
Ventricular pacing dependency after transcatheter aortic valve replacement: a prospective cohort
Highlight box
Key findings
• New VpDep occurred in less than 10% of all patients underwent TAVR.
• Among patients with pacemaker implanted before or within 1 month after TAVR, approximately one-third developed new VpDep.
• New VpDep was not linked with higher mortality.
What is known and what is new?
• Pacemaker implantation is one of the most common complications among patients underwent TAVR.
• Less than half of individuals with pacemakers following TAVR became pacemaker dependent.
What is the implication, and what should change now?
• Because the majority of TAVR patients with pacemakers are not VpDep, they should be regularly monitored and have their pacemaker properly programmed to prevent excessive ventricular pacing.
Introduction
Background and rationale
Atrioventricular (AV) conduction disturbance is a common consequence of transcatheter aortic valve replacement (TAVR), and many require permanent pacemaker (PPM) implantation (1,2). However, conduction disturbances may resolve over time and ventricular pacing dependency (VpDep) may occur only in a proportion of patients with PPMs (3-5). VpDep could lead to ventricular dysfunction (6) and affect the long-term prognosis of patients who undergo TAVR.
Knowledge gap
The incidence of VpDep has been calculated based on variable selection criteria (5). However, most of them excluded patients who already had a PPM even though there was no VpDep.
Objective
This study is sought to analyze the incidence, predictors, and outcomes of new VpDep in a cohort of patients with and without prior PPM who underwent TAVR using all types of devices.
The study has been registered in Thai Clinical Trial Registration (study ID: TCTR20220726005). We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-63/rc).
Methods
The Ramathibodi transcatheter aortic valve replacement registry (RACR) consecutively collected data on all TAVRs performed in a tertiary care cardiac center in Thailand. The registry was designed to provide information on short- and long-term clinical outcomes in patients treated with government-approved TAVR devices. Patients with severe, symptomatic, aortic stenosis were screened and selected by a multidisciplinary heart team using clinical and anatomical imaging information.
The first 130 consecutive patients who underwent transfemoral TAVR between 2015–2020 were studied for VpDep at 1 month and all-cause mortality at the end of the follow-up period in 2021. Patients who had already been implanted with a cardiac implantable electronic device (CIED) that required ventricular pacing were excluded from the analysis (Figure 1). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University (No. COA. MURA2021/673; August 16, 2021). Informed consent was taken from all the patients.
Procedures and TAVR devices
Transesophageal echocardiography and/or computed tomography was used for anatomical guidance and aortic annulus sizing. The device selection and sedation choice were left to the cardiac team’s discretion. The procedure was performed in a cardiac catheterization laboratory or hybrid operating room. After the procedure, all patients were monitored with continuous electrocardiography (ECG) for at least 72 h.
Implantation depth was assessed angiographically after device deployment. A total of 10 mL of the contrast agent was injected to assess the position of the prostheses. The maximum distance between the intraventricular end of the prosthesis and the aortic annulus at the level of each of the three cusps was measured using the HeartVision 2 system (GE Medical Systems SCS, France). The measurements were performed by an interventional cardiologist who was blinded to the results.
Data collection
Patient characteristics, valvular parameters, procedural data, and clinical outcomes were collected from a dedicated database. All patients were followed-up for 30 days for clinical improvement and major adverse cardiovascular events (MACEs), and another follow-up during 2021 for all-cause mortality. For those with CIEDs, device settings data and VpDep were recorded at the index procedure (if implanted before), and 1 month and 1 year after the index procedure. Two groups of authors were tasked with assessing VpDep status and the occurrence of MACEs. Both were uninformed of the other’s outcomes. All follow-ups were performed on the basis of clinical visits or phone calls.
Definitions
Major bleeding, major vascular complications, and acute kidney injury were defined according to the standardized endpoints by the Valve Academic Research Consortium-2 consensus (7). Procedure-related conduction disturbances included new or worsening AV conduction requiring an electrophysiological study (EPS) or PPM implantation.
MACEs at 30 days consisted of death, stroke, myocardial infarction, valve dysfunction, hospitalization for valve-related symptoms or worsening heart failure (HF), and the need for cardiovascular intervention, as defined by the Valve Academic Research Consortium-2 consensus (7).
Severe 1st degree atrioventricular block (AVB) was diagnosed in a patient of 1st degree AVB with a PR interval of >300 ms (8).
VpDep was defined as the occurrence of symptoms and signs that create emergent or urgent clinical situations upon abrupt cessation of pacing, or the absence of an intrinsic ventricular rate of >30 bpm (9). New VpDep included patients with newly implanted CIEDs within 1 month of the index procedure who had developed VpDep. Among patients with prior CIEDs, those who did not have VpDep, but developed it 1 month after the procedure were also considered to have new VpDep. VpDep was evaluated in the pacemaker clinic by either a cardiac electrophysiologist or device specialist.
EPS and PPM
A persistently high-grade AVB after 48 h was the primary indication for PPM implantation. In patients who developed new-onset or worsening left bundle branch block (LBBB) that persisted beyond 48 hours, we performed an EPS and prophylactically implanted a PPM for HV interval ≥65 ms.
The CIED types were selected according to standard guidelines (8). Briefly, single-chamber PPMs were selected for patients with chronic atrial fibrillation (AF), cardiac resynchronization therapy (CRT) for those who needed frequent ventricular pacing and in those with left ventricular systolic function (LVEF) <50%, and dual-chamber PPMs for those without the above conditions.
Pacemaker programming was tailored to each patient’s specific condition and was left to the discretion of the electrophysiology team. Atrioventricular delay (AVD) was extended to allow intrinsic ventricular conduction, but was not too excessive to avoid hemodynamic disadvantages. An algorithm to reduce unnecessary ventricular pacing was used, as appropriate. In patients with chronotropic incompetence, the response rate was turned on.
Statistical analysis
All analyses were performed using SAS software, version 9.04.01 (SAS OnDemand for Academics; SAS Institute Inc., Cary, NC, USA). Categorical variables are expressed as numbers and percentages, and continuous variables are expressed as means and standard deviations (SDs). Fisher’s exact test and one-way analysis of variance were used to compare the differences in baseline characteristics.
The new VpDep predictors were analyzed using multivariate binary logistic analysis. Variables included in the model were age, sex, and variables that had a significance level of <0.01 in the univariate analyses.
The effect of the variables on all-cause mortality was estimated using the Cox proportional hazards model. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. Kaplan-Meier survival curves were plotted and compared using the log-rank test. The proportional assumption was validated using the Schoenfeld residuals. Statistical significance was set at P<0.05.
Results
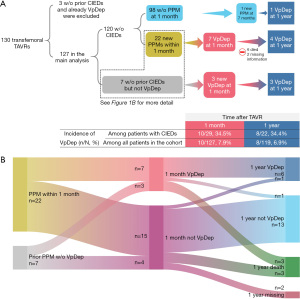
Of the 130 consecutive patients who underwent transfemoral TAVRs, 3 were excluded because they had CIEDs implanted before the procedure, and had VpDep. Therefore, 127 patients were included in the analysis (Figure 1A).
The study population (Table 1) was mainly elderly (81.8 years; SD, 6.3 years) females (n=79, 62.2%) with an intermediate STS mortality score (6.1%; SD, 4.5%). CIEDs were implanted in 7 patients (5.5%). Pre-existing conduction abnormalities were recorded as follows: 10 patients (7.9%) with right bundle branch block (RBBB) and 7 (5.5%) with severe 1st degree or Mobitz I AVB (Sev 1st/Mobitz I). More information on population characteristics is available in Table S1.
Table 1
| Characters | Total (n=127) | VpDep at 1 month | ||
|---|---|---|---|---|
| Independent (n=117) | Dependent (n=10) | P | ||
| Age (years), mean (SD) | 81.8 (6.3) | 81.7 (6.1) | 83.7 (8.0) | 0.075 |
| Female, n (%) | 79 (62.2) | 75 (64.1) | 4 (40.0) | 0.131 |
| LVEF (%), mean (SD) | 62.3 (13.7) | 62.8 (13.4) | 56.3 (15.6) | 0.125 |
| AVA (cm2), mean (SD) | 0.7 (0.20) | 0.7 (0.20) | 0.7 (0.18) | 0.535 |
| MPG (mmHg), mean (SD) | 48.9 (11.90) | 49.6 (11.85) | 40.1 (8.91) | 0.025 |
| STS mortality score (%), mean (SD) | 6.1 (4.5) | 6.2 (4.7) | 4.7 (1.7) | 0.722 |
| LVOT calcification, n (%) | 15 (11.8) | 15 (16.9) | 0 (0.0) | 0.273 |
| Bicuspid valve, n (%) | 3 (2.4) | 3 (2.6) | 0 (0.0) | 0.608 |
| Pre-existing severe 1st degree or Mobitz I AVB, n (%) | 7 (5.5) | 4 (3.4) | 3 (30.0) | <0.001 |
| Pre-existing RBBB, n (%) | 10 (7.9) | 6 (5.1) | 4 (40.0) | <0.001 |
| Valve type, n (%) | 0.587 | |||
| BE | 86 (67.7) | 80 (68.4) | 6 (60.0) | |
| SE | 41 (32.3) | 37 (31.6) | 4 (40.0) | |
| Implantation depth (mm) | ||||
| At NCC, mean (SD) | 3.9 (2.45) | 3.9 (2.49) | 4.0 (2.04) | 0.419 |
| At RCC, mean (SD) | 4.8 (2.54) | 4.8 (2.61) | 5.1 (1.58) | 0.169 |
| At LCC, mean (SD) | 4.2 (2.73) | 4.7 (2.76) | 5.4 (2.35) | 0.206 |
| Mean (SD) | 4.5 (2.48) | 4.5 (2.54) | 4.8 (1.85) | 0.664 |
| HV interval (ms) | 0.073 | |||
| Numbers of patients with data (%) | 13 (10.2) | 11 (9.4) | 2 (20.0) | |
| Mean (SD) | 62.9 (17.7) | 61.3 (18.9) | 72.0 (0.0) | |
| Indication for PPM implantation after TAVR, n (%) | 0.755 | |||
| Complete AVB | 18 (72.0) | 12 (10.3) | 6 (60.0) | |
| New LBBB and HV interval ≥65 ms | 5 (20.0) | 4 (3.4) | 1 (10.0) | |
| High grade AVB and HV interval ≥65 ms | 1 (4.0) | 1 (0.9) | 0 (0.0) | |
| Sick sinus syndrome | 1 (4.0) | 1 (0.9) | 0 (0.0) | |
| Ventricular pacing at 30 days (%), mean (SD) | 40.9 (22.8) | 12.2 (15.9) | 95.6 (7.2) | <0.001 |
| Ventricular pacing at 1 year (%), mean (SD) | 46.9 (44.1) | 31.1 (36.8) | 85.3 (37.6) | 0.004 |
| Death, n (%) | 18 (14.2) | 16 (13.7) | 2 (20.0) | 0.633 |
VpDep, ventricular pacing dependence; SD, standard deviation; LVEF, left ventricular ejection fraction; AVA, aortic valve area; MPG, mean peak gradient; STS, Society of Thoracic Surgeons; LVOT, left ventricular outflow tract; AVB, atrioventricular block; RBBB, right bundle branch block; BE, balloon-expandable; SE, self-expanding; NCC, non-coronary cusp; RCC, right coronary cusp; LCC, left coronary cusp; TAVR, transcatheter aortic valve replacement; PPM, permanent pacemaker; LBBB, left bundle branch block.
TAVR was successfully performed in 126 (99.2%) patients. One patient (0.8%) experienced an annulus rupture and died during the procedure. Periprocedural stroke, cardiac tamponade, and major bleeding occurred in 2 (1.6%), 4 (3.1%), and 4 (3.1%) patients, respectively (Table S2). The implantation depths at non-, right-, and left-coronary cusps were 3.9 mm (SD, 2.45 mm), 4.8 mm (SD, 2.54 mm), and 4.2 mm (SD, 2.73 mm), respectively (Table 1).
New conduction abnormalities and VpDep
New conduction abnormalities were observed in 37 patients (29.1%), with complete AVB being the most common abnormality (n=13, 10.2%). A total of 25 patients (19.7%) underwent PPM implantation after the procedure. Most implantations (n=22, 17.3%) were performed within the first 7 days (Figure S1). Of these, seven patients (31.8%) were classified to have VpDep 1 month after the procedure (Figure 1A). Among the patients who had CIEDs implanted prior to the procedure (n=7), three (42.9%) had VpDep at the 1-month follow-up. Therefore, a new 1-month VpDep occurred in 10 patients (7.9% of all patients in the cohort). The rate of VpDep, calculated based on the total number of patients with CIEDs, was 34.5% at 1-month and 34.4% at 1-year. Among patients implanted with PPM within 1 month or before TAVR (n=29), six patients died and two were unable to be reached at a 1-year follow up. Of the 21 patients remained, 19 (90.5%) had the same VpDep status at 1 month and 1 year (Figure 1B). The indications, pacing device types, and pacemaker settings of all patients treated with CIEDs are shown in Tables S3,S4.
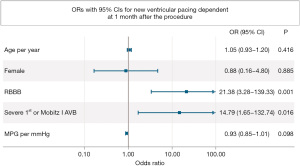
Patients with new VpDep at 1-month follow-up (Table 1) were more likely to have pre-existing RBBB (n=4, 40%) than those without new VpDep (n=6, 5.1%; P<0.001). They were also more likely to have pre-existing Sev 1st/Mobitz I (n=3, 30%) than those without new VpDep (n=4, 3.4%; P<0.001). In the multivariate analysis, pre-existing RBBB [odds ratio (OR), 21.38; 95% CI: 3.28–139.33; P=0.001]and Sev 1st/Mobitz I (OR, 14.79; 95% CI: 1.65–132.74; P=0.016) were independently associated with the occurrence of new VpDep (Figure 2).
TAVR device types and new VpDep
The most common device type was a balloon-expandable (BE) device (n=86, 67.7%). The details of all the valve models are presented in Table S2. The mean implantation depth (Table S5) was deeper in self-expanding (SE) device than in BE device (6.6 mm, SD, 3.22 vs. 3.5 mm, SD, 1.22 mm, respectively; P<0.001). The rate of new pacemaker implantation within 1 month) was significantly lower in patients treated with BE (n=10, 12.0%) than in those treated with SE (n=12, 32.4%; P=0.008). However, the incidence of new VpDep at 1-month follow-up was similar between the groups (BE: n=6, 7.0% vs. SE: n=4, 9.8%; P=0.587; Table 2).
Table 2
| New PPM or new VpDep at 1 mo | Value, n/N, % | P |
|---|---|---|
| New PPM at 1 mo | 0.008 | |
| BE device without prior CIED (n=83) | 10/83, 12.0 | |
| SE device without prior CIED (n=37) | 12/37, 32.4 | |
| New VpDep at 1 mo | 0.587 | |
| BE device | ||
| BE device with prior CIED (n=3) | 1/3, 33.3 | |
| BE device without prior CIED (n=83) | 5/83, 6.0 | |
| Total (n=86) | 6/86, 7.0 | |
| SE device | ||
| SE device with prior CIED (n=4) | 2/4, 50.0 | |
| SE device without prior CIED (n=37) | 2/37, 5.4 | |
| Total (n=41) | 4/41, 9.8 |
VpDep, ventricular pacing dependency; TAVR, transcatheter aortic valve replacement; PPM, permanent pacemaker; mo, month; BE, balloon-expandable; CIED, cardiac implantable electronic device; SE, self-expanding.
Outcomes
Thirty days after the index procedure, 2 patients (1.6%) died, 4 (3.1%) had a stroke, and 1 (0.8%) developed pacemaker infection requiring removal (Table S2). After a follow-up period of 25.8 months (range, 0–117 months; SD, 21.2 months), 18 patients (14.2%) had died. Kaplan-Meier curves with between-group comparisons are shown in Figures S2,S3. New pacemaker implantation (HR, 2.11; 95% CI: 0.78–5.72; P=0.143) and VpDep at 1-month (HR, 1.34; 95% CI: 0.28–6.45; P=0.720) follow-up were not associated with an increased risk of death (Table 3).
Table 3
| Variables | HRs (95% CI) | P |
|---|---|---|
| Age (per year) | 1.02 (0.95–1.10) | 0.613 |
| Female | 0.86 (0.54–1.38) | 0.535 |
| Baseline LVEF (per 1%) | 0.97 (0.94–1.00) | 0.032 |
| STS mortality score (per 1 point) | 1.05 (1.02–1.08) | 0.003 |
| Old CVA | 3.29 (1.01–10.79) | 0.049 |
| AF or AFL | 3.99 (1.13–14.16) | 0.032 |
| Pre-existing RBBB | 2.90 (0.83–10.17) | 0.096 |
| SE TAVR device | 0.86 (0.30–2.44) | 0.773 |
| New pacemaker implantation within 30 days | 2.11 (0.78–5.72) | 0.143 |
| New VpDep at 1 month | 1.34 (0.28–6.45) | 0.720 |
HR, hazard ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; STS, Society of Thoracic Surgeons; CVA, cerebrovascular accident; AF, atrial fibrillation; AFL, atrial flutter; RBBB, right bundle branch block; SE, self-expanding; TAVR, transcatheter aortic valve replacement; VpDep, ventricular pacing dependency.
Discussion
Key findings
In this real-world cohort of patients who underwent TAVR without prior VpDep, 7.9% (n=10/127) developed new VpDep 1 month after the procedure. Patients with pre-existing RBBB or Sev 1st/Mobitz I had a >10-fold risk of developing new VpDep. The occurrence of new VpDep was not associated with an increased risk of death.
Explanations of findings and comparison to similar researches
TAVR has become the default therapy for severe aortic stenosis in selected patients, mostly older patients and/or patients with intermediate and high surgical risks. The procedure has now expanded to include younger and low-risk patients (10). One of the major concerns is the need for ventricular pacing after the procedure. Long-term right ventricular pacing is known to increase the risk of HF and all-cause mortality (6,11). Therefore, new VpDep development after TAVR in patients with or without prior PPM could be prognostic. In a large cohort from Israel (12), a high pacing burden was associated with worsened LVEF, but not with higher mortality. Pacemaker dependency reportedly occurs more frequently in patients with baseline RBBB (5,13). Here, we showed that new VpDep occurred in less than 10% of all cases and was not associated with higher mortality after 2 years of follow-up. Underlying conduction abnormalities were strongly associated with new VpDep. Approximately 40% of patients with pre-existing Sev 1st/Mobitz or RBBB developed new VpDep 1 month after the procedure.
Previous studies (5,13,14) have reported a wide range of VpDep rates. In a meta-analysis (5), the average VpDep rate in patients with PPM at 1-year follow-up was 47.5% (7–89%). Among these studies, the definition of VpDep has not been uniform and the population has been varied. In the REPRISE III trial (14), a cutoff point of 30 bpm in the absence of native rhythm was used to declare VpDep. All devices implanted in REPRISE III were SE; the reported VpDep rates were 43% and 50% at 1 month and 1 year, respectively. In a trial evaluating the incidence of VpDep following Lotus valve implantation (3), a cutoff point of 40 bpm was used to define VpDep. At 30-day and 1-year, 57% and 38% of patients were pacing-dependent, respectively. In a large single-center cohort including patients treated with both BE and SE devices (13), the rates of VpDep using a cutoff point of 40 bpm were 35.7% and 33.3% at 1 month and 1 year, respectively. However, none of these trials included patients with prior use of CIEDs. In the present study, we used a cutoff point of 30 bpm to diagnose VpDep in the absence of a native rhythm. Both the BE and SE devices were included. Patients with prior CIEDs use who did not have VpDep were also included. The VpDep rates in our analysis, calculated based on all patients with CIEDs, were approximately 34% at both 1 month and 1 year. Among patients with prior CIEDs use (n=7), the rate increased to 42.9% at both 1 month and 1 year.
Deep implantation has been associated with conduction disturbances (15,16) and pacemaker dependency (14). The significance of implantation depth between different devices also varied. In addition, the optimal depth has not been consistently defined. The average implantation depths in patients with new conduction disturbances were reportedly 7.1 mm in BE and 5.2 mm in SE (15,16). In the REPRISE III trial (14), a comparison between the Lotus valve and the CoreValve systems showed that one of the predictors of pacemaker dependency at 30 days was implantation depth. The mean implantation depth in REPRISE III was >6 mm compared to our average depth of <5 mm. We chose different TAVR valves based on anatomical suitability and found no significant differences in the implantation depth between the VpDep and non-VpDep groups.
The rate of PPM implantation within 1 month of the procedure was 17%. The number is comparable to the rate reported in the registry that included both BE and SE devices (2). SE device was associated with higher rate of PPM implantation than BE device (32% vs. 12%, P=0.008), similar to previous studies (1,2,17,18). Our pacemaker rate was relatively high as we had relatively low thresholds for pacemaker implantation. Most pacemaker implantations were performed during the index TAVR procedure visit (88% within 7 days of the index procedure). However, we showed that the incidence of new VpDep did not differ between the BE and SE devices.
Implications
Our results support pacemaker interrogation and adjustment as early as 1 month. Of all patients with PPM after TAVR, approximately two-thirds did not depend on ventricular pacing at 1 month, and most remained independent of ventricular pacing at 1 year. An appropriate pacemaker setup in this group of patients would reduce unnecessary ventricular pacing and likely improve long-term outcomes.
Strengths and limitations
One of our study’s strengths was the real-world setting. All TAVR devices were registered. Despite the fact that the number of people with prior PPM was small, they were excluded from the majority of trials.
Our study has several limitations. The sample size was relatively small. The trial design was not powered to detect an effect on mortality and was not allowed to assume causality. The device selection and approach to PPM implantation were based on a single-center experience. Although the maximum follow-up time was more than 9 years, the mean follow-up time was 25.8 months, which might not be long enough to detect the consequences of VpDep. The associations of new conduction abnormalities and VpDep were reported with wide CI, indicating that a larger sample size is required to make any firm inferences from the data.
Conclusions
In a real-world cohort of TAVR patients, new VpDep occurred in fewer than 10% of all patients and one-thirds in patients with CIEDs, and was not linked with increased mortality. Patients who had no prior conduction abnormalities were less likely to be VpDep. Pacemaker programming should therefore be adjusted in this group of patients to avoid unnecessary ventricular pacing. More research with a larger sample size and a longer follow-up period are needed to corroborate these findings.
Acknowledgments
We would like to thank Editage (https://www.editage.com/) for English language editing. This paper was archived in the preprint server, identified by the following doi: https://doi.org/10.1101/2022.09.12.22279879.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-63/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-63/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-63/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the local human research ethics committee (No. COA. MURA2021/673; August 16, 2021) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fadahunsi OO, Olowoyeye A, Ukaigwe A, et al. Incidence, Predictors, and Outcomes of Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement: Analysis From the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv 2016;9:2189-99. [Crossref] [PubMed]
- Wenaweser P, Stortecky S, Heg D, et al. Short-term clinical outcomes among patients undergoing transcatheter aortic valve implantation in Switzerland: the Swiss TAVI registry. EuroIntervention 2014;10:982-9. [Crossref] [PubMed]
- Alasti M, Rashid H, Rangasamy K, et al. Long-term pacemaker dependency and impact of pacing on mortality following transcatheter aortic valve replacement with the LOTUS valve. Catheter Cardiovasc Interv 2018;92:777-82. [Crossref] [PubMed]
- Ng L, Nair R, Ali F, et al. Dependence on permanent pacemakers inserted after transcatheter aortic valve implantation: predictive factors in a ten-year retrospective analysis: Rates and predictors of pacemaker dependence after TAVI. AsiaIntervention 2021;7:98-102. [Crossref] [PubMed]
- Ravaux JM, Di Mauro M, Vernooy K, et al. One-year pacing dependency after pacemaker implantation in patients undergoing transcatheter aortic valve implantation: Systematic review and meta-analysis. JTCVS Open 2021;6:41-55.e15. [Crossref] [PubMed]
- Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932-7. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg 2013;145:6-23. [Crossref] [PubMed]
- Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:932-87. [Crossref] [PubMed]
- Levine PA. Pacemaker dependency after pacemaker implantation. Cardiol J 2007;14:318-20. [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev Esp Cardiol (Engl Ed) 2022;75:524. [Crossref] [PubMed]
- Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002;288:3115-23. [Crossref] [PubMed]
- Hochstadt A, Merdler I, Meridor Y, et al. Effect of pacemaker implantation after transcatheter aortic valve replacement on long- and mid-term mortality. Heart Rhythm 2021;18:199-206. [Crossref] [PubMed]
- Costa G, Zappulla P, Barbanti M, et al. Pacemaker dependency after transcatheter aortic valve implantation: incidence, predictors and long-term outcomes. EuroIntervention 2019;15:875-83. [Crossref] [PubMed]
- Meduri CU, Kereiakes DJ, Rajagopal V, et al. Pacemaker Implantation and Dependency After Transcatheter Aortic Valve Replacement in the REPRISE III Trial. J Am Heart Assoc 2019;8:e012594. [Crossref] [PubMed]
- Binder RK, Webb JG, Toggweiler S, et al. Impact of post-implant SAPIEN XT geometry and position on conduction disturbances, hemodynamic performance, and paravalvular regurgitation. JACC Cardiovasc Interv 2013;6:462-8. [Crossref] [PubMed]
- Pellegrini C, Husser O, Kim WK, et al. Predictors of Need for Permanent Pacemaker Implantation and Conduction Abnormalities With a Novel Self-expanding Transcatheter Heart Valve. Rev Esp Cardiol (Engl Ed) 2019;72:145-53. [Crossref] [PubMed]
- Nai Fovino L, Cipriani A, Fabris T, et al. Anatomical Predictors of Pacemaker Dependency After Transcatheter Aortic Valve Replacement. Circ Arrhythm Electrophysiol 2021;14:e009028. [Crossref] [PubMed]
- Hokken TW, Muhemin M, Okuno T, et al. Impact of membranous septum length on pacemaker need with different transcatheter aortic valve replacement systems: The INTERSECT registry. J Cardiovasc Comput Tomogr 2022;16:524-30. [Crossref] [PubMed]


