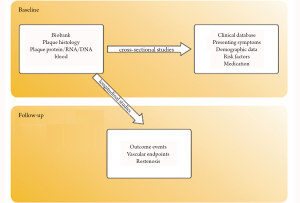
10-years experience with the Athero-Express study
Introduction and concept
Atherosclerotic disease is the most important cause of mortality in the Western society and the incidence is rapidly increasing in Eastern Europe, Asia and South America. With the growth of the ageing population, the incidence of the established risk factors for atherosclerotic disease, diabetes and kidney failure, is also rapidly increasing which will come with a further acceleration of cardiovascular (co) morbidity. In order to prevent life threatening events caused by atherosclerosis, such as myocardial infarction and stroke, identification of those who are at increased risk is warranted. Traditional risk factors such as age, gender, smoking, hypertension, diabetes, hypercholesterolemia and obesity can to some extent predict cardiovascular outcome on a population level (1). However, this is not sufficient to identify those individuals who require aggressive treatment and monitoring in order to prevent an impending atherosclerotic event. Although many studies have been performed in order to unravel the pathophysiology of atherosclerotic plaque destabilisation, this has had little influence on clinical management.
In treatment allocation for patients who already are known to have significant atherosclerotic disease, the nature of the underlying process, i.e. the composition of the atherosclerotic plaque, is not yet employed as a parameter that determines the necessity and modality of treatment. The main indication to perform an invasive arterial procedure is clinical symptoms with percentage luminal stenosis. In comparison to the field of oncology, this would be analogous to allocating treatment depending on tumor size only, making no difference between benign and malignant. This notion further clarifies the need for translation of previous fundamental research in the field of plaque biology towards clinical utility.
Previous pathology and animal studies have provided important insights in the association between plaque characteristics and the clinical presentation. Post-mortem studies in patients suffering acute coronary death revealed that the coronary occlusion did not occur as a result of gradual luminal narrowing, but rather as a result of disruption of an atherosclerotic plaque leading to acute thrombosis. Interestingly, most of these plaques did not cause a haemodynamically significant stenosis before the event. These plaques shared some important characteristics: large lipid core with a thin overlying fibrous cap, and marked presence of inflammatory cells, especially macrophages. Based on the results, it was postulated that, in thus far asymptomatic patients, this type of plaque would pose an increased risk of future rupture. These plaques were accordingly called “vulnerable plaques” (2-6). However, despite the fact that there is a clear association between a recent acute manifestation of cerebral or coronary artery disease and the features that define the vulnerable plaque, this does not imply that these features can also function as a surrogate for increased risk for events. High sensitivity or specificity values do not always go hand in hand with acceptable positive predictive and negative predictive values, measures that reflect the diagnostic value.
Local plaque determinants for local outcome
So far, the crucial missing link that should bring our understanding of plaque biology into the clinical setting has been the lack of prospective studies investigating the relation between plaque composition and future complications related to that plaque characteristic. We have to keep in mind that the aforementioned vulnerable plaque characteristics have been deduced from cross sectional studies, in which cause and consequence cannot be separated. Although it is reasonable to assume that the "vulnerable plaque" concept is valid, prospective clinical evidence is needed to validate these concepts. One hallmark study provided evidence that vulnerable plaque characteristics are indeed more frequently associated with local destabilization during follow up. Stone et al. reported that although nonculprit lesions that were responsible for unanticipated events were frequently angiographically mild, most were thin-cap fibroatheromas or were characterized by a large plaque burden, a small luminal area, or some combination of these characteristics, as determined by gray-scale and radiofrequency intravascular ultrasonography (7). These data support the idea that plaque characteristics may allow identification of those plaques at high risk to cause future cardiovascular events and thus provide a basis to optimize therapy allocation, as well as optimal choice of treatment modality.
Systemic determinants for systemic outcome
The identification for patients at risk for adverse cardiovascular events is not limited to the directly underlying pathological substrate, i.e. the atherosclerotic plaque. In a key paper, the concept of the “vulnerable patient” was introduced (8). This was defined as “patients in whom disruption of a vulnerable plaque is likely to result in a clinical event”. This concept also takes into account other factors such as thrombogenicety of the blood and vulnerability of the end organ to ischemia. Because detecting all vulnerable plaques in a patient is not feasible, the most commonly followed research approach to identify the vulnerable patient is by assessment of biomarkers in circulating blood that find their origin in pre-clinical basic research on atherosclerosis. Several studies have been performed on new inflammatory markers as well as markers for thrombogenecity. Predictive value of these approaches has been limited so far, even when using a multiple markers (9). This is probably due to expression of the targets in the blood is relatively low and obscured by larger production from other sources.
The Athero-Express concept: local plaque determinants for systemic outcome
Besides correlating local plaque composition with local thrombosis and systemic biomarkers with systemic outcome, there is a third option. Local plaque characteristics can serve as a fingerprint reflecting the stability or progression of the atherosclerotic process throughout the entire cardiovascular system in order and hence predict systemic vascular outcome (Figure 1). There is supporting evidence for this concept. Post mortem observations in patients who died following myocardial infarction revealed that next to the culprit plaque also other coronary plaques demonstrated significantly increased inflammation in comparison to controls (10). Thus, a single atherosclerotic plaque could provide information on the inflammatory status of plaques elsewhere in the vascular system (11).
The concept of using the characteristics of a local atherosclerotic plaque as predictor of systemic cardiovascular outcome is the main innovative feature that has been clearly demonstrated in the Athero-Express study. In this large longitudinal plaque biobank study we correlated baseline plaque composition of the dissected plaques to the occurrence of future cardiovascular events during follow up. The combination of systematic sample collection, large patient numbers and clinical follow-up allowed the identification of local plaque markers that predict adverse events in other vascular territories. Results of this follow up study will be discussed later in this paper.
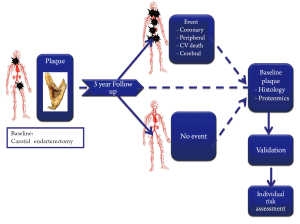
Different studies executed within the Athero-Express cohort
Apart from the main goal of predicting cardiovascular outcome based on local plaque composition, the Athero-Express study also facilitates a multitude of expression and other cross-seectional studies.
The current paper aims to describe the studies which have been conducted as a part of the Athero-Express biobank, from its beginning in 2002 until 2012. The design of the Athero-Express biobank will be described shortly, followed by the main observations until date. In addition, we will address the clinical perspectives and future directions of biobank studies.
Athero-Express study design
The Athero-Express study is an ongoing longitudinal cohort study, which includes patients undergoing carotid endarterectomy (CEA) in two Dutch hospitals: the University Medical Center Utrecht and St. Antonius Hospital Nieuwegein. The study protocol was approved by the medical ethics committee in both centers. All patients provide written informed consent. Both participating hospitals are high volume centers with longstanding experience in performing CEA. From the start of recruitment in April 2002, all consecutive patients undergoing CEA were asked to participate. By early 2012, more than 1700 patients have been included. Apart from the CEA study, we included patients undergoing femoral vascular procedures (N>550) and patients undergoing aneurysm surgery (n>450) in separate sub studies. An extensive description of the study protocol has been published previously (12). Below, the essentials of the study protocol will be discussed briefly (Figure 2).
Plaque characterisation
Histological assessment of the dissected atherosclerotic plaque is performed according to a standardized protocol on sections of the culprit segment for quantitative and semi-quantitative analysis of: macrophages (CD68), smooth muscle cells (alpha actin), and neovascularity (CD34 endothelial staining), and semi-quantitative analysis of collagen (picro-sirius), calcifications (H&E), luminal thrombus (H&E, EvG), intra-plaque bleeding (H&E, EvG) and lipid core size (H&E, picro-sirius). These measurements show good intra-observer and inter-observer reproducibility (13).
Protein and RNA are extracted from the plaques. The material is aliquoted and stored at –80 °C. A Blood sample is drawn from each patient at baseline. Cholesterol levels and hs-CRP are routinely assessed.
Clinical data
Each patient fills out an extensive questionnaire regarding medical history, symptoms of cardiovascular disease, medication use and life style. All patients are examined by a neurologist during the pre-operative work up. Furthermore, baseline clinical data are gathered from review of the medical records. Restenosis of the carotid artery is assessed by duplex sonography 1 year after CEA. The primary outcome iss defined as >70% restenosis (14).
The clinical outcome is defined as any major vascular event of vascular intervention during follow up. This includes any death of presumed vascular origin, non-fatal stroke, non-fatal myocardial infarction, and any arterial vascular intervention (both open surgery and endovascular procedures). All patients receive follow-up questionnaires each year after CEA for at least 3 years. If a questionnaire is answered positively, additional data (i.e. medical records, electrocardiograms, laboratory and imaging studies) are retrieved and assessed for the occurrence of an outcome event by two independent members of the study group, who are blinded for plaque analysis. If patients do not respond, their general practitioner is contacted. Furthermore, patient charts are reviewed for the occurrence of outcome events occurring peri-operatively and during hospital stay.
Main findings of the athero express study
Demographics
Based on the most recent data summary describing 1,385 patients, the mean age of the Athero-Express study population is 68 years (15). The majority (68%) are male. Most patients suffered from symptomatic carotid stenosis (TIA: 61%, stroke 24%), whereas 15% of the study population consisted of asymptomatic patients. There was a high prevalence of cardiovascular risk factors such as hypertension, hypercholesterolemia and smoking. Mean body mass index was 26.3, and 19% were diabetic. Most patients used aspirin (84%). In the older patient groups, use of coumarins and clopidogrel was more prevalent. The majority has also been prescribed statins prior to surgery (>70%).
Cross sectional studies
We investigated the association between composition of the atherosclerotic carotid plaques and the most relevant baseline clinical parameters. Below, the main findings from these cross sectional studies will be summarised.
Plaque characteristics and presenting symptoms (16)
In patients presenting with TIA or stroke we observed a higher prevalence of plaques with a large lipid core. Furthermore, plaque dissected from the symptomatic patients showed higher levels of the pro-inflammatory IL-8 and proteases MMP-8 and MMP-9. This was analogous to findings in post-mortem studies of the coronary arteries in patients who suffered coronary death. Surprisingly, patients presenting with amaurosis fugax (AFX; ocular ischemic symptoms) did not differ from the asymptomatic group with regard to plaque composition and IL-8 and MMP levels. This demonstrates that patients presenting with AFX represent a separate pathophysiological entity that merit careful consideration when included in clinical studies.
Interval between symptoms and CEA (17)
Plaque composition (histology, inflammatory mediators, MMPs and caspase) was investigated in relation to the time span between the most recent cerebrovascular event (attributed to the carotid artery stenosis) and CEA. By doing so, the natural history of changes in plaque composition after an event could be studied. This study revealed that macrophage infiltration gradually decreased after the event (especially after stroke), which was preceded by a strong >50% decrease in IL-6, IL-8 and Caspase levels. Overall the data suggested that plaques stabilize following an acute thrombotic event.
Age
Patients presenting with significant carotid artery stenosis with advanced age have a larger lipid pool, and less smooth muscle cells in their dissected plaques, compared to younger patients (15). Notably, advancing age was also associated with calcification and intraplaque hemorrhage, while gender and symptom status are not. Furthermore, there was a borderline significant increase of MMP-9 with advancing age, whereas activity of stabilizing MMP-2 was lower in elderly patients (18). Overall, elderly patients have a slightly more "vulnerable" plaque phenotype compared to younger patients.
Gender (19)
Female patients have more stable, less inflammatory plaques compared with men. Overall, the size of the lipid pool and macrophage infiltration are greater in males, while the difference in infiltration of smooth muscle cells is less gender related. Especially asymptomatic women have very stable plaques, with small lipid pools, and large smooth muscle cell and collagen content. IL-8 and MMP-8 levels are statistically significant lower in plaques that were harvested in females.
Emboli during CEA (20)
A large lipid pool in the excised carotid plaque was associated with increased risk of peri-operative adverse neurovascular events. In these plaques, the number of emboli as measured by per-operative transcranial Doppler monitoring, was higher during the dissection phase (i.e. before the carotid artery is clamped), although this was not statistically significant. However, during the other per, - and post operative phases a higher number of emboli was observed in the patient group with fibrous plaques. The most likely explanation for these findings is that the origin of the emboli alters during surgery: debris is dislodged from plaques during the dissection phase which, may more occur in vulnerable, lipid rich plaques, in comparison to air emboli and newly formed platelet aggregates which become apparent during clamp release and wound closure phases.
Restenotic plaques: early and late restenosis primary plaques
Restenosis occurs in a substantial proportion of patients after CEA. Plaques excised from patients who experienced early recurrent stenosis after previous CEA showed a markedly different plaque composition compared to primary plaques, being fibrous with high content of smooth muscle cells. On the other hand, the plaque composition in late restenosis (>5 years after previous CEA) was not different from primary plaques.
Medication
Somewhat unexpectedly, we did not observe decreased macrophage infiltration in plaques dissected from patients receiving statins compared with patients who had not been treated with statins prior to surgery. Interestingly, we did observe that the activation of macrophages in terms of cytokine production and MMP-9 was lower in patients on statin therapy (21). These results require careful interpretation since selection bias and subsequent confounding may be an issue comparing patients with and without statin prescriptions prior to surgery. In a study performed more recently, we also observed that statin use was associated with decreased incidence of intraplaque bleeding, while coumarin use was associated with increased incidence of intraplaque bleeding (22).
Other cross-sectional studies and expression studies
Alcohol use has been suggested to be beneficial for patients suffering from atherosclertotic disease but data are not consistent. The association between alcohol consumption, plaque characteristics and clinical outcome was investigated among patients undergoing CEA and patients undergoing femoral vascular surgery. In femoral patients, alcohol consumption was associated with lower amount of plaque lipid and inflammation, and better overall outcome (23). In the CEA cohort, none of these associations were present.
Other studies correlated stages of intra plaque hemorrhage and neutrophil influx with the other histological plaque characteristics (24,25). The expression of many proteins that could play a role in plaque destabilisation have been studied in relation to plaque composition in the Athero-Express biobank among which are: Forkhead box protein P1 (26), Telomer length (27), Neutrophil gelatinase associated lipocalin (28), hyaluronic acid (29), myeloid-related protein 8/14 (30), TGF-beta and endoglin (31), caveolin-1 (32), agiopoietin-1 and -2 (33), fibronectin extra domain A (34), Nogo-B (35), and MMP-2,8,9 (36).
Longitudinal follow-up studies
Plaque composition and clinical outcome (37)
As described above, the most novel concept of the Athero-Express study is the investigation of atherosclerotic plaque characteristics in relation to systemic clinical outcome. For this purpose, we examined data obtained from 1,002 patients undergoing carotid endarterectomy. From this patient group, long term follow-up was available in 818 patients. The main finding was that plaque hemorrhage and plaque neovascularisation significantly related to adverse cardiovascular outcome during clinical follow-up. Patients with plaque hemorrhage revealed a cumulative risk of 30.6% of a secondary vascular event, compared to 17.2% in patients who did not show plaque hemorrhage at the culprit lesion. Further analyses showed that the association was both evident for stroke as well as non-stroke cardiovascular events (i.e. myocardial infarction, peripheral vascular intervention). The relation between plaque composition and clinical outcome remained unaltered in multivariable analysis adjusting for multiple potential confounders. The other plaque characteristics, such as calcifications, and macrophage infiltration, were not associated with clinical events in other vascular territories.
This study was the first to show that the local atherosclerotic plaque houses prognostic information for the occurrence of future vascular events in any vascular territory. Further, it supports the hypothesis that plaque vascularization and plaque hemorrhage are an important source of plaque progression, as well as an important mechanism of plaque disruption leading to atherothrombotic events (38-41).
Plaque composition and restenosis (42)
We also studied if baseline plaque composition was related with restenosis development in 500 patients. This patient number was reached, following a power analyses, at March 14, 2006, within four years of the start of inclusion. Plaque histology of the dissected plaque was related to restenosis 1 year after the intervention, measured by duplex ultrasound.
At 1 year after CEA, 17% of patients developed >50% restenosis and 8% developed >70% restenosis. Marked plaque macrophage infiltration and a large lipid pool independently showed an inverse relation with the occurrence of future restenosis. Patients having plaques with marked macrophage infiltration showed a risk of 4.5% for developing >70% restenosis, compared to 12.6% in the patients who showed plaques with low macrophage numners. Large lipid core (>40%) was associated with 5.6% risk of >70% restenosis, compared to 14.9% in patients having plaques with very small (<10%) or no lipid pools. In multivariable analysis, this predictive value was independent of age, sex, clinical presentation and patch use.
The explanation for the relation between the composition of a dissected plaque, and restenosis of the operated artery seems difficult to comprehend. The underlying mechanism is not likely to be related to remainders of the plaque in operated carotid artery, because a thin layer of the media was present in the majority of specimen. Medial and adventitial cells also play an important role in atherosclerosis lesion progression. The inflammatory status of these layers has been related to plaque inflammation and we hypothesized that different composition of the medial and adventitial layers (e.g., presence of inflammatory cells and proteolytic activity) could lead to a different remodeling response after CEA.
To further explore the mechanisms behind this difference, we performed a small exploratory follow-up study in which we measured the two parameters determining restenosis: neo-intima formation and vessel constrictive remodeling. This substudy showed that the dissection of an inflammatory, lipid rich plaque was often followed by a tendency towards decreased neo-intima formation and increased expensive remodeling, both protective of restenosis.
Local Plaque protein targets that are related with systemic events
Matrix metalloproteinases are important in the homeostatis of the extracellular matrix. Over-expression of certain MMPs, especially MMP-9, has been implied as a major factor contributing to degradation of the fibrous cap and thereby to atherosclerotic plaque rupture (43). Interestingly, local MMP-2 and MMP-9 plaque expressions did not show any association with clinical outcome, whereas increased MMP-8 activity was significantly associated with the occurrence of adverse cardiovascular events during follow-up (44).
Proteomics studies of local plaques in relation to clinical outcome
Building upon the prospective design of the Athero-Express study, we executed proteomics analyses, in order to be able to identify novel protein targets related to vascular outcome. The following approach was followed: from our study cohort, we randomly selected 100 patients who suffered from an event during follow-up. We then selected matched control patients who did not suffer an event. The plaque proteins of these groups were pooled and subjected to proteomics analysis, in order to find proteins expressed differently between the two groups. The predictive value of these differentially expressed proteins was validated in the whole study cohort, as well as in the femoral study cohort.
Following this approach, we succeeded to identify a list of several proteins with differential expression between cases and controls, and therefore with potential predictive value with regard to future secondary manifestations of atherosclerotic disease. The process of validation in the study cohort has been completed for a number of proteins, including Osteopontin (OPN), fatty acid binding protein 4 (FABP4), and myeloid-related protein (MRP) 8/14.
Osteopontin, also known as early T-lymphocyte activation 1, is an excreted protein that has many functions, including inflammatory modulation, and is expressed by macrophages (45,46). The validation of OPN in the carotid study cohort showed that there was an expression dependent increase in future vascular events with increasing plaque OPN levels (47). Patients having plaques with OPN expression in the highest quartile had a 4-fold increased risk of future cardiovascular events compared to patients who showed OPN plaque levels in the lowest quartile. The relation between OPN and outcome was observed in stroke, coronary and peripheral vascular events. Furthermore, external validation in the femoral cohort confirmed the predictive value of plaque OPN for vascular outcome. FABP4 and MRP 8/14 also showed statically significant association with clinical outcome in the validation studies (48,49).
Genomics
Research on the association between the genetic signature and plaque phenotyes is ongoing in the Athero-Express study. DNA from 974 carotid plaques and 836 femoral samples has been extracted to perform a genome-wide association study. For this purpose, the Affymetrix genome wide single nucleotide polymorphism (SNP) array has already been used in 600 patients, which can detect 500,586 SNPs and features 420,000 non-polymorphic probes that can measure copy number variations. The Athero-Express will allow identification of SNPs that are associated with plaque characteristics which would be an excellent tool for validation of molecular targets that are thought to be involved in atherosclerotic disease progression. This study is in progress, and results from the GWAS study can be expected in the near future.
Clinical implications and future perspectives
The Athero-Express vascular biobank has become a successful project over the past 10 years, that allowed studies of human atherosclerotic plaque composition in an unprecedented number of patients. The large patient numbers and the clinical follow-up resulted for the first time in the establishment of the relation between atherosclerotic local plaque composition and clinical events in all vascular territories. This relation between local plaque markers and systemic events was both established in terms of histology - plaque hemorrhage and neovascularity being linked to adverse vascular outcome - and protein expression levels, where we discovered new predictive plaque biomarkers by the comparison of plaques from patients with future vascular events with plaques from controls by means of proteomics. Furthermore, the biobank has allowed the cross sectional study of plaques in relation to clinical determinants relevant to the benefit of vascular procedures and has provided important insight as to how plaque composition might influence the benefit and choice of vascular procedures.
Plaque biobanking - influence on choice of vascular interventions
The benefit of CEA is attributed to excision of the plaque that could be a source of future embolization. Therefore, it is reasonable to assume that excision of a "high risk" plaque that is highly likely to cause future embolization and associated clinical events portrays more benefit than excision of a stable, low risk plaque. The results from the Athero-Express study show that patients groups that benefit most from CEA, i.e. patients with advanced age, male gender, symptomatic presentation, with short time between presenting symptoms and CEA (50), have the highest prevalence of high risk plaques (plaques containing large lipid pools, high macrophage infiltration, and decreased smooth muscle cell content). The finding that patient groups with high benefit from CEA possess more "high risk" plaques confirms our hypothesis that differences in benefit from CEA are attributable to differences in carotid plaque phenotype. As stated in the introduction, plaque composition is currently not taken into account in clinical decision making. Our findings strongly suggest that plaque composition is an important determinant of the outcome of CEA. It should therefore be considered to routinely study plaque specimen at a pathology department when samples have been dissected.
Furthermore, data from clinical studies indicate that carotid artery stenting has a relatively high risk of peri-interventional embolic complications in elderly patients (51-54). Once again, the data from our biobank may provide important clues as to the underlying mechanism. Advancing age is related with a larger lipid pool, and a lower content of smooth muscle cells. These “vulnerable” plaques may be more prone to serious disruption by manipulation with guide wires and stent placement, leading to adverse events. In contrast, during CEA the internal carotid artery is clamped, which can prevent embolization in these patients.
On the other hand, carotid artery stenting seems a more appealing alternative in patients with fibrous plaques and patient with (early) restenotic plaques (55,56). Fibrous plaques show an increased risk of restenosis after CEA compared to lipid rich plaques, which is partly due to constrictive remodelling. Transcranial Doppler monitoring has shown that stenting results in a multitude of embolic signals compared to CEA. Biasi and colleages have shown that the extent of CAS related embolic signals is related to pre-procedural ultrasound measurement of plaque echogenicity, which is correlated with the size op the plaque lipid core (51,52). Plaques with a small or absent lipid core are thus less likely to produce embolization during CAS than lipid rich plaques.
Overall, our results suggest pre-clinical assessment of plaque composition could facilitate stratification of patients for either surgical or non invasive treatment. High risk plaques could be treated more aggressively (i.e. also when causing less then 70% or even less than 50% stenosis), preferably with CEA, while low risk plaques should be treated more conservatively and preferably with stenting. This hypothesis should be investigated in the future when imaging techniques are widely available to routinely assess plaque characteristics before surgery or stenting.
Using non-invasive pre-operative imaging, such as MRI, it is possible to determine lipid content and thrombus (57). Clinical studies correlating pre-interventional plaque composition to choice of treatment modality and clinical outcome are warranted to validate these findings.
Plaque biobanking and prediction of systemic outcome
The Athero-Express study is the first study to relate atherosclerotic plaque characteristics to systemic cardiovascular outcome. In this approach, the atherosclerotic plaque, excised during vascular surgery procedures, serves as a fingerprint of the entire vascular system, in order to determine the vulnerability for future vascular events.
This approach showed that the local atherosclerotic plaque indeed holds prognostic information with regard to local disease progression (i.e. restenosis) as well as systemic cardiovascular outcome during follow-up.
Non-invasive markers: circulating markers, molecular imaging
The patient group undergoing vascular surgery is limited in size compared to the entire patient group experiencing atherosclerosis. Therefore, plaque based biomarkers can only be applied to a limited patient group. In order to extend the clinical utility, these plaque-based biomarkers should be detected non-invasively. Molecular imaging could be a helpful tool for this purpose. Several imaging techniques, such as SPECT and MRI are potentially able to detect specific protein targets in plaques using contrast agents coupled to specific antibodies (58,59). Unfortunately, at present, most of these techniques are in a pre-clinical stadium, and not ready to be used in large population based studies. Intra plaque hemorrhage can be visualized using MRI, specifically using contrast agents, and can be used to show any association between these plaque feautures and subsequent risk for cardiovascular events (60).
New computer aided techniques
In addition to simple comparison of protein expression between different patients groups, advancing computer algorithms allow advanced correlation of expression patterns in relation to patient characteristics and outcome using novel techniques using artificial intelligence and network analyses. This work is ongoing and may provide new insights in the near future.
In conclusion, plaque biobanking in the Atherp-Express study has resulted in a number of observations that help to understand the associations between clinical characteristics and plaque phenotype. In addition, many plaque proteins have been studied and associated with plaque characteristics, clinical presentation and outcome during follow up. The magnitude of the biobank allows the execution of association studies with sufficient statistical power on many characteristics and also the possibility to adjust for confounding.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 1951;41:279-81.[LinkOut]
- Burke AP, Farb A, Malcom GT, et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997;336:1276-82.[LinkOut]
- Davies MJ. The pathophysiology of acute coronary syndromes. Heart 2000;83:361-6.[LinkOut]
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657-71.[LinkOut]
- Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol 1997;17:1859-67.[LinkOut]
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664-72.[LinkOut]
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35.[LinkOut]
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation 2003;108:1772-8.[LinkOut]
- Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 2006;355:2631-9.[LinkOut]
- Mauriello A, Sangiorgi G, Fratoni S, et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol 2005;45:1585-93.[LinkOut]
- Hellings WE, Peeters W, Moll FL, et al. From vulnerable plaque to vulnerable patient: the search for biomarkers of plaque destabilization. Trends Cardiovasc Med 2007;17:162-71.[LinkOut]
- Verhoeven BA, Velema E, Schoneveld AH, et al. Athero-express: differential atherosclerotic plaque expression of mRNA and protein in relation to cardiovascular events and patient characteristics. Rationale and design. Eur J Epidemiol 2004;19:1127-33.[LinkOut]
- Hellings WE, Pasterkamp G, Vollebregt A, et al. Intraobserver and interobserver variability and spatial differences in histologic examination of carotid endarterectomy specimens. J Vasc Surg 2007;46:1147-54.[LinkOut]
- Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: grayscale and Doppler US diagnosis--Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229:340-6.[LinkOut]
- van Lammeren GW, Reichmann BL, Moll FL, et al. Atherosclerotic plaque vulnerability as an explanation for the increased risk of stroke in elderly undergoing carotid artery stenting. Stroke 2011;42:2550-5.[LinkOut]
- Verhoeven B, Hellings WE, Moll FL, et al. Carotid atherosclerotic plaques in patients with transient ischemic attacks and stroke have unstable characteristics compared with plaques in asymptomatic and amaurosis fugax patients. J Vasc Surg 2005;42:1075-81.[LinkOut]
- Peeters W, Hellings WE, de Kleijn DP, et al. Carotid atherosclerotic plaques stabilize after stroke: insights into the natural process of atherosclerotic plaque stabilization. Arterioscler Thromb Vasc Biol 2009;29:128-33.[LinkOut]
- van Oostrom O, Velema E, Schoneveld AH, et al. Age-related changes in plaque composition: a study in patients suffering from carotid artery stenosis. Cardiovasc Pathol 2005;14:126-34.[LinkOut]
- Hellings WE, Pasterkamp G, Verhoeven BA, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J Vasc Surg 2007;45:289-96; discussion 296-7.[LinkOut]
- Verhoeven BA, de Vries JP, Pasterkamp G, et al. Carotid atherosclerotic plaque characteristics are associated with microembolization during carotid endarterectomy and procedural outcome. Stroke 2005;36:1735-40.[LinkOut]
- Verhoeven BA, Moll FL, Koekkoek JA, et al. Statin treatment is not associated with consistent alterations in inflammatory status of carotid atherosclerotic plaques: a retrospective study in 378 patients undergoing carotid endarterectomy. Stroke 2006;37:2054-60.[LinkOut]
- Derksen WJ, Peeters W, Tersteeg C, et al. Age and coumarin-type anticoagulation are associated with the occurrence of intraplaque hemorrhage, while statins are associated less with intraplaque hemorrhage: a large histopathological study in carotid and femoral plaques. Atherosclerosis 2011;214:139-43.[LinkOut]
- Gisbertz SS, Derksen WJ, de Kleijn DP, et al. The effect of alcohol on atherosclerotic plaque composition and cardiovascular events in patients with arterial occlusive disease. J Vasc Surg 2011;54:123-31.[LinkOut]
- Derksen WJ, Peeters W, van Lammeren GW, et al. Different stages of intraplaque hemorrhage are associated with different plaque phenotypes: a large histopathological study in 794 carotid and 276 femoral endarterectomy specimens. Atherosclerosis 2011;218:369-77.[LinkOut]
- Ionita MG, van den Borne P, Catanzariti LM, et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol 2010;30:1842-8.[LinkOut]
- Bot PT, Grundmann S, Goumans MJ, et al. Forkhead box protein P1 as a downstream target of transforming growth factor-β induces collagen synthesis and correlates with a more stable plaque phenotype. Atherosclerosis 2011;218:33-43.[LinkOut]
- Huzen J, Peeters W, de Boer RA, et al. Circulating leukocyte and carotid atherosclerotic plaque telomere length: interrelation, association with plaque characteristics, and restenosis after endarterectomy. Arterioscler Thromb Vasc Biol 2011;31:1219-25.[LinkOut]
- te Boekhorst BC, Bovens SM, Hellings WE, et al. Molecular MRI of murine atherosclerotic plaque targeting NGAL: a protein associated with unstable human plaque characteristics. Cardiovasc Res 2011;89:680-8.[LinkOut]
- Bot PT, Pasterkamp G, Goumans MJ, et al. Hyaluronic acid metabolism is increased in unstable plaques. Eur J Clin Invest 2010;40:818-27.[LinkOut]
- Ionita MG, Vink A, Dijke IE, et al. High levels of myeloid-related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol 2009;29:1220-7.[LinkOut]
- Bot PT, Hoefer IE, Sluijter JP, et al. Increased expression of the transforming growth factor-beta signaling pathway, endoglin, and early growth response-1 in stable plaques. Stroke 2009;40:439-47.[LinkOut]
- Rodriguez-Feo JA, Hellings WE, Moll FL, et al. Caveolin-1 influences vascular protease activity and is a potential stabilizing factor in human atherosclerotic disease. PLoS One 2008;3:e2612.[LinkOut]
- Post S, Peeters W, Busser E, et al. Balance between angiopoietin-1 and angiopoietin-2 is in favor of angiopoietin-2 in atherosclerotic plaques with high microvessel density. J Vasc Res 2008;45:244-50.[LinkOut]
- van Keulen JK, de Kleijn DP, Nijhuis MM, et al. Levels of extra domain A containing fibronectin in human atherosclerotic plaques are associated with a stable plaque phenotype. Atherosclerosis 2007;195:e83-91.[LinkOut]
- Rodriguez-Feo JA, Hellings WE, Verhoeven BA, et al. Low levels of Nogo-B in human carotid atherosclerotic plaques are associated with an atheromatous phenotype, restenosis, and stenosis severity. Arterioscler Thromb Vasc Biol 2007;27:1354-60.[LinkOut]
- Sluijter JP, Pulskens WP, Schoneveld AH, et al. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke 2006;37:235- 9.[LinkOut]
- Hellings WE, Peeters W, Moll FL, et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation 2010;121:1941-50.[LinkOut]
- Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316-25.[LinkOut]
- Michel JB, Virmani R, Arbustini E, et al. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J 2011;32:1977-85, 1985a, 1985b, 1985c.
- Kockx MM, Cromheeke KM, Knaapen MW, et al. Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23:440-6.[LinkOut]
- Arbustini E, Morbini P, D’Armini AM, et al. Plaque composition in plexogenic and thromboembolic pulmonary hypertension: the critical role of thrombotic material in pultaceous core formation. Heart 2002;88:177- 82.[LinkOut]
- Hellings WE, Moll FL, De Vries JP, et al. Atherosclerotic plaque composition and occurrence of restenosis after carotid endarterectomy. JAMA 2008;299:547-54.[LinkOut]
- Galis ZS, Sukhova GK, Lark MW, et al. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994;94:2493-503.[LinkOut]
- Peeters W, Moll FL, Vink A, et al. Collagenase matrix metalloproteinase-8 expressed in atherosclerotic carotid plaques is associated with systemic cardiovascular outcome. Eur Heart J 2011;32:2314-25.[LinkOut]
- Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 2007;27:2302-9.[LinkOut]
- O’Regan A. The role of osteopontin in lung disease. Cytokine Growth Factor Rev 2003;14:479-88.[LinkOut]
- de Kleijn DP, Moll FL, Hellings WE, et al. Local atherosclerotic plaques are a source of prognostic biomarkers for adverse cardiovascular events. Arterioscler Thromb Vasc Biol 2010;30:612-9.[LinkOut]
- Ionita MG, Catanzariti LM, Bots ML, et al. High myeloid-related protein: 8/14 levels are related to an increased risk of cardiovascular events after carotid endarterectomy. Stroke 2010;41:2010-5.[LinkOut]
- Peeters W, de Kleijn DP, Vink A, et al. Adipocyte fatty acid binding protein in atherosclerotic plaques is associated with local vulnerability and is predictive for the occurrence of adverse cardiovascular events. Eur Heart J 2011;32:1758-68.[LinkOut]
- Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363:915-24.[LinkOut]
- International Carotid Stenting Study investigators, Ederle J, Dobson J, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 2010;375:985-97.[LinkOut]
- Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol 2008;7:893-902.[LinkOut]
- Mas JL, Trinquart L, Leys D, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol 2008;7:885-92.[LinkOut]
- Hobson RW 2nd, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg 2004;40:1106-11.[LinkOut]
- Grønholdt ML, Nordestgaard BG, Wiebe BM, et al. Echo-lucency of computerized ultrasound images of carotid atherosclerotic plaques are associated with increased levels of triglyceride-rich lipoproteins as well as increased plaque lipid content. Circulation 1998;97:34-40.[LinkOut]
- Biasi GM, Froio A, Diethrich EB, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation 2004;110:756-62.[LinkOut]
- Vancraeynest D, Pasquet A, Roelants V, et al. Imaging the vulnerable plaque. J Am Coll Cardiol 2011;57:1961-79.[LinkOut]
- Briley-Saebo KC, Shaw PX, Mulder WJ, et al. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation 2008;117:3206-15.[LinkOut]
- Davies JR, Rudd JH, Weissberg PL, et al. Radionuclide imaging for the detection of inflammation in vulnerable plaques. J Am Coll Cardiol 2006;47:C57-68.[LinkOut]
- Staub D, Schinkel AF, Coll B, et al. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging 2010;3:761-71.[LinkOut]