating the three brachiocephalic arteries; this can be done as part of brachiocephalic vessels debranching (using the branched graft) or as part of a two- or three-vessel island reattachment (using the straight tube graft). As mentioned above, if the innominate and left common carotid arteries are reattached in a 2-vessel island, a separate treatment for the LSCA is needed (i.e., bypass, reimplantation, or less commonly, left in its native state). Notably, debranching Y-graft techniques and 2-vessel island patches allow the distal anastomosis of the FET to be performed in a more proximal position. Importantly, “proximal” to the origin of the LSCA includes zone 2, zone 1, or even zone 0 arch repairs.
Additional approaches are described in the literature to facilitate the revascularization and “proximilization” of the LSCA. Okamura and colleagues reported their experience with the one-piece Frozenix hybrid FET device (Japan Lifeline, Tokyo, Japan) to treat 22 patients with acute DeBakey type I aortic dissection. After the aortic arch was transected proximal to the LSCA, the stent-graft portion of the FET device was deployed and a 10-mm fenestration was manually created under direct vision to restore blood flow to the LSCA, with fixation of the fenestration site using a piece of graft to surround the opening and secured by running suture (12). As part of an FET repair, Velayudhan and coauthors reported the use of a cranial extension of the sternal incision aided the anastomosis of the LSCA to a branch graft and enabled completion of the distal anastomosis between the LCCA and LSCA (Zone 2) (13).
Outside of FET repair, Donas and colleagues described a sutureless telescoping anastomosis approach in a series of 20 patients undergoing debranching of the brachiocephalic arteries to treat varied aortic disease; here, the branches of an octopus graft (with inflow of the graft’s main body in the proximal portion of the ascending aorta) were combined with small-diameter covered stents [either a Viabahn (as part of an open rebranching technique or VORTEC procedure) or a Hemobahn covered stent] that were deployed over a wire within each artery and bridged the gap to the octopus branches. Initially, the anastomosis between the branch graft and the fully deployed covered stent was secured with interrupted stitches to avoid slippage but was later deployed without any additional anastomosis. Once the arch was debranched, a stent-graft was commonly deployed, covering the anatomic origins of the brachiocephalic arteries (14).
Postoperative
If the primary surgical procedure involved ligation, several possibilities present themselves for potential delayed treatment of the LSCA. In the early postoperative period, careful observation of the LSCA is expected. If there is a significant decrease in left arm circulation, early intervention may be necessary. For much of the time, this involves return to the operating room and performing either a LSCA-to-LCCA transposition or a LCCA-to-LSCA bypass using standard techniques. Conservative management may be entirely appropriate if the patient’s left arm remains viable and the patient recovers from the initial procedure, provided the patient remains asymptomatic.
FET experience
Early in FET experience, the distal anastomosis was typically performed distal to the LSCA, and thus, the LSCA was commonly reattached to the main body of the graft within an island patch (15). However, as branched and collared FET devices became available, contemporary practice shifted such that many surgeons began to move the distal anastomosis forward (namely, between the LCCA and LSCA) and to also debranch the brachiocephalic arteries (Figure 3) (16-18). Regarding preoperative management of the LSCA, Fiorentino and colleagues reported the selective use of LSCA-LCCA bypass about 2 weeks prior to FET repair in hopes of reducing operative ischemia; these patients recovered with minimal complication (19). Describing intraoperative management of FET repair in the setting of chronic aortic dissection, Zhong and coauthors report managing the LSCA intraoperatively by transposing it to the LCCA after 2-vessel island reattachment of the innominate and left common carotid arteries (20). In the setting of acute aortic dissection, it appears the LSCA is more likely to be sacrificed (21,22); here, the artery is postoperative managed with bypass or fenestration only if complications develop (21,23). As mentioned earlier, Velayudhan et al. extend the incision to the left side of the neck which provides a superb access for revascularization of the left subclavian after or before the implantation of the elephant trunk (13). Lastly, there are emerging techniques for LSCA management that involve modification of the FET device itself to include the use of a fenestration with suture fixation around the fenestrations (12).

Conclusions
The LSCA must be included in the operative strategy during total arch replacement regardless of the technique. Whether preoperative, intraoperative, or postoperatively, it is critical to maintain circulation of the LSCA, particularly in those patients with a patent left internal mammary artery bypass, left dominant vertebral circulation, or left arm AV fistula for hemodialysis. The LSCA is also an important source of collaterals to the spinal cord and its sacrifice can increase the risk of spinal cord ischemia during FET approach.
Acknowledgments
The authors thank Scott A. Weldon, MA, CMI, FAMI, of the Michael E. DeBakey Department of Surgery at Baylor College of Medicine, for creating several of the illustrations. Mr. Weldon’s work is partly supported by the E. Stanley Crawford Endowment. We also thank Ginger M. Etheridge, BBA, Liza N. Hirsch, DBA, and Susan Y. Green, MPH from the Michael E. DeBakey Department of Surgery for providing editorial and administrative support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mohamad Bashir, Edward P Chen and Mohammed Idhrees) for the series “Frozen Elephant Trunk” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-248/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-248/coif). The series “Frozen Elephant Trunk” was commissioned by the editorial office without any funding or sponsorship. JSC serves as principal investigator, consults for, and receives royalties and a departmental educational grant from Terumo Aortic; consults and participates in clinical trials for Medtronic, Inc., and W.L. Gore & Associates; and participates in clinical trials for Abbott Laboratories, CytoSorbents, Edwards Lifesciences, and Artivion. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using "elephant trunk" prosthesis. Thorac Cardiovasc Surg 1983;31:37-40. [Crossref] [PubMed]
- Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94:II188-93. [PubMed]
- Neri E, Massetti M, Sani G. The "elephant trunk" technique made easier. Ann Thorac Surg 2004;78:e17-8. [Crossref] [PubMed]
- Preventza O, Wheatley GH 3rd, Williams J, et al. Can the Left Subclavian Artery be Safely Covered During Endovascular Repair of the Descending Thoracic Aorta? Innovations (Phila) 2008;3:147-50. [Crossref] [PubMed]
- Feezor RJ, Lee WA. Management of the left subclavian artery during TEVAR. Semin Vasc Surg 2009;22:159-64. [Crossref] [PubMed]
- Matsumura JS, Rizvi AZSociety for Vascular Surgery. Left subclavian artery revascularization: Society for Vascular Surgery Practice Guidelines. J Vasc Surg 2010;52:65S-70S. [Crossref] [PubMed]
- Ohkura K, Shiiya N, Washiyama N, et al. Vertebral artery variations in thoracic aortic patients. Eur J Cardiothorac Surg 2014;46:27-31. [Crossref] [PubMed]
- Onrat E, Uluışık IE, Ortug G. The left vertebral artery arising directly from the aortic arch. Transl Res Anat 2021;24:100122. [Crossref]
- Kazui T. Total arch replacement: technique of separate reimplantation of epi-aortic vessels. Multimed Man Cardiothorac Surg 2007;2007:mmcts.2006.001925.
- Kazui T, Washiyama N, Muhammad BA, et al. Total arch replacement using aortic arch branched grafts with the aid of antegrade selective cerebral perfusion. Ann Thorac Surg 2000;70:3-8; discussion 8-9. [Crossref] [PubMed]
- Spielvogel D, Strauch JT, Minanov OP, et al. Aortic arch replacement using a trifurcated graft and selective cerebral antegrade perfusion. Ann Thorac Surg 2002;74:S1810-4; discussion S1825-32. [Crossref] [PubMed]
- Okamura H, Kitada Y, Miyagawa A, et al. Clinical outcomes of a fenestrated frozen elephant trunk technique for acute type A aortic dissection. Eur J Cardiothorac Surg 2021;59:765-72. [Crossref] [PubMed]
- Velayudhan B, Bashir M, Idhrees M. Left subclavian artery management in frozen elephant trunk: A novel technique. J Card Surg 2020; Epub ahead of print. [Crossref] [PubMed]
- Donas KP, Rancic Z, Lachat M, et al. Novel sutureless telescoping anastomosis revascularization technique of supra-aortic vessels to simplify combined open endovascular procedures in the treatment of aortic arch pathologies. J Vasc Surg 2010;51:836-41. [Crossref] [PubMed]
- Baraki H, Hagl C, Khaladj N, et al. The frozen elephant trunk technique for treatment of thoracic aortic aneurysms. Ann Thorac Surg 2007;83:S819-23; discussion S824-31. [Crossref] [PubMed]
- Beckmann E, Martens A, Korte W, et al. Open total arch replacement with trifurcated graft and frozen elephant trunk. Ann Cardiothorac Surg 2020;9:170-7. [Crossref] [PubMed]
- Detter C, Demal TJ, Bax L, et al. Simplified frozen elephant trunk technique for combined open and endovascular treatment of extensive aortic diseases. Eur J Cardiothorac Surg 2019;56:738-45. [Crossref] [PubMed]
- Di Marco L, Pantaleo A, Leone A, et al. The Frozen Elephant Trunk Technique: European Association for Cardio-Thoracic Surgery Position and Bologna Experience. Korean J Thorac Cardiovasc Surg 2017;50:1-7. [Crossref] [PubMed]
- Fiorentino M, de Beaufort HWL, Sonker U, et al. Thoraflex hybrid as frozen elephant trunk in chronic, residual type A and chronic type B aortic dissection. Interact Cardiovasc Thorac Surg 2021;32:566-72. [Crossref] [PubMed]
- Zhong YL, Qi RD, Ma WG, et al. Frozen elephant trunk with modified en bloc arch reconstruction and left subclavian transposition for chronic type A dissection. J Thorac Dis 2018;10:5376-83. [Crossref] [PubMed]
- Goebel N, Holder SA, Huether F, et al. Left Subclavian Artery Sacrifice in Acute Aortic Dissection Repair using the Frozen Elephant Trunk. Thorac Cardiovasc Surg 2022;70:623-9. [Crossref] [PubMed]
- Leontyev S, Tsagakis K, Pacini D, et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: results of a multicentre study. Eur J Cardiothorac Surg 2016;49:660-6. [Crossref] [PubMed]
- Veranyan N, Dunn J, Bowdish M, et al. In-Situ Fenestration of a PTFE Thoracic Aortic Stent Graft for Delayed Left Subclavian Artery Revascularization Following Frozen Elephant Trunk Repair of Type A Aortic Dissection. Ann Vasc Surg 2020;63:459.e9-459.e15. [Crossref] [PubMed]


