
Comparison between a dedicated stent positioning system and conventional stenting of aorto-ostial lesions: a prospective, multi-center, randomized controlled study
Highlight box
Key findings
• The stent positioning system appears to have significant advantages over the conventional approach for stenting of coronary aorto-ostial lesions (AOLs).
What is known and what is new?
• Accurate placement of coronary stents in aorto-ostial locations is challenging.
• The stent positioning system improves the accuracy of stent placement and prevents stent slippage when positioning.
What is the implication, and what should change now?
• The stent positioning system was shown to be safe and effective. The effects of the stent-positioning system on restenosis need further investigation.
Introduction
A coronary aorto-ostial lesion (AOL) is defined as a stenosis of more than 50% within 3 mm of the coronary ostium (1). Ostial stenosis occurs in 0.18–2.7% of patients with coronary artery disease (2-5). Due to its anatomical location, coronary aorto-ostial stenosis may cause large myocardial ischemia. It is a high-risk lesion and a precursor to fatal myocardial infarction or sudden cardiac death. However, the accurate placement of coronary stents in aorto-ostial locations is challenging due to the complex 3-dimensional (3D) anatomy of the lesions. The ostia of the left and right coronary arteries are located with a variable take-off angle just above the aortic valve, as are the left and right sinuses of Valsalva. The shape of the ostia resembles a funnel, with a larger diameter in the proximal end (6,7). To achieve a full coverage of the ostial lesion, the stent must be placed with a slight degree of overhang into the aorta. Therefore, correct placement of the stent is difficult. Further, stent slippage before deployment increases the surgical difficulty. If a stent does not entirely cover a lesion, a “geographic miss” may occur that may increase the risk of in-stent restenosis (1,8). Additionally, excessive protrusion of the stent into the aorta may lead to difficulties in catheter reengagement, stent wiring, and endothelization, as well as stent deformation (9,10).
Alternative treatments for AOLs have been investigated (11-13). The FLASH Ostial system (Ostial Corp., Campbell, CA, USA) was designed to optimize implantation of aorto-ostial coronary stents by flaring the proximal stent struts against the aortic wall. The floating wire technique in right coronary ostial lesions provides a significant advantage over the single-wire technique. However, these techniques are not particularly precise (14,15), remain susceptible to stent deformation or dislodgement, and thus have not been widely adopted (16). Further studies might be necessary to assess whether these techniques are more effective than the conventional technique. In this study, we aimed to compare the efficacy and safety of a dedicated stent positioning system and the conventional single-wire technique for AOL. We present this article in accordance with the CONSORT reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-542/rc).
Methods
Design and patients
In this prospective, multi-center, open-label, randomized controlled study (ChiCTR2100053869), patients aged 18–80 years with aorto-ostial coronary artery stenosis indicated for stenting were recruited from November 2015 to April 2019 at Beijing Anzhen Hospital, People’s Hospital of Liaoning Province, Six Medical Center of PLA General Hospital, Beijing Chaoyang Hospital, and Renmin Hospital of Wuhan University, China. All procedures were indicated according to coronary angiography (17) and were performed by experienced operators. The exclusion criteria were as follows: anomalous aortic origin of a coronary artery, in-stent restenosis, severely calcified lesion, ST elevated myocardial infarction (STEMI), heart failure with left ventricular ejection fraction (LVEF) ≤35%, and other situations that were not suitable for participation. According to the relevant literature and pre-test data, we assumed that the range of stent slippage could be reduced by at least 1 mm by the stent positioning system. Considering the maximum possible 10% drop out during the study, according to the statistical principle, 70 patients needed to be enrolled in each group. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (No. 2015-042I), Beijing Chaoyang Hospital, Capital Medical University (No. 2017-Qi-29), The Six Medical Center of PLA General Hospital (No. HZQX-PJ-2018-1), Renmin Hospital of Wuhan University (No. 2018Q-W001), and The People’s Hospital of Liaoning Province (No. llhs2015019). All patients provided written informed consent.
Randomization and procedures
Using block randomization (the block size was 4), the corresponding randomization table was generated by specialized statisticians before commencement of the study, and the participants were randomized by a central randomization system, which was developed by the Medical Research and Biometrics Center, National Center for Cardiovascular Diseases. The examiners were blinded. The eligible patients were randomly assigned to a stent positioning system group and a conventional technique group (allocation ratio 1:1). After randomization, the patients underwent interventional therapy, and the relevant information was recorded, including target vessel location, reference vessel diameter, lesion type, and thrombolysis in myocardial infarction (TIMI) flow grade. In the standard technique, the procedure of stent implantation was performed through the femoral or radial artery. For ostial lesions, the stents were carefully positioned to maintain extension of the proximal end at approximately 1 mm outside the orifice. To optimize the detection of the aorto-ostial plane, fluoroscopic angles were used to visualize the left oblique (LAO) angles for the right coronary artery (RCA) and the LAO and cranial anterior–posterior angles for the LM (left main).
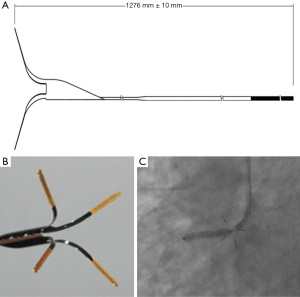
The dedicated stent positioning system (Beijing Advanced Medical Technologies, Beijing, China) was employed, which has a nitinol device that can extend beyond the guiding catheter. It has distal, self-expanding legs with markers that can be visualized under fluoroscopy. When the legs are expanded, the plane of the aortic wall is marked, and the tip of the guiding catheter can be aligned with the aorto-ostial plane instead of inserting it into the vessel (Figure 1). The essentials of the intervention in the stent positioning system group were as follows: (I) after the guidewire had penetrated the lesion, the stent positioning system was inserted into the guiding catheter in front of the stent and placed the stent positioning system approximately 4–10 cm away from the distal ostial area of the guiding catheter. Then, the stent was pushed along the guide wire to the distal end of the lesion; (II) the guiding catheter was withdrawn and a small amount of contrast agent was injected to confirm that the tip of the guiding catheter was in the aorta. Further, the stent positioning system was advanced until the legs were fully expanded. Then, the guiding catheter was pressed forward until the legs clearly touched the aortic wall. Next, contrast agent was injected to confirm the position of the guiding catheter, stent positioning system, and lesion. Then, the stent was retracted until the proximal marking point of the stent reached the proximal end, where the legs of the stent positioning system were located. A contrast agent was injected to confirm the position; (III) the stent was expanded; (IV) the balloon and the stent positioning system were withdrawn (Figure 2). Angiography (without additional equipment) was applied in the conventional manner before the stent release to ensure that the positioning of the stent was satisfactory.

Follow-up and outcomes
Follow-up was routinely performed 30 days after percutaneous coronary intervention (PCI) and documented in the database. Clinical events were collected via clinic visit, medical records, or telephone calls and recorded by an investigator who was blinded to the randomization. Every effort was made to collect the 1-year clinical follow-up data retrospectively, including cardiac death, myocardial infarction, and ischemia-driven revascularization, although the chronic outcomes (e.g., restenosis) were not included in the protocol. The primary endpoint was the range of stent slippage at positioning. The secondary endpoints were as follows: (I) guiding catheter swing during stent positioning (defined as the difference between the longest value and the shortest value of the distance between the tip of the guiding catheter and the reference branch, before stent release); (II) the rate of accurate stent placement (defined as the entire circumference of the proximal stent edge covering the area along the axis of the coronary artery located within 1 mm of the aorto-ostial plane); (III) the procedure time; and (IV) the incidence of major adverse cardiovascular events (MACEs), including cardiac death, myocardial infarction, target lesion revascularization, and stent thrombosis.
Statistical analysis
The analysis of the primary endpoint (the range of stent slippage when positioning) was carried out simultaneously on the basis of the full analysis set (FAS). In addition, postoperative follow-up event analysis was conducted on the basis of the FAS. All baseline demographic data, secondary efficacy analysis, and safety evaluation were performed on the basis of the FAS. We imputed missing values using the sequential regression multiple imputation method implemented by IVEware software version 0.2 (Survey Research Center, University of Michigan, Ann Arbor, MI, USA). The strategies employed for the management of missing data are presented in Table S1.
Continuous variables were expressed as mean ± standard deviation (SD) or median (first and third quartiles) depending on distribution type, and were compared by Student’s t-test or Wilcoxon rank-sum test. Categorical variables were presented as the number (percentage) and compared using χ2 statistics or Fisher’s exact test, as appropriate. All statistical analyses were performed with SAS version 9.2 or higher version software (SAS Institute, Cary, NC, USA). All statistical tests were 2-sided and a P value ≤0.05, was considered to indicate a statistically significant difference.
Results
Baseline demographics
During the study period, 139 patients with aorto-ostial coronary artery stenosis were included at 5 centers. A total of 69 patients were allocated to the stent positioning system group and 70 patients to the conventional technique group. The study flow chart is presented in Figure S1. The demographic and clinical characteristics of the 2 study groups were similar (Table 1). In the stent positioning system group, the mean age was 61.43±9.76 years, and 76.8% of participants were male. In the conventional technique group, the mean age was 64.05±8.80 years, and 68.6% were male. No differences between the 2 groups existed in the prevalence of diabetes, hyperlipidemia, hypertension, and prior PCI or coronary artery bypass grafting (CABG). No differences were present between the 2 groups in the left ventricular systolic function.
Table 1
| Parameters | Stent positioning system (n=69) | Conventional technique group (n=70) | P value |
|---|---|---|---|
| Age (years) | 61.43±9.76 | 64.05±8.80 | 0.10 |
| Gender | |||
| Male | 53 (76.8%) | 48 (68.6%) | 0.28 |
| Female | 16 (23.2%) | 22 (31.4%) | |
| BMI (kg/m2) | 25.43±2.76 | 25.21±2.56 | |
| Cases, n [missing] | 65 [4] | 67 [3] | 0.64 |
| Hypertension | 41 (59.4%) | 43 (61.4%) | 0.81 |
| Diabetes | 30 (43.5%) | 31 (44.3%) | 0.92 |
| Hyperlipidemia | 10 (14.5%) | 13 (18.6%) | 0.52 |
| Prior PCI | 24 (34.8%) | 24 (34.3%) | 0.95 |
| Prior CABG | 4 (5.8%) | 3 (4.3%) | 0.72 |
| LVEF (%) | 54.67±10.32 | 57.75±9.36 | |
| Cases, n [missing] | 63 [6] | 65 [5] | 0.08 |
Comparisons of qualitative indicators between groups were done by likelihood ratio chi-square test or Fisher’s exact probability test; Comparisons of quantitative indicators between groups were done by student t-test. BMI (kg/m2) = weight (kg)/(height(cm)/100)2. BMI, body mass index; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction.
Angiography and procedure
Table 2 displays the data comparison of the lesion and procedural characteristics between the 2 groups. In the stent positioning system group, 48 cases (69.6%) had right coronary AOL, and 21 cases (30.4%) had left main AOL. No significant difference between the groups was observed in the distribution of target lesions. The reference vessel diameters (3.42±0.48 vs. 3.49±0.49 mm; P=0.43) and the degrees of stenosis (81.29%±13.21% vs. 84.56%±11.66%; P=0.12) in the 2 groups were similar. The lesion classification [American College of Cardiology/American Heart Association (ACC/AHA)], and TIMI flow grade of the 2 groups were also similar. Stent overlap occurred in about a half of the patients in each of the groups. There was no significant difference between the groups in the stent length and diameter. The ischemia syndromes during the procedure were similar between groups (further details are provided in the Results section and Table 2).
Table 2
| Parameters | Stent positioning system (n=69) | Conventional technique group (n=70) | P value |
|---|---|---|---|
| Target vessel location | |||
| Left main ostial | 21 (30.4%) | 31 (44.3%) | 0.09 |
| Right coronary ostial | 48 (69.6%) | 39 (55.7%) | 0.09 |
| Reference vessel diameter (mm) | 3.42±0.48 | 3.49±0.45 | 0.43 |
| Stenosis (%) | 81.29±13.21 | 84.56±11.66 | 0.12 |
| Lesion type | 0.54 | ||
| A | 8 (11.6%) | 4 (5.7%) | |
| B1 | 12 (17.4%) | 13 (18.6%) | |
| B2 | 6 (8.7%) | 4 (5.7%) | |
| C | 43 (62.3%) | 49 (70.0%) | |
| TIMI flow | |||
| 0 | 11 (15.9%) | 12 (17.1%) | |
| 1 | 2 (2.9%) | 1 (1.4%) | 0.76 |
| 2 | 4 (5.8%) | 7 (10.0%) | |
| 3 | 52 (75.4%) | 50 (71.4%) | |
| Number of stent overlap | 34 (49.3%) | 37 (52.9%) | 0.67 |
| Ostial stent diameter (mm) | 3.41±0.45 | 3.46±0.40 | 0.51 |
| Ostial stent length (mm) | 24.59±7.21 | 24.33±0.82 | 0.84 |
| Ischemic symptoms | 4 (5.8%) | 4 (5.7%) | 0.63 |
| Palpitation | 1 (1.4%) | 2 (2.9%) | |
| Chest tightness | 2 (2.9%) | 2 (2.9%) | |
| Chest pain | 1 (1.4%) | 0 |
Data are presented as n (%) or mean ± SD. Comparisons of qualitative indicators between groups were done by likelihood ratio chi-square test or Fisher’s exact probability test; comparisons of quantitative indicators between groups were done by student t-test. TIMI, thrombolysis in myocardial infarction.
Outcomes and complications
The overall technical success was 100%. It was revealed that after the stent positioning system reaches the ostial location of the target vessel and the distal legs are fully expanded, it can maintain stability of the guiding catheter position and facilitate the subsequent operations. The mean range of the stent slippage when positioning was significantly shorter in the stent positioning system group than in the conventional technique group [0.64 (0.22; 1.35) vs. 1.11 (0.48; 1.72) mm; P=0.01]. In the sensitivity analyses after missing value imputation and adjustment for center, target lesion location, and height, the range of the stent slippage was still shorter in the stent positioning system group (Table S1). The extent of swing of guiding catheters during the stent positioning showed no difference between the 2 groups [0.24 (0.19; 0.53) vs. 0.23 (0.19; 0.53) mm; P=0.95]. The rate of accurate placement of stents was higher in the stent positioning system group than in the conventional technique group (74.6% vs. 57.1%, P=0.03). Compared with the conventional technique group, use of the stent positioning system did not result in a significant prolongation of the procedure time [1.00 (0.50; 1.50) vs. 0.80 (0.50; 1.50) min, P=0.09] (Table 3).
Table 3
| Parameters | Stent positioning system | Conventional technique group | P value | |||
|---|---|---|---|---|---|---|
| Data | n [missing] | Data | n [missing] | |||
| The range of stent slippage, mm | 0.64 (0.22; 1.35) | 59 [10] | 1.11 (0.48; 1.72) | 70 [0] | 0.01 | |
| The extent of guiding catheter swing during stent positioning, mm | 0.24 (0.19; 0.53) | 66 [3] | 0.23 (0.19; 0.53) | 70 [0] | 0.95 | |
| The rate of accurate stent placement | 50 (74.6%) | 67 [2] | 40 (57.1%) | 70 [0] | 0.03 | |
| The procedure time, min | 1.00 (0.50; 1.50) | 69 [0] | 0.80 (0.50; 1.50) | 70 [0] | 0.09 | |
| MACE (during hospital) | 1 (1.4%) | 69 [0] | 2 (2.9%) | 70 [0] | >0.99 | |
| MACE (30 days after PCI) | 1 (1.5%) | 67 [2] | 2 (2.9%) | 68 [2] | >0.99 | |
Quantitative indicators were described by median (first and third quartiles). Comparisons of qualitative indicators between groups were done by likelihood ratio chi-square test or Fisher's exact probability test; Comparisons of quantitative indicators between groups were done by Wilcoxon rank-sum test. MACE, major adverse cardiovascular event; PCI, percutaneous coronary intervention.
Complete 30-day follow-up data were available for all patients. The risk for MACE during hospitalization and 30 days after PCI was not statistically different between the 2 groups (Table 3). In the stent positioning system group, 1 participant had creatine kinase-myoglobin binding (CK-MB) ≥2 × URL during the hospital stay, and 2 patients had CK-MB ≥2 × URL at 30-day follow-up examination, which were not related to the device. By the 1 year follow-up assessment, only 1 patient in the control group had undergone non-target vessel ischemia-driven revascularization at 2 months post-intervention.
Discussion
In this study, we found that the mean range of stent slippage during positioning was significantly shorter in the stent positioning system group. The rate of accurate placement of stents in the stent positioning system group was higher than in the conventional technique group.
Coronary AOLs were defined as stenosis >50% within 3 mm of the vessel orifice (1). The outcome of drug eluting stents (DES) placement in AOLs remains inferior to non-ostial lesions (18,19). The failure to precisely place the stent may contribute to a suboptimal outcome. The following technical difficulties and challenges are most common: (I) ischemia due to pressure dampening caused by the guiding catheter insertion; (II) risk of stents dislodgement; (III) geographic miss with incomplete coverage of the vessel ostium; (IV) excessive protrusion into the aorta preventing re-engagement of the vessel with a guiding catheter; (V) risk of stent deformation requiring careful and extensive operative experience.
In our study, the mean range of stent slippage during positioning was significantly shorter in the stent position system group. The stent position system may prevent the entry of the guiding catheter into the target vessel, and stabilize the whole stent-catheter system by aligning the tip of the guiding catheter with the aorto-ostial plane. Moreover, when the legs expand, the plane of the aortic wall is marked, which may facilitate the positioning of the stent with a slight degree of overhang in the aorta to ensure lesion coverage. Although we understand that there is a learning curve for the use of the stent positioning system, no prolongation of procedure time was observed in this study.
Several techniques using more than 1 guidewire or other equipment have been implemented to resolve this problem with some success. The anchor wire technique, also called the Szabo technique, employs a second angioplasty guidewire positioned in the aorta to anchor the stent at the ostial location by passing the proximal end of the anchor wire through the last cell of the stent (13). However, it may lead to stent deformation and dislodgement by bench testing and cases (14). The floating wire technique is another method for solving interventional problems in AOLS (15). This is a similar approach, in which a second guidewire is placed within the aortic sinus to mark the aorto-ostial plane and prevent the entry of the catheter. The application of the floating wire technique in right coronary AOLs was analyzed in a retrospective study including 126 patients (12). Although it was shown to provide a significant advantage over the single-wire technique, due to the design and the lesions selected in the study, its evidence power is limited. The FLASH Ostial System is a novel device designed to flare the proximal (aortic) part of the stent. It is a dual balloon angioplasty catheter that includes a larger proximal low-pressure balloon (anchoring balloon) and a higher-pressure distal balloon (angioplasty balloon). The system has 3 markers which can be visualized by fluoroscopy, facilitating the localization of the balloons (11). When a longer stent was implanted for adequate lesion coverage, the overhanging portion of the stent would eventually be flared by the system. However, if the stent was placed just distal to the lesion, the device could not prevent a geographical miss. Harding et al. described a novel technique to guide stent placement in coronary AOLs. An intravenous ultrasound (IVUS) catheter placed over a floating coronary guidewire in the aorta allowed real-time IVUS imaging and guidance of stent positioning at the ostium (20). Both benchtop testing and a clinical case series have demonstrated that this technique may allow precise stent placement in AOLs. A 7Fr guide catheter may increase the risk of bleeding, and thus careful operation is required to reduce the risk of catheter damage. In the future, 3D transesophageal echocardiography may be useful in the assessment of stent protrusion during the interventions of AOLs (21). These techniques, however, are not particularly precise, and have not been widely adopted.
Limitations
Although all the operators were experienced, the inherent limitations of the study design introduced the possibility of bias in the procedure time and contrast volume. Intracoronary imaging (e.g., IVUS) may be more applicable to detect the aorto-ostial plane and increase procedural success, and may also be better than angiography to evaluate the efficacy of the stent positioning system. However, intracoronary imaging was not routinely used in this study, even though we had satisfactory angiographic results. Further study is needed to explore the efficacy of this system assessed by intracoronary imaging. Additionally, routine angiographic follow-up examinations were not performed in this study. Hence, the effects of the stent-positioning system applied on restenosis need to be explored in further investigations.
Conclusions
The dedicated stent positioning system with a simple nitinol device proposed in this study provides more accurate placement of stents for coronary AOLs than conventional methods. This approach is safe and effective and can be employed in stenting for AOLs.
Acknowledgments
We would like to thank AME Editing Service for their help in polishing our paper.
Funding: This study was financed by research grant from Beijing Advanced Medical Technologies, Ltd. And partially funded by National Key R&D Program of China 12 (No. 2020YFC2004800) and Beijing Nova Program (No. Z201100006820087).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-542/rc
Trial Protocol: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-542/tp
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-542/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-542/coif). SN reports research grants to the institution from Boston Scientific, Abbott, Jiangsu Hengrui Pharmaceuticals, China Resources Sanjiu Medical & Pharmaceuticals, and East China Pharmaceuticals. Qing Liu reports that he is the chairman of the Beijing Advanced Medical Technologies, Ltd. Inc. He has no role in study design and conduct, data management, interpretation of the results, or decisions for publication. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zampieri P, Colombo A, Almagor Y, et al. Results of coronary stenting of ostial lesions. Am J Cardiol 1994;73:901-3. [Crossref] [PubMed]
- Stewart JT, Ward DE, Davies MJ, et al. Isolated coronary ostial stenosis: observations on the pathology. Eur Heart J 1987;8:917-20. [Crossref] [PubMed]
- Koh KK, Hwang HK, Kim PG, et al. Isolated left main coronary ostial stenosis in Oriental people: operative, histopathologic and clinical findings in six patients. J Am Coll Cardiol 1993;21:369-73. [Crossref] [PubMed]
- Srinivas SK, Sunil B, Bhat P, et al. Incidence, predictors, clinical profile, management and outcome of patients with isolated left main coronary artery ostial disease. Indian Heart J 2018;70:214-9. [Crossref] [PubMed]
- Ragosta M, Boehm R, Shields M, et al. Intentional removal of erroneously deployed coronary stents: A case series and review of the literature. Catheter Cardiovasc Interv 2021;97:670-4. [Crossref] [PubMed]
- Aviram G, Shmilovich H, Finkelstein A, et al. Coronary ostium-straight tube or funnel-shaped? A computerized tomographic coronary angiography study. Acute Card Care 2006;8:224-8. [Crossref] [PubMed]
- Pan W, Xu H, Liu Q, Fan J. Comparison of Clinical Value between Right Distal Radial Artery Access and Right Radial Artery Access in Patients Undergoing Coronary Angiography or Percutaneous Coronary Intervention. Cardiovasc Innov Appl 2020;5:103-7. [Crossref]
- Mathias DW, Mooney JF, Lange HW, et al. Frequency of success and complications of coronary angioplasty of a stenosis at the ostium of a branch vessel. Am J Cardiol 1991;67:491-5. [Crossref] [PubMed]
- Attizzani GF, Capodanno D, Ohno Y, et al. Mechanisms, pathophysiology, and clinical aspects of incomplete stent apposition. J Am Coll Cardiol 2014;63:1355-67. [Crossref] [PubMed]
- Chetcuti SJ, Moscucci M. Double-wire technique for access into a protruding aorto-ostial stent for treatment of in-stent restenosis. Catheter Cardiovasc Interv 2004;62:214-7. [Crossref] [PubMed]
- Nguyen-Trong PJ, Martinez Parachini JR, Resendes E, et al. Procedural outcomes with use of the flash ostial system in aorto-coronary ostial lesions. Catheter Cardiovasc Interv 2016;88:1067-74. [Crossref] [PubMed]
- Taştan A, Özel E, Öztürk A, et al. Comparison of floating wire and single wire techniques in right coronary ostial lesions in terms of procedural features and one-year clinical follow-up results. Anatol J Cardiol 2015;15:830-5. [Crossref] [PubMed]
- Kern MJ, Ouellette D, Frianeza T. A new technique to anchor stents for exact placement in ostial stenoses: the stent tail wire or Szabo technique. Catheter Cardiovasc Interv 2006;68:901-6. [Crossref] [PubMed]
- Vaquerizo B, Serra A, Ormiston J, et al. Bench top evaluation and clinical experience with the Szabo technique: new questions for a complex lesion. Catheter Cardiovasc Interv 2012;79:378-89. [Crossref] [PubMed]
- Chan CK, Fung RC. "Sepal wire technique"--a novel technique for aorto-ostial left main stenting. J Invasive Cardiol 2011;23:211-2. [PubMed]
- Jaffe R, Halon DA, Shiran A, et al. Percutaneous treatment of aorto-ostial coronary lesions: Current challenges and future directions. Int J Cardiol 2015;186:61-6. [Crossref] [PubMed]
- Section of Interventional Cardiology, Chinese Society of Cardiology of Chinese Medical Association. Editorial Board of Chinese Journal of Cardiology. Chinese guideline for percutaneous coronary intervention(pocket guideline). Zhonghua Xin Xue Guan Bing Za Zhi 2012;40:271-7.
- Hsieh IC, Chen CC, Chang SH, et al. Acute and long-term outcomes of drug-eluting stent implantations in aorto-ostial, left anterior descending artery-ostial, and nonostial lesions. Catheter Cardiovasc Interv 2013;82:727-34. [Crossref] [PubMed]
- Ojeda S, Luque A, Pan M, et al. Percutaneous coronary intervention in aorto-ostial coronary chronic total occlusion: outcomes and technical considerations in a multicenter registry. Rev Esp Cardiol (Engl Ed) 2020;73:1011-7. [Crossref] [PubMed]
- Harding SA, Webber B, Fairley S, et al. Real-time intravascular ultrasound guidance: A novel technique for accurate placement of ostial stents. Catheter Cardiovasc Interv 2022;99:699-705. [Crossref] [PubMed]
- Arisha MJ, Hsiung MC, Ahmad A, et al. Incremental benefit of three-dimensional transesophageal echocardiography in the assessment of left main coronary artery stent protrusion. Echocardiography 2017;34:915-8. [Crossref] [PubMed]


