
Outcome of percutaneous coronary intervention using ultrathin-strut biodegradable polymer sirolimus-eluting versus thin-strut durable polymer zotarolimus-eluting stents in patients with comorbid peripheral arterial disease: a post-hoc analysis from two randomized trials
Highlight box
Key findings
• Patients with obstructive coronary artery disease and concomitant peripheral arterial disease, who underwent percutaneous coronary intervention with ultrathin-strut biodegradable polymer sirolimus-eluting stents, showed numerically lower target vessel revascularization rates than patients treated with thin-strut durable polymer zotarolimus-eluting stents. This was mainly driven by lower rates of repeated revascularization in patients treated with the sirolimus-eluting stent in vessels smaller than 2.75 mm, while in larger vessels no between-stent difference was seen.
What is known and what is new?
• Following coronary stenting, a higher repeated revascularization risk was observed in patients with concomitant peripheral arterial disease. Yet, little is known about the potential impact of different new-generation drug-eluting stents on clinical outcome after percutaneous coronary intervention.
What is the implication, and what should change now?
• Use of the ultrathin-strut stent instead of coronary stents with a thicker strut might be a good choice in PCI patients with concomitant peripheral arterial disease. It makes particular sense that cardiologists consider using ultrathin-strut stents with probably a lower risk for repeated revascularization if these patients have target lesions in small coronary vessels.
Introduction
Over the years, percutaneous coronary intervention (PCI) with drug-eluting stents (DES) has undergone a process of refinement. New-generation DES have thinner struts, and they are coated with durable polymers that are more biocompatible than the early-generation devices or devices with non-permanent polymers that are biodegradable (1). Iterations of DES aimed at a reduction of both vascular injury and inflammation, while promoting fast and complete re-endothelialization in order to avoid excessive in-stent neointimal proliferation and stent thrombosis (2). Over the years, improved clinical long-term safety and efficacy have been shown for different DES (3). Yet, even in new-generation DES, restenosis does occur and often requires a repeated target lesion revascularization (TLR) (3). The BIO-RESORT (Comparison of Biodegradable Polymer and Durable Polymer Drug-Eluting Stents in an All Comers Population) and BIONYX (Bioresorbable Polymer-coated Orsiro Versus Durable Polymer-coated Resolute Onyx Stents) randomized trials assessed new-generation coronary DES in all-comers patients. Both trials compared ultrathin-strut biodegradable polymer-coated sirolimus-eluting stents (BP-SES) and thin-strut durable polymer-coated zotarolimus-eluting stents (DP-ZES) in all-comers, showing similar results for both DES groups (4-6).
A significant proportion of all patients (about 5–19%) who undergo PCI also have obstructive atherosclerotic lesions in arterial vessels other than the coronary arteries (7-9). In PCI patients with concomitant peripheral arterial disease (PADs) the overall atherosclerotic plaque burden is increased, and these patients have an increased cardiovascular event risk (and a higher cardiovascular mortality) as compared to PCI patients without PADs (10). Furthermore, studies that assessed PCI with early-generation DES found a worse clinical outcome in these patients (9).
So far, no study assessed the clinical outcome of different contemporary new-generation DES in PCI patients with concomitant PADs. We hypothesized that in PCI patients with PADs clinical outcome may be better after treatment with ultrathin-strut DES. Therefore, in the present patient-level pooled analysis of data from BIO-RESORT and BIONYX, we assessed differences in outcome after PCI with ultrathin-strut BP-SES vs. thin-strut DP-ZES among all-comers who had PADs. We present this article in accordance with the CONSORT reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-584/rc).
Methods
Study design
We assessed PCI patients with PADs, who were treated with ultrathin-strut biodegradable polymer-coated sirolimus-eluting stent (BP-SES) or thin-strut durable polymer-coated zotarolimus-eluting stents (DP-ZES) in the BIO-RESORT (TWENTE III, NCT01674803) and BIONYX (TWENTE IV, NCT02508714) trials. Details and designs of these randomized clinical trials have been previously published (11-14). In brief, both trials assessed all-comers, and all coronary syndromes were permitted. Patients were eligible for participation in the randomized trials if they were aged 18 years or older, capable of providing informed consent, and required a PCI. The patients were enrolled in 7 tertiary or secondary PCI centers in the Netherlands (Medisch Spectrum Twente, Enschede; Haga Hospital, The Hague; Rijnstate Hospital, Arnhem; Scheper Hospital, Emmen), Belgium (Jessa Hospital, Hasselt; Centre Hospitalier Universitaire de Charleroi, Charleroi), and Israel (Hillel Yaffe Medical Center, Haifa). All centers have a high, or at least moderate, annual volume of PCI procedures, and 5 centers have a cardiothoracic surgery department. Web-based randomization, with block size of 4 and 8, was performed in a 1:1:1 fashion in the BIO-RESORT and in a 1:1 fashion in the BIONYX. The BIO-RESORT stratified for diabetes while BIONYX stratified for diabetes and sex. Assessors, research staff, and patients were blinded to the type of stent used, while the treating clinical physicians were not. In the overall trial populations, non-inferiority of ultrathin-strut BP-SES and thin-strut DP-ZES was demonstrated regarding a composite primary clinical endpoint. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The original trials were approved by the Medical Ethics Committee Twente and the Institutional Review Boards of all participating centers (BIO-RESORT NL41558.044.12; BIONYX NL54076.044.15) and individual consent was taken from all individual participants.
In the present pooled post-hoc analysis, we examined 3-year clinical outcome data of patients with comorbid PADs, treated with BP-SES or DP-ZES in one of these trials. The BIO-RESORT and BIONYX trial recorded the presence of PADs at baseline. As the original randomized trials, our present analysis was based on a principle of intention-to-treat. Patients were classified as having PADs if they—by anamnesis or medical record—had a history of: symptomatic atherosclerotic lesion in the lower or upper extremities; atherosclerotic lesion in the aorta causing symptoms or requiring treatment; atherosclerotic lesion in the carotid or vertebral arteries related to a non-embolic ischemic cerebrovascular event; or symptomatic atherosclerotic lesion in a mesenteric artery. Whenever possible, the self-reported diagnosis was checked in medical records or by contacting the patients’ general practitioners. Of these PADs patients, we pooled from both trials the demographics, clinical and angiographic characteristics, and outcome data. In addition, a small coronary vessel was defined as a vessel with a reference diameter <2.75 mm, as measured by quantitative coronary angiography in the angiographic core lab.
Study devices
In the BIO-RESORT and BIONYX randomized trials, new-generation ultrathin-strut biodegradable polymer SES (Orsiro; Biotronik, Bülach, Switzerland) were compared with thin-strut durable polymer ZES (Resolute Integrity and Resolute Onyx, respectively; Medtronic, Santa Rosa, California, USA). The ultrathin-strut BP-SES releases the drug during slightly more than 3 months. Its poly(L-lactide) acid (PLLA) coating, which is resorbed during approximately 2 years, is more than twice as thick on the abluminal strut side (7.4 µm, compared to 3.5 µm luminal). Its metallic backbone is made from 60 µm cobalt-chromium struts in stents with a nominal diameter of less than 3.5 mm; in larger stents, strut thickness is 80 µm. DP-ZES release zotarolimus during a period of 6 months from a conformal (thickness 5.6 µm) durable polymer layer—the BioLinx polymer system. The Resolute Integrity ZES has round struts made from cobalt-chromium and the Resolute Onyx ZES has roundish (ellipsoid) struts made from cobalt-chromium with a platinum-iridium core. Both DP-ZES have thin-struts with a strut thickness of 81 µm in Resolute Onyx ZES <3.0 mm and 91 µm in larger Resolute Onyx ZES and in all Resolute Integrity ZES. Further technical details of the DES are presented in Table S1.
Study procedures, follow-up, monitoring, and event adjudication
Coronary interventional procedures were performed according to standard techniques. A choice of concomitant medication and antiplatelet therapy was made based on routine clinical practice, current international guidelines, and operator’s judgment. Blinded (for the used stent type) analysts of an angiographic core laboratory performed angiographic analyses and offline quantitative coronary angiographic measurements with dedicated software (Qangio XA version 7.3, Medis, Leiden, the Netherlands), according to current standards. Patient visits to outpatient clinics, questionnaires, and telephone follow-up were used to obtain clinical follow-up. Cardiovascular Research and Education Enschede (Enschede, the Netherlands) performed trial and data management. Data monitoring was executed by an independent clinical research organization (Diagram, Zwolle, the Netherlands). Independent, blinded clinical event committees adjudicated adverse clinical events: Diagram (Zwolle, the Netherlands) for BIO-RESORT, and a committee of experienced interventional cardiologists of the University of Amsterdam (Amsterdam, the Netherlands) for BIONYX.
Definitions of clinical endpoints
The main composite endpoint assessed in this study was target vessel failure (TVF), a composite of cardiac death, target vessel related myocardial infarction (MI) or clinically indicated target vessel revascularization (TVR). Secondary endpoints included the individual components of the main composite endpoint, supplemented by target lesion failure (cardiac death, target vessel related MI or clinically indicated TLR) and major adverse cardiac events (MACEs; all-cause death, any MI, emergent coronary artery bypass surgery or clinically indicated TLR). All clinical endpoints were defined according to the Academic Research Consortium (15,16).
Statistical analysis
Categorical variables were reported as frequencies with percentages and differences between groups were assessed by the Chi-squared test, whereas continues variables were reported as mean and standard deviation and differences assessed with the Student’s t-test or Wilcoxon Rank Sum test whichever was appropriate. Kaplan-Meier methods were used to assess the time to composite and secondary endpoints, and the log-rank test was used for between-group comparisons. Cox regression was performed to test for interaction between used DES and PADs for the endpoints TVF, TVR, and TLR (interaction term PADs*type DES) as well as for the interaction between small vessel target lesion and type of DES for the endpoints TVF, TVR, and TLR (interaction term small vessel*type DES). Cox proportional hazards analysis was assessed to compute two-sided hazard ratios (HRs). Potential confounders were identified, if in univariate analysis a P value of less than 0.15 was found. These variables were: previous coronary artery bypass surgery; treatment of left anterior descending artery; treatment of a bypass graft; and small vessel disease. Further adjustments were made for the included trial. The potential confounders were entered into a multivariable Cox regression model, using stepwise backward selection. As this approach did not determine any confounder. A P value of less than 0.05 was considered significant. Statistical analyses were performed with SPSS software (version 28, IBM, Armonk, NY, USA).
Results
Study population
From December 2012 to December 2016, a total of 4,830 trial participants underwent PCI with BP-SES or DP-ZES, of whom 360 (7.5%) had comorbid PADs. These patients represent the study population of the current analysis: 177 (49.2%) patients were treated with BP-SES and 183 (50.8%) with DP-ZES (Figure 1). During 3-year follow-up, 3 (0.8%) of all 360 patients were lost to follow-up and 8 (2.2%) withdrawal consent. Data prior to withdrawal were included in this analysis, as patients agreed upon the use of data that had been collected before the withdrawal. The treatment groups did not differ in age (68.3±9.4 vs. 67.8±9.9 years), sex (32.8% vs. 29.0% women), and various other baseline patient characteristics. There were also no differences in procedural characteristics, such as multivessel treatment and target lesion complexity. Yet, BP-SES treated patients underwent more often PCI in (small) vessels with diameters <2.75 mm, and DP-ZES treated patients were more often treated in bypass grafts (Table 1).
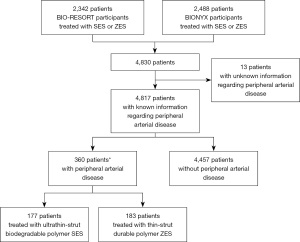
Table 1
| Characteristics | BP-SES (n=177) | DP-ZES (n=183) | P value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 68.3±9.4 | 67.8±9.9 | 0.63 |
| Premature CAD† | 5 (2.8) | 14 (7.7) | 0.041* |
| Woman | 58 (32.8) | 53 (29.0) | 0.43 |
| Body mass index (kg/m2) | 27.4±4.2 | 27.3±4.2 | 0.74 |
| Smoker | 56/173 (32.4) | 58/178 (32.6) | 0.97 |
| Diabetes mellitus | 45 (25.4) | 58 (31.7) | 0.19 |
| Renal failure‡ | 28 (15.8) | 22 (12.0) | 0.30 |
| Hypertension | 105 (59.3) | 118 (64.5) | 0.31 |
| Hypercholesterolemia | 95/174 (54.6) | 99/180 (55.0) | 0.94 |
| Previous stroke | 25 (14.1) | 31 (16.9) | 0.46 |
| LVEF <30% | 9/175 (5.1) | 8/183 (4.4) | 0.73 |
| Family history of coronary artery disease | 85/168 (50.6) | 87/174 (50.0) | 0.91 |
| Previous MI | 46 (26.0) | 54 (29.5) | 0.46 |
| Previous PCI | 58 (32.8) | 53 (29.0) | 0.43 |
| Previous coronary bypass surgery | 17 (9.6) | 31 (16.9) | 0.041* |
| Clinical syndrome at presentation | 0.19 | ||
| Stable angina pectoris | 66 (37.3) | 67 (36.6) | |
| Unstable angina pectoris | 33 (18.6) | 50 (27.3) | |
| Non-STEMI | 47 (26.6) | 43 (23.5) | |
| STEMI | 31 (17.5) | 23 (12.6) | |
| Procedural characteristics | |||
| Multivessel treatment | 40 (22.6) | 39 (21.3) | 0.77 |
| Target vessels | |||
| Left main stem | 7 (4.0) | 9 (4.9) | 0.66 |
| Right coronary artery | 74 (41.8) | 76 (41.5) | 0.96 |
| Left anterior descending artery | 89 (50.3) | 74 (40.4) | 0.06 |
| Left circumflex artery | 49 (27.7) | 58 (31.7) | 0.41 |
| Bypass graft | 2 (1.1) | 10 (5.5) | 0.022* |
| Total stent length (mm) | 43.2±29.4 | 41.8±30.8 | 0.66 |
| Post-dilation | 128 (72.3) | 131 (71.6) | 0.88 |
| Pre-dilatation | 77/79 (97.5) | 69/81 (85.2) | 0.006* |
| Thrombus aspiration | 10 (5.6) | 9 (4.9) | 0.76 |
| Rotational atherectomy | 5 (2.8) | 5 (2.7) | 0.96 |
| Complex lesion treatment | 142 (80.2) | 140 (76.5) | 0.39 |
| Ostial lesion treatment | 16 (9.0) | 16 (8.7) | 0.92 |
| Bifurcation treatment§ | 70 (39.5) | 59 (32.2) | 0.15 |
| Chronic total occlusion treatment | 7 (4.0) | 5 (2.7) | 0.52 |
| Total target lesion length >27 mm | 55 (31.1) | 41 (22.4) | 0.06 |
| Small vessel treatment¶ | 110 (62.1) | 90 (49.2) | 0.013* |
Values are mean ± SD, n (%) or n/N (%). Procedures present patient-level data. †, defined as CAD in men <50 and women <55 years; ‡, defined as previous renal failure, creatinine ≥130 μmol/L, or the need for dialysis; §, lesion classified as bifurcated if side branch ≥1.5 mm, according to criteria of Syntax Score; ¶, vessel classified as small if vessel diameter <2.75 mm; *, statistically significant. DES, drug-eluting stent; PADs, peripheral arterial disease; BP-SES, biodegradable polymer sirolimus-eluting stents; DP-ZES, durable polymer zotarolimus-eluting stents; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; non-STEMI, non-ST-segment-elevation myocardial infarction; STEMI, ST-segment-elevation myocardial infarction; SD, standard deviation.
Clinical outcome in different DES groups
Table 2 presents the 3-year clinical outcome of both treatment groups. The main endpoint TVF occurred in 19/177 (11.0%) patients assigned to BP-SES and in 32/183 (17.9%) assigned to DP-ZES (HR: 0.59, 95% CI: 0.33–1.04, P=0.07). Figure 2 displays the 3-year Kaplan-Meier event curves for TVF and its components. At 1- and 2-year follow-up, the proportions of patients experiencing TVF were numerically higher (statistically not significant) in patients treated with DP-ZES (Table 2). P values for interaction between PADs and type of DES were 0.11 (TVF), 0.041 (TVR), and 0.034 (TLR).
Table 2
| Outcome | BP-SES (n=177) | DP-ZES (n=183) | HR (95% CI) | Plog-rank |
|---|---|---|---|---|
| 1-year follow-up | ||||
| TVF† | 9 (5.1) | 18 (9.9) | 0.51 (0.23–1.13) | 0.09 |
| Cardiac death | 3 (1.7) | 5 (2.8) | 0.62 (0.15–2.58) | 0.50 |
| Target vessel related MI | 5 (2.8) | 8 (4.4) | 0.65 (0.21–1.97) | 0.44 |
| TVR | 1 (0.6) | 9 (5.0) | 0.11 (0.01–0.88) | 0.012* |
| TLR | 1 (0.6) | 8 (4.4) | 0.13 (0.02–1.01) | 0.020*° |
| Target lesion failure‡ | 9 (5.1) | 17 (9.4) | 0.54 (0.24–1.21) | 0.13 |
| Probable or definite stent thrombosis | 1 (0.6) | 1 (0.6) | 1.03 (0.07–16.49) | 0.98 |
| Bleeding | 1 (0.6) | 1 (0.6) | 1.03 (0.07–16.49) | 0.98 |
| MACE§ | 10 (5.6) | 20 (11.0) | 0.51 (0.24–1.09) | 0.08 |
| All-cause death | 4 (2.3) | 9 (5.0) | 0.46 (0.14–1.48) | 0.18 |
| MI | 6 (3.4) | 8 (4.4) | 0.77 (0.27–2.23) | 0.63 |
| 2-year follow-up | ||||
| TVF† | 17 (9.8) | 28 (15.6) | 0.61 (0.33–1.10) | 0.10 |
| Cardiac death | 6 (3.5) | 9 (5.0) | 0.68 (0.24–1.92) | 0.47 |
| Target vessel related MI | 6 (3.5) | 10 (5.6) | 0.61 (0.22–1.69) | 0.34 |
| TVR | 6 (3.5) | 15 (8.5) | 0.40 (0.15–1.02) | 0.046*° |
| TLR | 4 (2.3) | 12 (6.8) | 0.33 (0.11–1.03) | 0.044*° |
| Target lesion failure‡ | 15 (8.6) | 25 (13.9) | 0.60 (0.32–1.15) | 0.12 |
| Probable or definite stent thrombosis | 2 (1.2) | 3 (1.7) | 0.68 (0.11–4.07) | 0.67 |
| Bleeding | 2 (1.2) | 4 (2.2) | 0.51 (0.09–2.80) | 0.43 |
| MACE§ | 24 (13.6) | 30 (16.5) | 0.80 (0.47–1.37) | 0.42 |
| All-cause death | 15 (8.6) | 15 (8.5) | 1.02 (0.50–2.09) | 0.96 |
| MI | 7 (4.0) | 11 (6.2) | 0.65 (0.25–1.68) | 0.37 |
| 3-year follow-up | ||||
| TVF† | 19 (11.0) | 32 (17.9) | 0.59 (0.33–1.04) | 0.07 |
| Cardiac death | 7 (4.1) | 9 (5.0) | 0.80 (0.30–2.13) | 0.65 |
| Target vessel related MI | 7 (4.1) | 12 (6.8) | 0.60 (0.23–1.51) | 0.27 |
| TVR | 7 (4.1) | 19 (11.0) | 0.36 (0.15–0.86) | 0.016* |
| TLR | 4 (2.3) | 14 (8.0) | 0.28 (0.09–0.86) | 0.018* |
| Target lesion failure‡ | 17 (9.8) | 27 (15.1) | 0.63 (0.34–1.16) | 0.13 |
| Definite-or-probable stent thrombosis | 2 (1.2) | 4 (2.3) | 0.51 (0.09–2.78) | 0.43 |
| Bleeding | 12 (6.9) | 17 (9.6) | 0.72 (0.34–1.51) | 0.38 |
| MACE§ | 28 (15.9) | 37 (20.5) | 0.75 (0.46–1.23) | 0.26 |
| All-cause death | 17 (9.7) | 18 (10.0) | 0.96 (0.50–1.87) | 0.91 |
| MI | 9 (5.3) | 14 (8.0) | 0.67 (0.28–1.52) | 0.32 |
Data are n (%), unless otherwise indicated. †, the composite endpoint of target vessel failure is a composite of cardiac death, target vessel related myocardial infarction, and clinically indicated target vessel revascularization; ‡, target lesion failure is a composite of cardiac death, target vessel related myocardial infarction, and clinically indicated target lesion revascularization; §, major adverse cardiac events is a composite of all-cause death, any myocardial infarction, emergent coronary artery bypass surgery, and clinically indicated target lesion revascularization; *, statistically significant; °, because the log-rank P value is based on χ2, it does not correspond with the 95% CI because of the very low event rate in the Biodegradable group (P value based on Wald test: 0.051, 0.055, 0.056). BP-SES, biodegradable polymer sirolimus-eluting stents; DP-ZES, durable polymer zotarolimus-eluting stents; PADs, peripheral arterial disease; TVF, target vessel failure; MI, myocardial infarction; TVR, target vessel revascularization; TLR, target lesion revascularization; MACE, major adverse cardiac event; MI, myocardial infarction; HR, hazard ratio; CI, confidence interval.

In patients treated with BP-SES the 3-year rates of TVR and TLR were significantly lower compared to DP-ZES (HR: 0.36, 95% CI: 0.15–0.86, P=0.016 and HR: 0.28, 95% CI: 0.09–0.86, P=0.018). At 1- and 2-year follow-up, TVR also occurred significantly less often in BP-SES (HR: 0.11, 95% CI: 0.01–0.88, P=0.012; and HR: 0.40, 95% CI: 0.15–1.02, P=0.046). Other secondary endpoints such as cardiac death (HR: 0.80, 95% CI: 0.30–2.13, P=0.65), target vessel related MI (HR: 0.60, 95% CI: 0.23–1.51, P=0.27), definite-or-probable stent thrombosis (HR: 0.51, 95% CI: 0.09–2.78, P=0.43), and MACE (HR: 0.75, 95% CI: 0.46–1.23, P=0.26) showed no significant between-group difference at 3-year follow-up.
Clinical outcome in different DES groups after treating small vs. larger vessels
Among BP-SES and DP-ZES treated patients (i.e., within-stent groups), there was no statistically significant difference in TVF or secondary clinical outcomes between patients treated in small vessels (<2.75 mm) or in larger vessels. Details are presented in Table S2.
Clinical outcome after small vessel treatment with different DES
In between-stent group comparisons, BP-SES showed in patients with at least one small target vessel (<2.75 mm) lower rates of TVR and TLR than DP-ZES (HR: 0.32, 95% CI: 0.11–0.91, P=0.024 and HR: 0.17, 95% CI: 0.04–0.80, P=0.011). P values for interaction between small vessel target lesion and type of DES were statistically not significant (Table S3).
Clinical outcome after larger vessel treatment with different DES
In patients with target vessels of a larger size, no between-stent difference was seen (Table S4).
Discussion
Main findings
At 3-year follow-up of PCI patients with comorbid PADs, those who were treated with BP-SES had a numerically lower TVF rate than patients treated with DP-ZES (11.0% vs. 17.8%, P=0.07). Furthermore, both TVR and TLR were about two thirds lower in BP-SES treated patients than in patients treated with DP-ZES (TVR: 4.1% vs. 11.0%; TLR: 2.3% vs. 8.0%). Likewise, at 1- and 2-year follow-up, these repeat revascularization rates were lower in BP-SES treated patients. In addition, among patients who were treated in at least one small vessel, repeat revascularization rates were significantly lower after PCI with BP-SES (P<0.03). Yet, no between-stent difference in clinical outcome was found in vessels greater than 2.75 mm.
Previous studies
Most previous studies that compared PCI patients with and without concomitant PADs were performed with bare metal stents or early-generation DES. These studies showed in patients with PADs inferior clinical outcomes including all-cause mortality, TVR, or MACE compared to patients without this comorbidity (7,8,17,18). In PCI patients with PADs, treatment with early-generation DES showed an improved clinical outcome as compared to bare metal stents (7,19). Unfortunately, DES types were not specified but are likely new-generation DES. These studies found higher event rates for outcomes such as all-cause mortality, MI, TLR, bleeding, and major adverse cardiac and cerebrovascular events patients with PADs compared to patients without PADs (20-22). The present analysis is the first to compare the treatment results of PCI with different new-generation DES in patients with PADs.
Characteristics of DES and clinical outcome
The lower risk of repeat revascularization procedures after PCI with BP-DES in patients with PADs might be related to the strut thickness, the nature of the polymer coating, the geometry and flexibility of the metallic backbone, the drug and its release kinetics, or a combination thereof. Recent pathological assessment of the early biological responses after implantation of new-generation DP-DES and BP-DES has shown that BP-DES displays faster vessel healing and greater strut coverage than DP-DES (23). While BP-coatings could have some advantage, most trials and meta-analyses found overall no difference in clinical outcome between BP-DES- and DP-DES-treated patients (24-27). Two meta-analyses of sixteen and nine randomized trials, respectively, showed no medium- and long-term difference in safety and efficacy between BP-DES and second-generation DP-DES (24,28). Furthermore, one randomized trial compared DES with different coatings (BP-DES and DP-DES) but the same strut thickness and revealed no difference in outcome up to 5 years (27,29).
Yet, there are some signals that the ultrathin-strut thickness might represent a certain advantage for clinical outcome. A meta-analysis that compared the outcome of three ultrathin-strut DES (60–65 µm) with DES that had substantially thicker struts (81–120 µm), found a reduction in target lesion failure in patients treated with ultrathin-strut DES, resulting from a lower risk of MI (2). In contrast, the SORT OUT VII (Scandinavian Organization for Randomized Trials With Clinical Outcome) trial comparing the Orsiro ultrathin-strut BP-SES (60–80 µm) vs. a thick strut BP-DES (120 µm), showed no statistical significant difference in 2-year clinical outcome (30). Furthermore, in the BIOFLOW-II (Biotronik-Safety and Clinical Performance of the Drug Eluting Orsiro Stent in the Treatment of Subjects With Single De Novo Coronary Artery Lesions) study no statistically significant difference in optical coherence tomography-determined neointimal thickness was found between ultrathin-strut BP-DES (60 µm) and thin-strut DP-DES (81 µm) (31). In contrast, the BIOSTEMI (A Comparison of an Ultrathin Strut Biodegradable Polymer Sirolimus-Eluting Stent With a Durable Polymer Everolimus-Eluting Stent for Patients With Acute ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention) trial compared treatment with 60 µm strut BP-SES with a 81-µm strut DP-EES in patients with ST-segment elevation MI and found lower 2-year rates of TVF, clinically indicated TLR and TVR after treatment with the ultrathin-strut stent (32). While our findings in PCI patients with comorbid PADs (e.g., mortality and MI) are in accordance with most studies that found no statistical significant difference (28,30,31), we did observe lower TVR and TLR rates in patients treated with ultrathin-strut BP-SES. Notably, in our study, about 55% of all patients with PADs were treated in at least one small vessel (<2.75 mm).
After PCI in small vessels, DES with an ultrathin-struts stent have been suggested to show better clinical outcomes as a result of less relative lumen obstruction (33). The advantage of DES with ultrathin-struts, as seen in the present study, corroborates the findings of a meta-analysis by Bangalore and coworkers (2). In addition, in the BIO-RESORT trial, patients treated in coronary arteries with a lumen diameter of less than 2.5 mm experienced fewer repeated revascularizations if treated with ultrathin-strut BP-SES as compared to thin-strut DP-ZES (33). Furthermore, a subgroup analysis of patients enrolled in the RAIN study, who were treated with PCI in vessels with lumen diameters of 2.5 mm or less, found lower rates of target lesion failure, MI, and stent thrombosis after PCI with DES that had thinner struts (74 vs. 81 µm) (34). Finally, the small vessel subgroup analysis of the CENTURY-II (Clinical Evaluation of New TerUmo drug-elUting coRonary stent system in the treatment of patients with coro-narY artery disease) trial, which compared thin-strut DES with similar strut thickness (80 and 81 µm) but different coatings (BP and DP-coating), showed no between-stent difference in revascularization rate (35).
Strengths and limitations
To the best of our knowledge, this is the first analysis that assessed the impact of treatment with different new-generation DES, performed in patients who had a PCI and comorbid PADs. Therefore, the current study pooled patient-level data from two randomized controlled stent trials and assessed the outcome after PCI with BP-SES or DP-ZES in all-comer patients who had comorbid PADs. For both trials, the same in- and exclusion criteria, definitions and clinical endpoints were used. Clinical events were independently adjudicated by external clinical event committees. Nevertheless, this study has some limitations. The results of this analysis should be considered hypothesis generating due to the sample size and formal lack of power. The current study assessed patients who at the time of enrollment or previously had symptomatic PADs or clinically relevant aortic pathologies; therefore, patients with undiagnosed and asymptomatic disease will have been missed. No ankle-brachial index was performed and the definition of PADs was determined by the local site.
Conclusions
In PCI patients with PADs, the 3-year incidence of TVF was numerically lower in the ultrathin-strut BP-SES vs. the thin-strut DP-ZES group. Furthermore, TVR risk was significantly lower in ultrathin-strut BP-SES, mainly driven by a lower TVR rate in small vessels.
Acknowledgments
Funding: The BIO-RESORT trial was equally funded by Biotronik, Boston Scientific, and Medtronic. The BIONYX trial was equally funded by Biotronik, and Medtronic. Funders of the original randomized clinical trials had no impact on the design, conduct, analysis, and reporting of the present study. There was no external funding for performing the present study.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-584/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-584/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-584/coif). EHP reports that the Research Department of Thoraxcentrum Twente has received institutional research grants from these companies (Biotronik, Boston Scientific, and Medtronic) for performing the 2 RCTs on which the analyses of this manuscript are based. In addition, the Research Department of Thoraxcentrum Twente has received an institutional research grant for performing a stent study, not directly related with the content of the present manuscript. RLA reports a teaching grant from Biotronik, a license from Sanovi, a speaking fee from Abiomed and support from Amgen for attending a meeting, all outside the submitted work. CvB reports that the Research Department of Thoraxcentrum Twente has received institutional research grants from these companies (Biotronik, Boston Scientific, and Medtronic) for performing the 2 RCTs on which the analyses of this manuscript are based. In addition, the Research Department of Thoraxcentrum Twente has received an institutional research grant for performing a stent study, not directly related with the content of the present manuscript. The author is a DSMB member in a TAVI trial and a vascular surgical trial. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The original trials were approved by the Medical Ethics Committee Twente and the Institutional Review Boards of all participating centers (BIO-RESORT NL41558.044.12; BIONYX NL54076.044.15) and individual consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Byrne RA, Stone GW, Ormiston J, et al. Coronary balloon angioplasty, stents, and scaffolds. Lancet 2017;390:781-92. [Crossref] [PubMed]
- Bangalore S, Toklu B, Patel N, et al. Newer-Generation Ultrathin Strut Drug-Eluting Stents Versus Older Second-Generation Thicker Strut Drug-Eluting Stents for Coronary Artery Disease. Circulation 2018;138:2216-26. [Crossref] [PubMed]
- Sousa JE, Costa MA, Abizaid AC, et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation 2001;104:2007-11. [Crossref] [PubMed]
- Buiten RA, Ploumen EH, Zocca P, et al. Thin, very thin, or ultrathin strut biodegradable or durable polymer-coated drug-eluting stents: 3-year outcomes of BIO-RESORT. JACC Cardiovasc Interv 2019;12:1650-60. [Crossref] [PubMed]
- Buiten RA, Ploumen EH, Zocca P, et al. Thin Composite-Wire-Strut Zotarolimus-Eluting Stents Versus Ultrathin-Strut Sirolimus-Eluting Stents in BIONYX at 2 Years. JACC Cardiovasc Interv 2020;13:1100-9. [Crossref] [PubMed]
- Ploumen EH, Buiten RA, Zocca P, et al. First Report of 3-Year Clinical Outcome After Treatment With Novel Resolute Onyx Stents in the Randomized BIONYX Trial. Circ J 2021;85:1983-90. [Crossref] [PubMed]
- Ramzy J, Andrianopoulos N, Roberts L, et al. Outcomes in patients with peripheral vascular disease following percutaneous coronary intervention. Catheter Cardiovasc Interv 2019;94:588-97. [Crossref] [PubMed]
- Nikolsky E, Mehran R, Mintz GS, et al. Impact of symptomatic peripheral arterial disease on 1-year mortality in patients undergoing percutaneous coronary interventions. J Endovasc Ther 2004;11:60-70. [Crossref] [PubMed]
- Midwall S, Swaminathan RV, Charitakis K, et al. Impact of peripheral vascular disease on short- and long-term outcomes in patients undergoing non-emergent percutaneous coronary intervention in the drug-eluting stent era. J Invasive Cardiol 2013;25:132-6. [PubMed]
- Polonsky TS, McDermott MM. Lower Extremity Peripheral Artery Disease Without Chronic Limb-Threatening Ischemia: A Review. JAMA 2021;325:2188-98. [Crossref] [PubMed]
- von Birgelen C, Zocca P, Buiten RA, et al. Thin composite wire strut, durable polymer-coated (Resolute Onyx) versus ultrathin cobalt-chromium strut, bioresorbable polymer-coated (Orsiro) drug-eluting stents in allcomers with coronary artery disease (BIONYX): an international, single-blind, randomised non-inferiority trial. Lancet 2018;392:1235-45. [Crossref] [PubMed]
- von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet 2016;388:2607-17. [Crossref] [PubMed]
- Lam MK, Sen H, Tandjung K, et al. Comparison of 3 biodegradable polymer and durable polymer-based drug-eluting stents in all-comers (BIO-RESORT): rationale and study design of the randomized TWENTE III multicenter trial. Am Heart J 2014;167:445-51. [Crossref] [PubMed]
- van der Heijden LC, Kok MM, Zocca P, et al. Bioresorbable Polymer-Coated Orsiro Versus Durable Polymer-Coated Resolute Onyx Stents (BIONYX): Rationale and design of the randomized TWENTE IV multicenter trial. Am Heart J 2018;198:25-32. [Crossref] [PubMed]
- Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344-51. [Crossref] [PubMed]
- Vranckx P, Cutlip DE, Mehran R, et al. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention 2010;5:871-4. [Crossref] [PubMed]
- Chiu JH, Topol EJ, Whitlow PL, et al. Peripheral vascular disease and one-year mortality following percutaneous coronary revascularization. Am J Cardiol 2003;92:582-3. [Crossref] [PubMed]
- Dinser L, Meisinger C, Amann U, et al. Peripheral arterial disease is associated with higher mortality in patients with incident acute myocardial infarction. Eur J Intern Med 2018;51:46-52. [Crossref] [PubMed]
- Parikh SV, Saya S, Divanji P, et al. Risk of death and myocardial infarction in patients with peripheral arterial disease undergoing percutaneous coronary intervention (from the National Heart, Lung and Blood Institute Dynamic Registry). Am J Cardiol 2011;107:959-64. [Crossref] [PubMed]
- Sasaki M, Mitsutake Y, Ueno T, et al. Low ankle brachial index predicts poor outcomes including target lesion revascularization during the long-term follow up after drug-eluting stent implantation for coronary artery disease. J Cardiol 2020;75:250-4. [Crossref] [PubMed]
- Attar R, Wester A, Koul S, et al. Peripheral artery disease and outcomes in patients with acute myocardial infarction. Open Heart 2019;6:e001004. [Crossref] [PubMed]
- Al-Zakwani I, Al Siyabi E, Alrawahi N, et al. Association between Peripheral Artery Disease and Major Adverse Cardiovascular Events in Patients with Acute Coronary Syndrome: Findings from the Gulf COAST Registry. Med Princ Pract 2019;28:410-7. [Crossref] [PubMed]
- Kawagoe Y, Otsuka F, Onozuka D, et al. Early vascular responses to abluminal biodegradable polymer-coated versus circumferential durable polymer-coated newer-generation drug-eluting stents in humans: a pathological study. EuroIntervention 2023;18:1284-94. [PubMed]
- Kobayashi T, Sotomi Y, Suzuki S, et al. Five-year clinical efficacy and safety of contemporary thin-strut biodegradable polymer versus durable polymer drug-eluting stents: a systematic review and meta-analysis of 9 randomized controlled trials. Cardiovasc Interv Ther 2020;35:250-8. [Crossref] [PubMed]
- Meredith IT, Verheye S, Dubois C, et al. Final five-year clinical outcomes in the EVOLVE trial: a randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent. EuroIntervention 2018;13:2047-50. [Crossref] [PubMed]
- Wijns W, Vrolix M, Verheye S, et al. Randomised study of a bioabsorbable polymer-coated sirolimus-eluting stent: results of the DESSOLVE II trial. EuroIntervention 2015;10:1383-90. [Crossref] [PubMed]
- Kereiakes DJ, Windecker S, Jobe RL, et al. Clinical Outcomes Following Implantation of Thin-Strut, Bioabsorbable Polymer-Coated, Everolimus-Eluting SYNERGY Stents. Circ Cardiovasc Interv 2019;12:e008152. [Crossref] [PubMed]
- El-Hayek G, Bangalore S, Casso Dominguez A, et al. Meta-Analysis of Randomized Clinical Trials Comparing Biodegradable Polymer Drug-Eluting Stent to Second-Generation Durable Polymer Drug-Eluting Stents. JACC Cardiovasc Interv 2017;10:462-73. [Crossref] [PubMed]
- Wijns W, Valdes-Chavarri M, Richardt G, et al. Long-term clinical outcomes after bioresorbable and permanent polymer drug-eluting stent implantation: final five-year results of the CENTURY II randomised clinical trial. EuroIntervention 2018;14:e343-51. [Crossref] [PubMed]
- Jensen LO, Maeng M, Raungaard B, et al. Two-year outcome after biodegradable polymer sirolimus- and biolimus-eluting coronary stents (from the randomised SORT OUT VII trial). EuroIntervention 2018;13:1587-90. [Crossref] [PubMed]
- Lefèvre T, Haude M, Neumann FJ, et al. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: 5-year outcomes of the randomized BIOFLOW-II trial. JACC Cardiovasc Interv 2018;11:995-1002. [Crossref] [PubMed]
- Pilgrim T, Muller O, Heg D, et al. Biodegradable- Versus Durable-Polymer Drug-Eluting Stents for STEMI: Final 2-Year Outcomes of the BIOSTEMI Trial. JACC Cardiovasc Interv 2021;14:639-48. [Crossref] [PubMed]
- Buiten RA, Ploumen EH, Zocca P, et al. Outcomes in Patients Treated With Thin-Strut, Very Thin-Strut, or Ultrathin-Strut Drug-Eluting Stents in Small Coronary Vessels: A Prespecified Analysis of the Randomized BIO-RESORT Trial. JAMA Cardiol 2019;4:659-69. [Crossref] [PubMed]
- Franchin L, Piroli F, D'Ascenzo F, et al. Impact of stent thickness on clinical outcomes in small vessel and bifurcation lesions: a RAIN-CARDIOGROUP VII sub-study. J Cardiovasc Med (Hagerstown) 2021;22:20-5. [Crossref] [PubMed]
- Wöhrle J, Markovic S, Rottbauer W, et al. Bioresorbable polymer sirolimus-eluting coronary stent compared with permanent polymer everolimus-eluting coronary stent implantation for treatment of small vessel coronary artery disease: CENTURY II trial. EuroIntervention 2016;12:e167-74. [Crossref] [PubMed]


