
Multi-modality imaging of high-intensity plaques on non-contrast T1-weighted magnetic resonance imaging: a case report
Highlight box
Key findings
• Our three cases indicate the possible relationship of non-contrast T1-weighted imaging (T1WI)-derived coronary high-intensity plaque with the degree of lipidic plaque components.
What is known and what is new?
• Non-contrast T1WI-derived coronary high-intensity plaque has been shown to predict risk of future coronary events.
• Our cases characterize coronary plaques which exhibit high-intensity on non-contrast T1WI magnetic resonance imaging (MRI) imaging.
What is the implication, and what should change now?
• Lipidic materials may be an important contributor to the degree of plaque intensity on non-contrast T1WI MRI imaging. Lipid-lowering therapies should be further intensified in patients with high-intensity coronary plaques.
Introduction
Non-contrast T1-weighted imaging (T1WI) with cardiac magnetic resonance is a non-invasive imaging tool to visualize coronary atheroma. The observational study demonstrated that the extent of signal on non-contrast T1WI at coronary lesions was associated with an increased risk of coronary events (1). In particular, coronary high-intensity plaque (HIP) defined as T1WI-derived plaque-to-myocardial signal intensity ratio (PMR) >1.4 was an independent predictor for a composite of cardiac death and coronary events (1). In addition, lowering low-density lipoprotein cholesterol (LDL-C) with pitavastatin has been shown to modulate signal intensity associated with coronary events (2). These findings suggest the potential of non-contrast T1WI to stratify future events’ risk and integrate into the clinical trial to assess the efficacy of novel therapies. Despite these clinically applicable abilities of non-contrast T1WI, determinant of its signal intensity has not been fully elucidated yet. Recent study reported intra-luminal thrombus as a cause of coronary HIP (3). However, this non-contrast T1WI-derived plaque feature is observed at coronary lesions without any intra-luminal thrombus (4). This observation suggests that plaque component itself may be another contributor to coronary high-intensity plaque. Given that a significant reduction of PMR was observed under lipid-lowering therapy which modulates lipidic plaque materials (2), coronary high-intensity plaque may be attributable to lipidic components within plaques.
Near-infrared spectroscopy (NIRS) imaging has enabled to quantitatively measure the degree of lipidic burden in vivo (5). This modality provides the opportunity to assess lipid-rich plaque at coronary lesions with coronary high-intensity plaque. This case series summarize three cases with and without coronary high-intensity plaque who were imaged by NIRS-intravascular ultrasound (IVUS) and optical coherence tomography (OCT). Multi-modality imaging was conducted to predict a risk of distal embolization following percutaneous coronary intervention (PCI) in all three cases. We present this article in accordance with the CARE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-125/rc).
Case presentation
Case 1
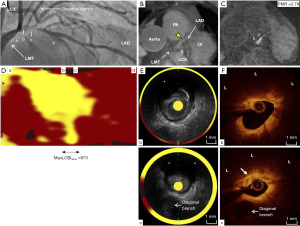
A 57-years-old man was hospitalized due to exertional chest pain in January, 2017 (Table 1). Treadmill test demonstrated definitive ST-segment depression at precordial leads. He had a history of hypertension and dyslipidemia. He was already treated with 100 mg aspirin, 1.25 mg bisoprolol, 2.5 mg enalapril and 2 mg pitavastatin. Elective coronary angiography (CAG) identified a significant stenosis at the proximal segment of left anterior descending (LAD) artery (Figure 1A). Following elective CAG, magnetic resonance angiography (MRA) and non-contrast T1WI were conducted to evaluate culprit lesion. MRA visualized culprit site in his LAD artery (Figure 1B, yellow triangle). In addition, on non-contrast T1WI, coronary high-intensity plaque was observed at the corresponding lesion and its PMR was 2.79 (Figure 1C, Video S1). Elective PCI was performed under the guidance of NIRS-IVUS and OCT imaging. NIRS-derived chemogram imaging illustrated that maximum lipid core burden index in 4 mm (maxLCBI4mm) at culprit lesion was 873 (Figure 1D). The cross-sectional image of IVUS exhibited ultrasonic signal attenuation (asterisk) at culprit site (Figure 1E, Video S2). Despite the presence of coronary high-intensity plaque on T1WI, OCT imaging did not identify any obvious intra-luminal thrombus (b and c in Figure 1F) but the presence of lipid-rich plaque (L) harboring cholesterol crystal (white arrow) (Figure 1F, Video S3).
Table 1
| Characteristics | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age (years) | 57 | 81 | 65 |
| Gender | Male | Male | Male |
| Race | Japanese | Japanese | Japanese |
| BMI (kg/m2) | 26.1 | 23.4 | 19.2 |
| Hypertension | Yes | Yes | No |
| Dyslipidemia | Yes | Yes | Yes |
| Type 2 diabetes mellitus | No | No | No |
| Smoking | No | No | No |
| Diagnosis of CAD | Angina pectoris | Silent myocardial ischemia | Angina pectoris |
| Duration of CAD since its diagnosis (months) | 10 | 6 | 8 |
| Culprit lesion | Severe stenosis at the proximal segment of LAD | Severe stenosis at the middle segment of LAD | Severe stenosis at the middle segment of LAD |
| Statin use | 2 mg pitavastatin | 2.5 mg rosuvastatin | 10 mg atorvastatin |
| LDL-C (mg/dL) | 63 | 70 | 96 |
| HDL-C (mg/dL) | 39 | 85 | 39 |
| Triglyceride (mg/dL) | 131 | 86 | 130 |
| HbA1c (%) | 5.4 | 6.0 | 5.7 |
| eGFR (mL/min/1.73 m2) | 68.2 | 45.4 | 71.7 |
BMI, body mass index; CAD, coronary artery disease; LAD, left anterior descending artery; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HbA1c, glycated hemoglobin; eGFR, estimated glomerular filtration rate.
Case 2
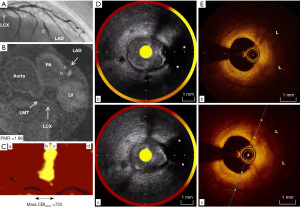
An 81-years-old gentleman presented with cerebral infarction in the middle cerebral artery territory attributable to severe right internal carotid artery stenosis (Table 1) in August, 2017. Stress myocardium perfusion scintigraphy prior to the scheduled carotid artery stenting showed the presence of cardiac ischemia at anterior myocardium. He was taking 100 mg aspirin, 30 mg edoxaban, 20 mg azilsartan, 2.5 mg amlodipine and 2.5 mg rosuvastatin due to a history of hypertension, dyslipidemia and paroxysmal atrial fibrillation. CAG revealed one severe stenosis at the middle segment of LAD artery (Figure 2A). Non-contrast T1WI visualized the presence of coronary high-intensity plaque at this lesion (PMR =1.88; Figure 2B, Video S4). On NIRS-IVUS imaging, maxLCBI4mm at culprit site was 725 (Figure 2C). Some ultrasonic signal attenuation and low-echoic plaque were also identified (Figure 2D, Video S5). Lipid-rich plaque (L) without any thrombus was observed at the corresponding site on OCT (Figure 2E, Video S6).
Case 3
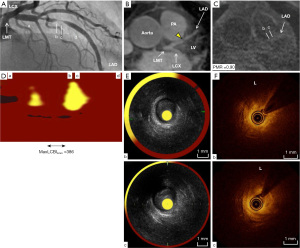
A 65-years-old man was referred to our institute due to chest pain on exertion in March, 2017 (Table 1). He has a history of dyslipidemia. Myocardial ischemia was noted by stress myocardium perfusion scintigraphy. CAG demonstrated the presence of severe stenosis at the middle segment of LAD artery (Figure 3A). Further evaluation of culprit plaque with MRA visualized the corresponding lesion in LAD artery (yellow arrowhead), whereas there was no HIP within his LAD artery on non-contrast T1WI (PMR =0.90) (Figure 3B,3C, Video S7). NIRS-IVUS and OCT imaging were undergone prior to PCI procedure. MaxLCBI4mm at this lesion was 386, which was numerically lower compared to other two cases (Figure 3D). Furthermore, the cross-sectional images of culprit lesion on IVUS showed the presence of low-echoic plaque without any ultrasonic attenuation (Figure 3E, Video S8). OCT demonstrated the presence of lipid plaque (L) (Figure 3F, Video S9). However, the arc of lipid plaque was marginally smaller compared to cases 1 and 2.
Ethical statement
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Non-contrast T1WI has enabled to quantitatively visualize high-risk coronary atheroma causing future events. While this non-invasive approach has a great potential to optimize risk stratification and preventive therapies, it remains to be determined which plaque features correspond to PMR on non-contrast T1WI. In our case series, a larger maxLCBI4mm was observed at coronary lesion with coronary HIP, whereas this NIRS measure was least at the another lesion without any coronary HIP. These findings indicate that lipidic material may contribute to PMR imaged by non-contrast T1WI.
Recent study using OCT reported the importance of intraluminal thrombus in PMR (3). In this analysis including 26 patients, lesions with coronary HIP were more likely to exhibit the presence of thrombus at the surface of the lesion. In addition, the frequency of lipid-rich plaque was numerically higher at lesions containing coronary HIP. In our cases, however, despite the absence of intraluminal thrombus on OCT imaging, coronary HIP was observed at culprit lesions in cases 1 and 2. Of note, these two lesions exhibited lipid-rich features on both NIRS and OCT imaging. Similar observation was reported by our recent study which analyzed 137 lesions (4). In this study, 34% of plaques without any obvious intraluminal thrombus exhibited coronary HIP. Moreover, the presence of OCT-derived lipid-rich plaque as well as healed plaque feature predicted coronary HIP in subjects with stable coronary artery disease (4). These findings highlight that not only intraluminal thrombus but also plaque component could affect PMR on non-contrast T1WI.
Current cases suggest that the degree of lipidic materials may be an important determinant for coronary HIP. Mechanistically, fat-containing substances has been shown to cause high intensity signal due to shortening of the T1 relaxation time (6). Multi-modality imaging in our cases identified that two lesions with coronary HIP exhibited a greater maxLCBI4mm. NIRS-derived maxLCBI4mm is a histologically validated quantitative measure to evaluate lipidic burden and predict future cardiac events (5,7). Moreover, recent OCT imaging study showed that lipid arc was significantly associated with coronary HIP (4). Collectively, these evidences as well as our cases may support the association of lipidic component with coronary HIP.
There are several caveats to interpret our findings. Firstly, we imaged only LAD artery but not other vessels. Three-vessel intravascular imaging is required to further clarify the association of magnetic resonance imaging (MRI)-derived PMR with NIRS/IVUS and OCT images. The definition of coronary HIP on MRI imaging varies in each published paper. While we defined it as T1WI-derived PMR >1.4, other paper used 1.0. The standardization of its definition on MRI is needed. Given that we presented only three cases with multi-modality imaging observation at single time point, it remains unknown about the longitudinal association of PMR with NIRS/IVUS and OCT features. Clinical study with appropriate study sample size is warranted to elucidate it. We selected three patients with evaluable images. This causes selection bias of patients. It remains unknown whether current findings consistently exist in consecutively enrolled patients.
Conclusions
Current three cases suggest the possible relationship of non-contrast T1WI-derived coronary HIP with the degree of lipidic plaque components in vivo. Given that lipid-rich plaque is a precursor lesion causing acute coronary syndrome, the presence of greater amount of lipid tissues at coronary HIP may account for its association with future coronary events.
Acknowledgments
Funding: This case report was supported by the Nakatani foundation for advancement of measuring technologies in biomedical engineering 2021, and JSPS KAKENHI (Grant No. JP20K08415).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-125/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-125/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-125/coif). YK has received honoraria and research support from Kowa, Nipro and Abbott. YK serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2021 to August 2023. FO has received honoraria from Nipro and Abbott. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study were in accordance with the ethical standards of the institutional research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Noguchi T, Kawasaki T, Tanaka A, et al. High-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J Am Coll Cardiol 2014;63:989-99. [Crossref] [PubMed]
- Noguchi T, Tanaka A, Kawasaki T, et al. Effect of Intensive Statin Therapy on Coronary High-Intensity Plaques Detected by Noncontrast T1-Weighted Imaging: The AQUAMARINE Pilot Study. J Am Coll Cardiol 2015;66:245-56. [Crossref] [PubMed]
- Ehara S, Hasegawa T, Nakata S, et al. Hyperintense plaque identified by magnetic resonance imaging relates to intracoronary thrombus as detected by optical coherence tomography in patients with angina pectoris. Eur Heart J Cardiovasc Imaging 2012;13:394-9. [Crossref] [PubMed]
- Kanaya T, Noguchi T, Otsuka F, et al. Optical coherence tomography-verified morphological correlates of high-intensity coronary plaques on non-contrast T1-weighted magnetic resonance imaging in patients with stable coronary artery disease. Eur Heart J Cardiovasc Imaging 2019;20:75-83. [Crossref] [PubMed]
- Kataoka Y, Puri R, Andrews J, et al. In vivo visualization of lipid coronary atheroma with intravascular near-infrared spectroscopy. Expert Rev Cardiovasc Ther 2017;15:775-85. [Crossref] [PubMed]
- Ginat DT, Meyers SP. Intracranial lesions with high signal intensity on T1-weighted MR images: differential diagnosis. Radiographics 2012;32:499-516. [Crossref] [PubMed]
- Waksman R, Di Mario C, Torguson R, et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet 2019;394:1629-37. [Crossref] [PubMed]


