
Branched endovascular iliac artery repair using the Zenith® Branch Endovascular Iliac Bifurcation graft: outcomes and reinterventions in our retrospective cohort
Highlight box
Key findings
• Endovascular treatment using the ZBIS device was performed in 63 patients with iliac artery aneurysms and we observed no in-hospital mortality. The internal iliac artery’s diameter and a prior abdominal aortic intervention were identified as significant variables in the competing risk regression model for a reintervention.
What is known and what is new?
• ZBIS is a feasible and safe treatment option for patients with iliac aneurysms.
• We identified the internal iliac artery’s diameter as a significant predictor for a secondary intervention.
What is the implication, and what should change now?
• We recommend close follow-up of patients following branched iliac artery repair.
Introduction
Endovascular repair of aneurysms extending beyond the iliac bifurcation remains a therapeutic challenge (1,2). One option to create sufficient distal landing zone is to intentionally occlude the internal iliac artery with either a plug or coil and deploy the stent graft into the external iliac artery. It is, however, an approach that has resulted in significant morbidity including impotence and claudication (3,4). Moreover, it may compromise the collateral spinal blood flow and complicate potential future aortic repair by increasing the risk for spinal cord ischemia (5). The use of iliac branch devices has, therefore, become an effective treatment option by preserving antegrade blood flow into the internal iliac artery (3,4).
The aims of this study were to analyze outcomes after endovascular iliac branch repair for aneurysms of the iliac arteries extending beyond the iliac bifurcation, and to evaluate the secondary interventions and associated outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-564/rc).
Methods
Patients and follow-up protocol
Our retrospective cohort study investigates patients who underwent branch repair for aneurysms of the iliac arteries using the Zenith® Branch Endovascular Iliac Bifurcation (ZBIS; Cook Medical Europe Ltd., Limerick, Ireland) graft device between 07/2013 and 11/2020 in one single center, which is a large university hospital. All procedures were carried out by or under direct supervision of one of two endovascular experts. All patients, which received the ZBIS stentgraft during this period in our center were included in the study. All patients underwent preoperative computed tomography angiography (CTA). Postoperative follow-up included CTA before discharge, if the renal function was acceptable and ultrasound at 6 and 12 months, and yearly thereafter. If the ultrasound was not diagnostic, a CTA was obtained.
The primary outcomes of this study were in-hospital death, technical success and long-term patency rates after ZBIS implantation and the secondary outcomes of this study were risk factors for reinterventions.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our institutional review board (IRB) approved this study, and the need for informed consent was waived due to the retrospective nature of the study (IRB number: 20-1302; approval date: February 4, 2021).
Endovascular approach
ZBIS implantation follows the manufacturer’s instructions for use, and this procedure is routine in our hybrid operating room. It is carried out by a board-certified vascular surgeon and a surgical resident. Nowadays, we routinely access the femoral arteries percutaneously via pre-closure techniques (Proglide, Abbott Medical, Chicago, Illinois, USA), while open surgical cut-down was more common at the beginning of the study period.
Definition of parameters and data collection
Data were collected retrospectively using our center’s prospectively maintained databases. We evaluated baseline and characteristics, previous aortic procedures, intraoperative details, clinical outcomes, and follow-up data. Diameter progression was defined as an increase in total iliac diameter of more than 5 mm within 6 months. Immediate technical success was defined as exclusion of the iliac aneurysm with preservation of the internal iliac artery and the absence of malposition or migration of the stent-graft or conversion to open repair.
Statistical analysis
Data are presented as absolute and relative frequency or as median [first quartile, third quartile]. A student t-test or the Mann-Whitney U test were used to compare continuous variables as appropriate. Categorical variables were compared using the χ2 test by calculating exact values. In case of small group sizes (n<5), Fisher’s exact test was used. A competing risk analysis was performed to analyze the influence of clinically selected variables (age, connective tissue disease, previous infrarenal aortic intervention, diagnosis of an intraoperative endoleak, and the internal iliac artery diameter) on the risk for reintervention. A P value of <0.05 was considered to represent a statistically significant difference. Statistical analysis was conducted using IBM SPSS Statistic 21.0 (IBM-SPSS Inc., Armonk, NY, USA) and R version 3.5.1 (The R Foundation for Statistical Computing, Austria).
Results
Patient characteristics
A total of 63 patients were included in our study. Mean [± standard deviation (SD)] age was 73±11 years. The majority of the aneurysms were felt to be degenerative in nature, except in two patients (3%) who had a well-established diagnosis of Marfan’s syndrome. The indications for treatment were common iliac aneurysm in 62 (98%) patients, 20 (32%) of them bilateral and internal iliac aneurysm in 32 (51%) patients, 5 (8%) of them bilateral. The underlying pathologies are summarized in Table 1. Five patients (8%) had already undergone open abdominal-aorta surgery, while ten patients (16%) had undergone prior endovascular aortic repair (EVAR). All patient demographics and risk factors are summarized in Table 1.
Table 1
| Characteristics | All patients (n=63) | No reintervention (n=40) | Reintervention (n=23) | P |
|---|---|---|---|---|
| Age (years) | 73 [64, 78] | 74 [64, 78] | 74 [65, 78] | 0.372 |
| Male | 61 [97] | 40 [100] | 21 [91] | 0.130 |
| Diabetes mellitus type 2 | 3 [5] | 2 [5] | 1[4] | 1.000 |
| Hyperlipidemia | 28 [44] | 19 [48] | 9 [39] | 0.603 |
| Hypertension | 48 [76] | 33 [83] | 15 [65] | 0.137 |
| Coronary artery disease | 30 [48] | 21 [53] | 9 [39] | 0.432 |
| History of smoking | 10 [16] | 7 [18] | 3 [13] | 0.734 |
| Smoking | 10 [16] | 8 [20] | 2 [9] | 0.302 |
| COPD | 9 [14] | 5 [13] | 4 [17] | 0.713 |
| History of stroke | 5 [8] | 3 [8] | 2 [9] | 1.000 |
| Dialysis | 1 [2] | 1 [3] | 0[0] | 1.000 |
| Connective tissue disease | 2 [3] | 1 [3] | 1[4] | 1.000 |
| AAA operation before | 15 [24] | 11 [28] | 4 [17] | 0.540 |
| Open surgery | 5 [8] | 4 [10] | 1 [4] | 0.424 |
| EVAR | 10 [16] | 4 [10] | 6 [26] | 0.093 |
| Maximal diameters in mm | – | |||
| CIA right | 35 [24, 39] | 34 [25, 37] | 37 [25, 39] | |
| CIA left | 33 [26, 39] | 32 [24, 40] | 33 [27, 39] | |
| IIA right | 27 [23, 37] | 26 [22, 32] | 33 [24, 42] | |
| IIA left | 29 [24, 36] | 28 [21, 31] | 35 [30, 42] | |
| Abdominal aorta | 46 [36, 53] | 47 [39, 53] | 43 [31, 54] | |
| Pathology | – | |||
| Aortoiliac aneurysm | 30 [48] | 17 [43] | 13 [57] | |
| Concomitant IIA aneurysm | 12 [19] | 6 [15] | 6 [26] | |
| Unilateral | 16 [25] | 10 [25] | 6 [26] | |
| Bilateral | 14 [22] | 7 [18] | 7 [30] | |
| Isolated iliac aneurysm | 30 [48] | 21 [53] | 9 [39] | – |
| Concomitant IIA aneurysm | 13 [21] | 9 [23] | 4 [17] | |
| Unilateral | 22 [35] | 15 [38] | 7 [30] | |
| Bilateral | 8 [13] | 6 [15] | 2 [9] | |
| Isolated IIA aneurysm | 3 [5] | 2 [5] | 1 [4] | – |
Values are presented as n [%] or median [first quartile, third quartile] or mm. COPD, chronic obstructive pulmonary disease; AAA, abdominal aortic aneurysm; EVAR, endovascular abdominal aortic repair; CIA, common iliac artery; IIA, internal iliac artery.
Periprocedural details
A total of 71 ZBIS stent-grafts were implanted. Eight patients (13%) required bilateral ZBIS implantation and 40 patients (63%) underwent additional EVAR to treat an aneurysm of the infrarenal aorta or to gain a sufficient proximal landing zone. In the majority of cases, we used the femoral arteries as access vessels, whereas the subclavian artery was employed in ten patients (16%). Median fluoroscopy time was 35±25 minutes. A perioperative endoleak was present and accepted in 20 patients (32%). Nine of these patients required a reintervention during follow-up, four during the same hospital stay and five after discharge. Of these reinterventions after discharge, only one was caused by the 20 before mentioned accepted endoleaks, the others were staged procedures or a thrombosis of the stentgraft, mainly internal iliac artery. All periprocedural details are illustrated in Table 2.
Table 2
| Intervention details | All patients (n=63) | No reintervention (n=40) | Reintervention (n=23) | P |
|---|---|---|---|---|
| ZBIS right | 32 [51] | 18 [45] | 14 [61] | 0.192 |
| ZBIS left | 39 [62] | 27 [68] | 12 [48] | 0.276 |
| ZBIS bilateral | 8 [13] | 5 [13] | 3 [5] | 0.950 |
| Abdominal aortic stent-graft | 40 [63] | 27 [68] | 13 [56] | 0.778 |
| Access | ||||
| Left CFA cut down | 20 [32] | 9 [23] | 11 [48] | 0.051 |
| Left CFA percutaneous | 37 [59] | 28 [70] | 9 [39] | 0.020 |
| Right CFA cut down | 15 [24] | 8 [20] | 7 [30] | 0.373 |
| Right CFA percutaneous | 34 [54] | 25 [63] | 9 [39] | 0.115 |
| Subclavian artery | 10 [16] | 5 [13] | 5 [22] | 0.476 |
| X-ray time in minutes | 35 [23, 49] | 35 [16, 47] | 35 [28, 57] | 0.221 |
| Endoleak perioperative | ||||
| Ia | 4 [6] | 1 [3] | 3 [13] | 0.134 |
| Ib IIA | 1 [2] | 0 [0] | 1 [4] | 0.365 |
| Ib CIA | 0 [0] | 0 [0] | 0 [0] | – |
| II | 12 [19] | 8 [20] | 4 [17] | 1.000 |
| III | 3 [5] | 3 [8] | 0 [0] | 0.293 |
Values are presented as n [%] or median [first quartile, third quartile]. ZBIS, Zenith® Branch Endovascular Iliac Bifurcation; CFA, common femoral artery; IIA, internal iliac artery; CIA, common iliac artery.
Outcome characteristics after ZBIS
Postoperative outcome following ZBIS implantation was satisfactory with no in-hospital death, stroke, need for dialysis or myocardial infarction. All outcome characteristics are listed in Tables 3,4.
Table 3
| Outcome | All patients (n=63) | No reintervention (n=40) | Reintervention (n=23) |
|---|---|---|---|
| In-hospital death | 0 [0] | 0 [0] | 0 [0] |
| Stroke | 0 [0] | 0 [0] | 0 [0] |
| Dialysis | 0 [0] | 0 [0] | 0 [0] |
| Myocardial infarction | 0 [0] | 0 [0] | 0 [0] |
| Hospital stay (days) | 9 [7, 10] | 8 [7, 10] | 11 [9, 15] |
Values are presented as n [%] or median [first quartile, third quartile].
Table 4
| Reason for reintervention | Values |
|---|---|
| First reintervention | 23 [37] |
| Endoleak | 14 [61] |
| Thrombus | 3 [13] |
| Other | 6 [26] |
| Reintervention successful | 21 [91] |
| Days between first intervention & reintervention | 91 [12, 203] |
| Second reintervention | 7 [11] |
| Endoleak | 4 [57] |
| Thrombus | 2 [29] |
| Other | 1 [14] |
| Reintervention successful | 6 [86] |
| Days between first intervention & reintervention | 584 [250, 983] |
| Third reintervention | 1 [2] |
| Endoleak | 1 [100] |
| Reintervention successful | 1 [100] |
| Days between first intervention & reintervention | 1,346 |
Values are presented as n [%] or median [first quartile, third quartile].
Details on reinterventions
The patients were followed-up for a total of 129 patient-years, with a median follow-up of 19 [5, 39] months. 39 patients (62%) had at least a 6-month follow-up. Eight (13%) patients were lost to follow-up and four patients (6%) died during follow-up. In total 32 reinterventions were performed in 23 patients (37%). There was no statistically significant difference in patient characteristics between patients with or without iliac reinterventions. Indications for reinterventions were endoleaks in 19 cases (61%), and occlusive thrombus formation within the stent-graft in 5 cases (16%). Seven patients (23%) underwent a staged procedure. Reinterventions were primarily done endovascularly with a 90% success rate in 28 cases. Two patients (6%) needed to be converted to open surgery because of multiple endoleaks, and one patient (3%) required an additional endovascular reintervention because of persistent endoleak. Overall, 90% of the reinterventions succeeded. All details on the reinterventions are summarized in Tables 3,4, and a detailed list of the indications for each patient’s intervention is found in Table 5.
Table 5
| Patient | Reason for reintervention | Reintervention successful | Days between first intervention & reintervention |
|---|---|---|---|
| 1 | Planned reintervention | Yes | 113 |
| 2 | Thrombus | Yes | 145 |
| 3 | Thrombus | Yes | 805 |
| 4 | Planned reintervention | Yes | 91 |
| Thrombus | Yes | 268 | |
| 5 | Endoleak type I b | Yes | 21 |
| Endoleak type I a & rupture | No | 944 | |
| 6 | Endoleak type I b | No | 15 |
| Endoleak type III | Yes | 566 | |
| 7 | Endoleak type I b | Yes | 987 |
| 8 | Endoleak type II | Yes | 1,248 |
| 9 | Endoleak type I a | Yes | 8 |
| 10 | Endoleak type I a | Yes | 5 |
| 11 | Endoleak type II | Yes | 870 |
| Endoleak type II | Yes | 1,099 | |
| 12 | Planned reintervention | Yes | 41 |
| 13 | Thrombus | Yes | 140 |
| Multiple endoleaks | Yes | 601 | |
| 14 | Endoleak type II | No | 1,106 |
| Endoleak type II | Yes | 1,158 | |
| Endoleak type III | Yes | 1,346 | |
| 15 | Endoleak type Ia | Yes | 7 |
| 16 | Endoleak type II | Yes | 217 |
| 17 | Endoleak type Ia | Yes | 8 |
| Endoleak type Ib | Yes | 1,996 | |
| 18 | Endoleak type III | Yes | 188 |
| 19 | Endoleak type II | Yes | 142 |
| 20 | Planned reinterventions | Yes | 28 |
| 21 | Endoleak type IIb | Yes | 76 |
| Endoleak type IIb | Yes | 196 | |
| 22 | Thrombus | Yes | 6 |
| 23 | Endoleak type 1 stent-graft dislocation | Yes | 7 |
Patency
In the last follow-up CTA, which was done after a median follow-up of 19 [5, 39] months, 68 stent-grafts in the common iliac artery (96%) were patent, and the stent-graft in the internal iliac artery was patent in 66 cases (93%). Reintervention did not affect patency rates.
Competing risk model
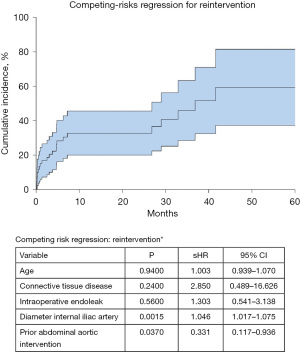
The internal iliac artery’s diameter [subdistribution hazard ratio (sHR): 1.046; P=0.002] and a prior abdominal aortic intervention (sHR: 0.3331; P=0.037) were identified as significant variables in our competing risk regression model for a reintervention (Figure 1). The risk for reintervention was 33% (95% CI: 20–46%), and 46% (95% CI: 28–63%) after 12 and 36 months, respectively (Figure 1).
Discussion
This study’s most important findings are that: (I) branched iliac artery repair is a safe and effective treatment for aneurysms of the iliac arteries extending beyond the bifurcation with high internal iliac artery patency rates; (II) the risk for secondary interventions in these patients is considerable, and the procedures are associated with low morbidity and mortality; (III) the internal iliac arteries diameter is a significant predictor of secondary intervention; and (IV) close follow-up of patients following branched iliac artery repair is recommended.
This study’s population is comparable to other reports addressing the issue of iliac artery disease with a predominantly male population and a high incidence of cardiovascular risk factors (2,6,7). This high disease burden accompanied by the study cohort’s relatively advanced age and a comparatively high incidence of prior abdominal aortic interventions well justifies endovascular treatment as preferred strategy to address the underlying iliac artery disease process. Open vascular surgery in the deep pelvic region is technically more difficult (especially in obese patients) and burdened by higher rate of postoperative complications and longer hospital stay, compared to the endovascular approach (8,9).
We treated two patients with an underlying connective tissue disease (Marfan’s syndrome) endovascularly in this study. Both patients had already undergone open abdominal graft implantation or thoracoabdominal aortic replacement, respectively, creating an ideal artificial proximal landing zone for downstream endovascular repair. In general, we refrain from EVAR in patients with connective tissue disorders with native landing zones because of their high risk of late endoleak formation, negative aortic remodelling, and subsequent treatment failure (10-12). However, we carry out endovascular aortic or iliac repair liberally when a sufficient, artificial proximal landing zone is present. In so doing, we defer and potentially prevent a later distal reintervention (while ensuring sufficient and close clinical follow-up of these patients), or we lower the risk for subsequent open surgical completion (11,13). Of note, the benefit of an artificial proximal landing zone is also reflected in our competing risk regression model that reveals a lower hazard ratio for reinterventions in these patients.
In this study, 8 patients underwent bilateral ZBIS, and 40 patients received an additional abdominal stent-graft. This is in line with other studies (2,6,14) and highlights the general vasculopathy that usually affects the patient’s entire vasculature. Also, branched iliac artery repair is an ideal treatment option to preserve collateral spinal blood flow (5) and to prevent buttock claudication and impotence especially in patients with bilateral iliac artery disease (7). Moreover, ten patients required subclavian artery access for ZBIS implantation because of unsuitable femoral arteries or unfavorable anatomy for cross-over access. Subclavian access is safe and commonly used for transcatheter aortic valve implantation in similar clinical scenarios or for the antegrade treatment of thoraco-abdominal aortic aneurysms (15,16). These points emphasize the value of a multidisciplinary vascular team to enable tailored treatment approaches for these complex patients.
We observed no in-hospital death, stroke, myocardial infarction, or kidney failure in our cohort, which is also in line with other studies (2,6,8,17) highlighting the ZBIS procedure’s safety. Even when a patient needed a reintervention, their outcome was excellent and associated with low morbidity and mortality.
In this study, although we tolerated a noteworthy number of intraoperative endoleaks, we detected no statistically significant effect of tolerated endoleaks on secondary interventions. Since our dedicated outpatient clinic routinely follows-up all our patients after 6 months, we are capable of carefully and timely assessing the remodeling of the aorto-iliac axis, and are thus able to intervene appropriately during follow-up. Our competing risk regression model did not show that an intraoperative endoleak is predictive for a secondary intervention after discharge. Our indications for reinterventions were heterogeneous with different types of endoleaks, rupture, thrombosis and most reinterventions were unexpected during follow-up.
This study also shows that patients undergoing reinterventions had larger pre-interventional iliac arteries and larger diameters in their last pre-intervention follow-up CTA. In addition, the internal iliac artery’s diameter was predictive for a reintervention during follow-up. These findings are in line with other studies investigating the use of branched iliac devices (1,14,18). This is important to know before planning the procedure. Maybe patients with large internal iliac artery aneurysms should be considered for another therapeutic option, or if they undergo ZBIS implantation, carefully monitored during follow-up. Of note, we are unable to define a clear cut-off for the internal iliac artery’s diameter because of the retrospective study design and the patient number included into this study. Hence, prospective studies are required to close this knowledge gap. However, implanting surgeons need to be aware of an increasing risk proportional to the internal iliac artery when choosing ZBIS treatment.
Lastly, this study delivers convincing evidence of excellent technical success and long-term patency rates following branched iliac artery repair which well reflects the patency rates ranging from 90% to 100% reported in the literature (17,19,20,21,22).
Limitations and strengths
Our study is limited by retrospective nature, its sample size, and the relatively brief follow-up period. Therefore, the competing risk regression model should be interpreted carefully within this context. Furthermore, we accepted mild intraoperative type I and III endoleaks (defined in interdisciplinary consent with the vascular surgeon and the interventional radiologist) and included them in the technical success group, what is unusual. However, this investigation contributes valuable knowledge on positive outcomes after branched iliac artery repair, and highlights the need for continuous follow-up of these patients. In addition, due to the retrospective character of the study, we were able to identify patients with clearly intended reintervention but we were unable to identify patients with anticipated or expected reinterventions.
Conclusions
Branched iliac artery repair is a safe and effective treatment for aneurysms of the iliac arteries extending beyond the bifurcation, as it is associated with high mid-term patency rates during follow-up. Although the risk these patients carry for secondary interventions is considerable, the procedures are associated with excellent outcomes. We identified the diameter of the internal iliac artery as a predictor for a reintervention during follow-up. Lastly, we recommend close follow-up of patients following branched iliac artery repair.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-564/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-564/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-564/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-564/coif). MK reports that he has received speaking honoraria from Terumo Aortic. MC reports that he is a consultant for Terumo Aortic, Medtronic and Cryolife, received speaking honoraria from Bentley and is a minority shareholder of TEVAR Ltd. and a shareholder of Ascense Medical. BR reports that he performs proctor activities for Terumo Aortic and is a shareholder of Ascense Medical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our institutional review board (IRB) approved this study, and the need for informed consent was waived due to the retrospective nature of the study (IRB number: 20-1302; approval date: February 4, 2021).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gray D, Shahverdyan R, Jakobs C, et al. Endovascular aneurysm repair of aortoiliac aneurysms with an iliac side-branched stent graft: studying the morphological applicability of the Cook device. Eur J Vasc Endovasc Surg 2015;49:283-8. [Crossref] [PubMed]
- Mylonas SN, Ioannides G, Ahmad W, et al. Comparison of Two Iliac Branch Devices and Their Midterm Performance in Maintaining Blood Flow to the Internal Iliac Artery. J Endovasc Ther 2020;27:818-25. [Crossref] [PubMed]
- Taudorf M, Grønvall J, Schroeder TV, et al. Endovascular Aneurysm Repair Treatment of Aortoiliac Aneurysms: Can Iliac Branched Devices Prevent Gluteal Claudication? J Vasc Interv Radiol 2016;27:174-80. [Crossref] [PubMed]
- Wanhainen A, Verzini F, Van Herzeele I, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg 2019;57:8-93. [Crossref] [PubMed]
- Kari FA, Saravi B, Krause S, et al. New insights into spinal cord ischaemia after thoracic aortic procedures: the importance of the number of anterior radiculomedullary arteries for surgical outcome. Eur J Cardiothorac Surg 2018;54:149-56. [Crossref] [PubMed]
- Delay C, Deglise S, Lejay A, et al. Zenith Bifurcated Iliac Side Branch Device: Mid-term Results and Assessment of Risk Factors for Intraoperative Thrombosis. Ann Vasc Surg 2017;41:141-50. [Crossref] [PubMed]
- Marques de Marino P, Botos B, Kouvelos G, et al. Use of Bilateral Cook Zenith Iliac Branch Devices to Preserve Internal Iliac Artery Flow During Endovascular Aneurysm Repair. Eur J Vasc Endovasc Surg 2019;57:213-9. [Crossref] [PubMed]
- Patel NV, Long GW, Cheema ZF, et al. Open vs. endovascular repair of isolated iliac artery aneurysms: A 12-year experience. J Vasc Surg 2009;49:1147-53. [Crossref] [PubMed]
- Igari K, Kudo T, Toyofuku T, et al. Comparison between endovascular repair and open surgery for isolated iliac artery aneurysms. Surg Today 2015;45:290-6. [Crossref] [PubMed]
- Kari FA, Russe MF, Peter P, et al. Late complications and distal growth rates of Marfan aortas after proximal aortic repair. Eur J Cardiothorac Surg 2013;44:163-71. [Crossref] [PubMed]
- Schoenhoff FS, Schmidli J. TEVAR in Patients With Marfan Syndrome: From Bailout to Strategy. Eur J Vasc Endovasc Surg 2020;59:586. [Crossref] [PubMed]
- Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg 2019;55:133-62. [Crossref] [PubMed]
- Kreibich M, Siepe M, Berger T, et al. Downstream thoracic endovascular aortic repair following zone 2, 100-mm stent graft frozen elephant trunk implantation. Interact Cardiovasc Thorac Surg 2022;34:1141-6. [PubMed]
- Simonte G, Parlani G, Farchioni L, et al. Lesson Learned with the Use of Iliac Branch Devices: Single Centre 10 Year Experience in 157 Consecutive Procedures. Eur J Vasc Endovasc Surg 2017;54:95-103. [Crossref] [PubMed]
- Overtchouk P, Modine T. Alternate Access for TAVI: Stay Clear of the Chest. Interv Cardiol 2018;13:145-50. [Crossref] [PubMed]
- Zimmermann A, Menges AL, Rancic Z, et al. E-nside Off-the-Shelf Inner Branch Stent Graft: Technical Aspects of Planning and Implantation. J Endovasc Ther 2022;29:167-74. [Crossref] [PubMed]
- Lebas B, Galley J, Legall M, et al. Preservation of the Internal Iliac Arteries with Branched Iliac Stent Grafts (Zenith Bifurcated Iliac Side): 5 Years of Experience. Ann Vasc Surg 2016;33:18-22. [Crossref] [PubMed]
- Parlani G, Verzini F, De Rango P, et al. Long-term results of iliac aneurysm repair with iliac branched endograft: a 5-year experience on 100 consecutive cases. Eur J Vasc Endovasc Surg 2012;43:287-92. [Crossref] [PubMed]
- Maurel B, Bartoli MA, Jean-Baptiste E, et al. Perioperative evaluation of iliac ZBIS branch devices: a French multicenter study. Ann Vasc Surg 2013;27:131-8. [Crossref] [PubMed]
- Donas KP, Inchingolo M, Cao P, et al. Secondary Procedures Following Iliac Branch Device Treatment of Aneurysms Involving the Iliac Bifurcation: The pELVIS Registry. J Endovasc Ther 2017;24:405-10. [Crossref] [PubMed]
- Masciello F, Fargion AT, Pratesi G, et al. A propensity score-matched comparison of two commercially available iliac branch devices in patients with similar clinical and anatomic preoperative features. J Vasc Surg 2020;71:1207-14. [Crossref] [PubMed]
- Dueppers P, Duran M, Floros N, et al. The JOTEC iliac branch device for exclusion of hypogastric artery aneurysms: ABRAHAM study. J Vasc Surg 2019;70:748-55. [Crossref] [PubMed]


