
Differentially expressed miR-127, miR-150, and miR-145 in serum extracellular vesicles are novel diagnostic biomarkers of unstable angina
Highlight box
Key findings
• MiR-127, miR-150, and miR-145 in serum extracellular vesicles (EVs) are closely linked with unstable angina (UA) and serve as novel diagnostic biomarkers.
What is known and what is new?
• The diagnostic potentials of miRNAs in UA have been previously analyzed.
• Our study explored the differentially expressed miRNAs in serum exosomes of UA patients. We used high-throughput sequencing, followed by verification via quantitative reverse transcription polymerase chain reaction, and analyzed Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment to the target genes of miRNAs. The present study aims to explore their diagnostic potentials and their biological functions in UA, as well as the correlation between conventional biochemical indexes of UA.
What is the implication, and what should change now?
• MiR-127, miR-150, and miR-145 in serum EVs are novel diagnostic biomarkers of UA.
• Observations of long-term prognoses and predictions of the incidence of adverse cardiovascular events in patients with UA with low miR-127, miR-150, and miR-145 expression.
Introduction
Background
Unstable angina (UA) is a condition of myocardial ischemia at rest or after activity that damages myocardial cells. Clinical manifestations of UA include chest pain at rest that lasts longer than 10 min and chest tightness (1). In the United States, 10% of patients presenting to the emergency room with acute chest pain are diagnosed with UA (2).
Rationale and knowledge gap
Compared with patients with non-ST-elevation myocardial infarction (NSTEMI), UA patients do not benefit more from intensive anti-platelet therapy or invasive therapy within 72 h (1). In 2008, the World Health Organization declared that UA was diagnosed in cases of worsening symptoms of ischemia, ischemic electrocardiogram (ECG) changes, and normal biomarkers, such as creatine kinase-MB (CK-MB) or cardiac troponin I (cTnI) (3). However, high-sensitivity cardiac troponin I (hs-cTnI), the recommended test for distinguishing UA from NSTEMI, is often affected by age, kidney function, presence of disease (such as aortic dissection or pulmonary embolism), duration of chest pain, and sex (1). Therefore, the diagnosis of UA currently relies on medical history and physician experience (4), whereas evidence is lacking of the diagnostic potential of risk scores or clinical predictors. Therefore, the identification of novel diagnostic biomarkers for UA is urgently required. extracellular vesicles (EV) are small vesicles secreted by cells that are transported into the extracellular environment or body fluids. Deoxyribonucleic acids (DNAs), Ribonucleic acids (RNAs), microRNAs (miRNAs), proteins, and lipids contained in EVs contribute to the transmission of information from donor cells to recipient cells. EVs protect circulating RNAs from RNase-induced degradation, thereby maintaining their functional stability (5). miRNAs exert specific biological effects by transferring genetic information carried by vehicles to other cells (6). Owing to their specific characteristics, EV-derived miRNAs are potential non-invasive diagnostic biomarkers.
Objective
This study aimed to identify the potential of miRNAs in serum EVs for UA diagnosis. We present this article in accordance with the STARD reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-575/rc).
Methods
Subjects
A total of 147 UA patients, 8 NSTEMI patients, and 8 stable angina (SA) patients treated at Nanjing Hospital of Traditional Chinese Medicine Affiliated to Nanjing University of Chinese Medicine between January 2019 and February 2022 were recruited, excluding 5 UA patents for they evoluting to NSTEMI. Fifty-eight healthy volunteers (HVs) were recruited to the control group during the same period. All participants were of Han nationality from Nanjing. Symptoms of myocardial ischemia occurred within 3 h, and reperfusion therapy was not provided after the onset of symptoms. UA and NSTEMI were diagnosed based on the 2015 European Society of Cardiology guidelines (2). Recruited SA patients were graded as having Canadian Cardiovascular Society class I–II angina pectoris. HVs were those who received physical examinations in our hospital during the same period. They did not have history of chronic diseases and the current ECG and echocardiography findings were normal. Patients with ST-elevation myocardial infarction, aortic dissection, pulmonary embolism, hypertensive heart disease, transient ischemic attack, congenital heart disease, dilated cardiomyopathy, valvular heart disease, pulmonary heart disease, blood disorders, chronic liver disease, tumors, rheumatic immune system diseases, infectious diseases, or a history of psychiatric disorders were excluded. Pregnant or lactating women were also excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Nanjing Hospital of Traditional Chinese Medicine affiliated with Nanjing University of Chinese Medicine (No. KY2019067). Written informed consent was obtained from all subjects. This study is a prospective study and participants were recruited randomly.
Serum samples
Peripheral blood samples were obtained from all subjects before the intervention and placed in serum separator tubes. They were centrifuged at 4 ℃ and 3,600 rpm for 15 min, and the supernatant was isolated for measuring CK-MB and cTnI levels.
Extraction of miRNAs from exosomes
Serum samples from 8 NSTEMI patients, 8 SA patients, 8 randomly selected UA patients and 8 HVs were subjected to EV-derived total RNA extraction using an exoRNeasy Serum/Plasma Maxi Kit (QIAGEN, Shanghai, China; 77064). EV-derived miRNAs were isolated using the miRNeasy Serum/Plasma Maxi Kit (QIAGEN, 217184) and purified using a miRNeasy 96 Kit (QIAGEN, 217061). After reverse transcription using a miScript II RT Kit (QIAGEN, 218160), cDNA samples were amplified using a miScript PreAMP PCR Kit (QIAGEN, 331451) and miScript PreAMP Pathway Primer Mix and subjected to quantitative reverse transcription polymerase chain reaction (qRT-PCR) using miScript miRNA PCR arrays and a miScript SYBR Green PCR Kit (QIAGEN, 218073). Following the extraction of miRNA, quality control was performed. Qubit 2.0 was initially used for quantification, and the length of miRNA was measured by Agilent 2100. Qualified miRNAs were subjected to the following experiments. The effective concentration of the detected miRNA was accurately quantified by qRT-PCR (effective concentration >2 nmol/L). Sequencing of pooled libraries was finally performed on the Illumina system.
qRT-PCR
Screening of serum EV miRNAs as UA biomarkers. After high-throughput sequencing, differentially expressed miRNAs were identified between 8 UA patients and HVs, 8 NSTEMI patients and HVs, and 8 SA patients and HVs. Serum EVs miRNAs serving as UA biomarkers were identified by depicting the Venn diagram. Differentially expressed miRNAs in the serum EVs were subjected to qRT-PCR analysis. Serum EVs and total RNAs were extracted using the relevant kits, and the mixed solution was prepared using the Bulge-LoopTM miRNA qRT-PCR Starter Kit (Ribobio, Guangzhou, China; R1008). The mixture was immediately centrifuged, and subjected to the amplification of nucleic acids at 42 ℃ for 60 min and 70 ℃ for 10 min. Target miRNA primers (Ribobio, R0813, 2131, 201311) were added into the RT reaction solution following the instructions of the Bulge-LoopTM miRNA qRT-PCR Starter Kit (Ribobio, R1008). After pre-denature at 95 ℃ for 10 min and 40 cycles of 95 ℃ for 2 s and 60 ℃ for 30 s, melting curve was immediately plotted.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses
Target genes of differentially expressed miRNAs in serum EVs were predicted using the multiMiR package, which hosts 8 online databases for predicting miRNA target interactions, including DIANA-microT-CDS, ElMMo, MicroCosm, miRanda, miRDB, PicTar, PITA, and TargetScan. After validation in miRecords, miRTarBase, and TarBase, differentially expressed miRNAs with experimental data were subjected to GO and KEGG pathway enrichment analyses using the clusterProfiler package.
Statistical analysis
The statistical analysis was performed using SPSS 20.0 (IBM Inc., Armonk, NY, USA). Categorical data were expressed as n and compared using the chi-square test or Fisher’s exact test. Measurement data are expressed as mean ± standard deviation, while normally distributed data were compared using Student’s t-test; otherwise, they were compared using the Mann-Whitney U test. Receiver operating characteristic (ROC) curves were plotted to assess the diagnostic potential of miRNAs in serum exosomes, and those with an area under the curve (AUC) ≥0.5 were considered to have diagnostic potential (7). Risk factors for UA were identified using univariable and multivariable logistic regression analyses, and odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated. The correlation between risk factors for UA and differentially expressed miRNAs was determined using Spearman’s rank correlation test. Statistical significance was set at P<0.05.
Results
Baseline characteristics
A total of 142 UA patients, 8 NSTEMI patients, and 8 SA patients treated between January 2019 and February 2022 at Nanjing Hospital of Traditional Chinese Medicine Affiliated with Nanjing University of Chinese Medicine were recruited. Fifty-eight HVs were recruited during the same period as the control group. We recorded participants’ age, sex, medical history, and laboratory test data. No significant differences were observed in age, sex, history of hypertension, type 2 diabetes mellitus (T2DM), pre-cerebral infarction, smoking, drinking, or anti-platelet therapy between the UA, NSTEMI, and SA patients and the HVs (Table 1).
Table 1
| Characteristics | HVs (n=58) | UA (n=142) | P value (UA vs. HVs) | NSTEMI (n=8) | P value (NSTEMI vs. HVs) | SA (n=8) | P value (SA vs. HVs) |
|---|---|---|---|---|---|---|---|
| Age, years | 60.50±7.99 | 62.30±6.80 | 0.10 | 61.12±5.46 | 0.84 | 63.01±4.59 | 0.40 |
| Male sex | 30 (51.7) | 75 (52.8) | 0.80 | 4 (50.0) | 0.90 | 4 (50.0) | 0.90 |
| PCI history | 0 | 12 (8.5) | 0.02* | 3 (37.5) | 0.001* | 2 (25.0) | 0.013* |
| CAD history without PCI | 0 | 25 (17.6) | <0.001* | 6 (75.0) | <0.001* | 3 (37.5) | 0.001* |
| Hypertension history | 0 | 10 (7.0) | 0.066 | 1 (12.5) | 0.121 | 1 (12.5) | 0.121 |
| T2DM history | 0 | 9 (6.3) | 0.061 | 1 (12.5) | 0.121 | 1 (12.5) | 0.121 |
| CCS class | |||||||
| I | 0 | 21 (14.8) | 0.001 | 0 | – | 6 (75.0) | <0.001* |
| II | 0 | 87 (61.3) | <0.001* | 0 | – | 2 (25.0) | 0.013* |
| III | 0 | 34 (23.9) | <0.001* | 1 (12.5) | 0.121 | 0 | – |
| IV | 0 | 0 | – | 7 (87.5) | <0.001* | 0 | – |
| Pre-cerebral infarction | 0 | 7 (4.9) | 0.197 | 1 (12.5) | 0.121 | 1 (12.5) | 0.121 |
| Smoking | 13 (22.4) | 27 (19.0) | 0.08 | 2 (25.0) | 0.587 | 3 (37.5) | 0.296 |
| Drinking | 14 (24.1) | 32 (22.5) | 0.854 | 4 (50.0) | 0.133 | 3 (37.5) | 0.415 |
| Anti-platelet therapy | 8 (13.8) | 21 (14.8) | 0.525 | 3 (37.5) | 0.122 | 2 (25.0) | 0.349 |
| CK-MB, U/L | 6.12±2.65 | 6.56±2.65 | 0.300 | 35.87±13.51 | <0.001* | 6.62±2.61 | 0.616 |
| cTnI, ng/mL | 0.002±0.14 | 0.05±0.03 | <0.001* | 0.06±0.02 | <0.001* | 0.02±0.11 | 0.304 |
Data are shown as n (%) or mean ± standard deviation. *, P<0.05 vs. HVs. For each parameter, 158 patients were included, unless data were not available (the number of values is shown in the left column). P values refer to χ2, Wilcoxon, and t-tests for binary, skewed continuous, and symmetrical continuous variables. All analyses were performed using SPSS 20.0 (IBM Inc.). UA, unstable angina; NSTEMI, non-ST-elevation myocardial infarction; SA, stable angina; HVs, healthy volunteers; CAD, coronary artery disease; PCI, percutaneous coronary intervention; T2DM, type 2 diabetes mellitus; CCS, Canadian Cardiovascular Society; CK-MB, creatine kinase-MB; cTnI, cardiac troponin I.
Identification of differentially expressed miRNAs in serum exosomes
Differentially expressed miRNAs in the serum EVs of 8 UA patients, 8 NSTEMI patients, 8 SA patients, and 8 HVs were detected by high-throughput sequencing (Table 2). Compared with HVs, miR-127, miR-150, and miR-145 were downregulated in UA patients but upregulated in NSTEMI patients. Their expression levels were comparable in the serum EVs of the HVs and SA patients. Venn diagram plotting revealed that miR-127, miR-150, and miR-145 were the differentially expressed miRNAs in the serum EVs of UA patients (Figure 1).
Table 2
| miRNA | UA vs. HVs | NSTEMI vs. HVs | SA vs. HVs | |||||
|---|---|---|---|---|---|---|---|---|
| FC | P value | FC | P value | FC | P value | |||
| miR-141 | 2.95 | 0.028* | 0.31 | 0.092 | 1.62 | 0.073 | ||
| miR-1270 | 2.68 | 0.018* | 0.61 | 0.062 | 0.78 | 0.087 | ||
| miR-1290 | 2.07 | 0.009* | 1.42 | 0.084 | 1.37 | 0.085 | ||
| miR-154 | 1.47 | 0.071 | −1.24 | 0.095 | 3.98 | 0.047* | ||
| miR-12 | 1.32 | 0.08 | 1.29 | 0.032* | 1.24 | 0.075 | ||
| miR-135b | 1.28 | 0.02* | 1.29 | 0.17 | 1.04 | 0.13 | ||
| miR-1275 | 1.21 | 0.65 | −0.78 | 0.32 | −3.99 | 0.027* | ||
| miR-1285 | 1.04 | 0.068 | −3.4 | 0.001* | −1.41 | 0.094 | ||
| miR-18b | 0.96 | 0.49 | −3.58 | 0.046* | 0.93 | 0.089 | ||
| miR-214 | 0.81 | 0.13 | −1.47 | 0.13 | 1.97 | 0.025* | ||
| miR-136 | 0.72 | 0.053 | −2.05 | 0.004* | 1.45 | 0.071 | ||
| miR-1343 | 0.67 | 0.082 | 0.93 | 0.72 | −3.97 | 0.003* | ||
| miR-2116 | 0.45 | 0.062 | 1.06 | 0.092 | 2.05 | 0.014* | ||
| miR-205 | 0.32 | 0.091 | 0.92 | 0.063 | −2.17 | 0.013* | ||
| miR-1292 | −0.87 | 0.94 | 1.47 | 0.16 | 3.62 | 0.015* | ||
| miR-150 | −1.55 | 0.012* | 1.28 | 0.02* | 1.49 | 0.073 | ||
| miR-127 | −1.81 | 0.015* | 1.62 | 0.01* | −1.25 | 0.062 | ||
| miR-145 | −1.85 | 0.04* | 2.52 | 0.014* | −1.29 | 0.07 | ||
| miR-1249 | −1.97 | 0.001* | 0.74 | 0.31 | −1.29 | 0.068 | ||
*, P<0.05 vs. HVs. T-tests were calculated using SPSS 20.0. miRNA, microRNA; EVs, extracellular vesicles; UA, unstable angin; NSTEMI, non-ST-elevation myocardial infarction; SA, stable angina; HVs, healthy volunteers; FC, fold change.
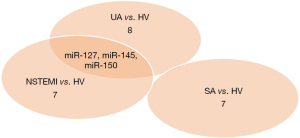
Validation of miR-127, miR-150 and miR-145 in serum exosomes
We validated the differentially expressed miRNAs in serum EVs using qRT-PCR. Compared with those of the HVs (n=50), the relative miR-127 (Figure 2A), miR-150 (Figure 2B), and miR-145 (Figure 2C) levels in the serum EVs of the UA patients (n=134) were significantly lower (P<0.05, Figure 2).

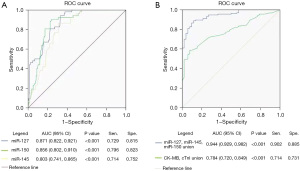
Diagnostic potential of miR-127, miR-150, and miR-145 in UA
The diagnostic potential of miR-127, miR-150, and miR-145 in UA was assessed by the plotting of ROC curves in 95% CIs. The AUC of miR-127, miR-150, and miR-145 for distinguishing UA patients from HVs were 0.871 (95% CI: 0.822–0.921), 0.856 (95% CI: 0.802–0.910), and 0.803 (95% CI: 0.741–0.865), respectively. Notably, the AUC of the combination of the three differentially expressed miRNAs in UA diagnosis was 0.944 (95% CI: 0.929–0.982), higher than that of the combination of CK-MB and cTnI (0.944 vs. 0.784). This suggests that all three miRNAs possessed acceptable diagnostic potential for UA (Figure 3).
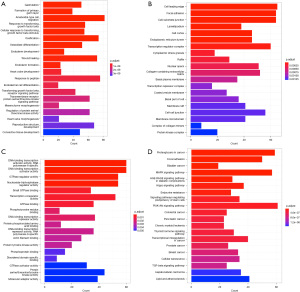
GO and KEGG pathway enrichment analyses
GO (http://www.geneontology.org/) and KEGG (https://www.kegg.jp/) pathway enrichment analyses were performed of miR-127, miR-150, and miR-145. A total of 994 biological processes, 1,032 cellular locations, 1,002 molecular functions, and 465 signaling pathways enriched in miR-127, miR-150, and miR-145 were identified (Figure 4).
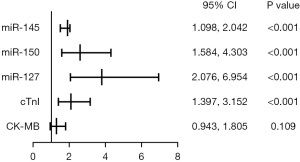
Risk factors for UA
Univariable logistic regression analysis showed that CK-MB, cTnI, miR-127, miR-150, and miR-145 were significantly associated with the early diagnosis of UA (P<0.05). Furthermore, a multivariate logistic regression analysis showed that cTnI (P=0.0006), miR-127 (P=0.0001), miR-150 (P=0.0004), and miR-145 (P=0.0005) were independent risk factors for UA (Table 3, Figure 5).
Table 3
| Factors | Univariable logistic regression analysis | Multivariable logistic regression analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| miR-145 | 1.647 (1.394–1.946) | 0.005 | 1.497 (1.098–2.042) | <0.001 | |
| miR-150 | 2.186 (1.712–2.777) | <0.001 | 2.611 (1.584–4.303) | <0.001 | |
| miR-127 | 2.823 (2.023–3.912) | <0.001 | 3.799 (2.076–6.954) | <0.001 | |
| cTnI | 1.658 (1.371–2.005) | <0.001 | 2.098 (1.397–3.152) | <0.001 | |
| CK-MB | 1.149 (1.002–1.316) | 0.046 | 1.304 (0.943–1.805) | 0.109 | |
Univariable and multivariable logistic regression analyses were performed using SPSS20. UA, unstable angina; OR, odds ratio; CI, confidence interval; cTnI, cardiac troponin I; CK-MB, creatine kinase-MB.
Correlation between CK-MB, cTnI, and differentially expressed miRNAs in the serum Evs.
The correlation between CK-MB, cTnI, and differentially expressed miRNAs in the serum EVs of UA patients was examined using Spearman’s rank correlation test. Only cTnI was significantly correlated with miR-127 (r=0.1988, P=0.0067; Table 4).
Table 4
| miR-127 | miR-145 | miR-150 | ||||||
|---|---|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |||
| CK-MB | 0.06534 | 0.3769 | 0.1106 | 0.1338 | 0.08271 | 0.2630 | ||
| cTnI | 0.1988 | 0.0067 | 0.1217 | 0.0989 | 0.1407 | 0.0560 | ||
Spearman’s correlations were calculated using SPSS 20.0. CK-MB, creatine kinase-MB; cTnI, cardiac troponin I; miRNA, microRNA; UA, unstable angina.
Discussion
Key findings
In this study, we found different correlations between miR-127, miR-150, and miR-145 and the diagnosis of UA. These miRNAs may serve as new biomarkers for a UA diagnosis.
Strengths and limitations
Here we measured the differentially expressed miRNAs in the serum EVs of patients with UA. Clinical symptoms and myocardial injury markers of UA are similar to those of NSTEMI, which greatly enhances the difficulty of the non-invasive diagnosis of UA. To precisely screen out patients with NSTEMI and UA, serum samples were collected to identify differentially expressed miRNAs in serum EVs. Here, miR-127, miR-150, and miR-145 were downregulated in UA patients but upregulated in NSTEMI patients versus HVs. No significant differences in expression levels were detected between SA patients and HVs. Subsequently, the relative levels of miR-127, miR-150, and miR-145 in the serum EVs of 134 UA patients and 50 HVs were measured by qRT-PCR to validate our sequencing findings. These genes were weakly expressed in patients with UA. ROC curves were plotted to explore the diagnostic potential of UA. Notably, the AUC of the combination of the three differentially expressed miRNAs in the UA diagnosis was higher than that of the combination of CK-MB and cTnI. The GO analysis revealed that miR-127, miR-150, and miR-145 were mainly enriched in cell adhesion and migration, Hinterdobler et al. suggested that acute stress enhances leucocyte influx into mouse atherosclerotic plaques by modulating endothelial cells, increases adhesion molecule expression (8). Whereas the KEGG pathway enrichment analysis showed that they were enriched in the PI3K-Akt, MAPK, and Hippo signaling pathways. The introduction of CK-MB, cTnI, miR-127, miR-150, and miR-145 into the logistic regression analyses revealed that increased cTnI levels and downregulated miR-127, miR-150, and miR-145 were significantly associated with an increased risk of UA. Finally, Spearman’s rank correlation test showed a significant correlation between cTnI and miR-127 in patients with UA (P=0.0067).
This study had some limitations, e.g., (I) this study is a single-center study, Because of the relatively small profiling sample size, differently expressed miRNAs are expressed in high and low levels, which do not yet provide a very accurate cut-off value; (II) it did not include observations of long-term prognoses and predictions of the incidence of adverse cardiovascular events in patients with UA with low miR-127, miR-150, and miR-145 expression. Those are the goals of our future research.
Comparison with similar researches
The diagnostic potentials of miRNAs in UA have been previously analyzed. Zeller et al. compared serum miRNA levels in patients with chest pain and no obstructive coronary artery disease, and UA patients. Most of them are downregulated in UA patients. Among them, miR-132, miR-150, and miR-186 exert the diagnostic potential in UA, and their combination detection produces a higher diagnostic potential (9). Ali Sheikh et al. demonstrated that miR-21 may be a potential diagnostic marker for coronary heart disease (10). Our study mainly identified differentially expressed miRNAs in serum exosomes of UA patients, and consistently, miRNA-150 was found to be lowly expressed in UA patients. Moreover, the combination detection of miR-127, miR-150 and miR-145 exerted the best diagnostic potential in UA. We also analyzed the diagnostic potential of conventional biochemical indexes in UA, and their potential correlation. Through analyzing the molecular mechanisms of target genes involved in UA patients, our findings provide a new direction to the future explorations.
Explanations of findings
Patients with UA and NSTEMI exhibit similar clinical symptoms and ECG characteristics. Since the beginning of the 21st century, UA and NSTEMI have collectively been referred to as non-ST-segment elevation acute coronary syndrome (11). The diagnosis of UA must rely on “a clinical assessment and a careful and full clinical history” (3). If UA or NSTEMI cannot be differentiated in the emergency department with chest pain, patients must remain in the hospital for observation for 12 h after the occurrence of myocardial ischemia. During this time, peripheral blood collection must be performed to test for highly sensitive cardiac troponin (hs-cTN) levels immediately and at 0, 1, 3 (or 2), and 6 h after the occurrence in addition to ECG examinations (1). Consequently, the active exploration of objective indicators that can help quickly and accurately diagnose UA is imperative. Moreover, miRNAs in EVs are involved in the pathological processes of cardiovascular diseases (12-14), and all cells can secrete EVs into the blood. Furthermore, miRNAs in EVs function stably and can be reliably quantified, indicating that they are potential non-invasive biomarkers of cardiovascular diseases (15).
The diagnostic potential of miRNAs in coronary artery diseases has been explored (16-18). Here we discovered that miR-127, miR-150, and miR-145 levels were low in patients with UA and high in those with NSTEMI. This may be due to several reasons. First, after blood clots completely block coronary arteries, local pressure in the chest cavity increases considerably; Leistner et al. suggested that miRNA profiles are correlated with pressure gradients in the coronary arteries (19). Second, miRNAs are dynamically expressed during the later stages of pathological progression (20). Unlike acute myocardial infarction, UA does not involve myocardial necrosis (1); instead, it results in myocardial ischemia through a chain reaction induced by inflammatory factors and procoagulants released by parts in which the plaque is disrupted due to vulnerable plaque ulceration, rupture, and hemorrhage. The typical pathological characteristics of vulnerable plaques are thin fibrous caps, large lipid cores, and active inflammation (21). Inflammatory cell adhesion and infiltration, cell necrosis, apoptosis, and pathological angiogenesis are the main mechanisms involved in vulnerable plaque formation. MiR-127 is present in vulnerable plaques (22). Moreover, miR-127 is involved in macrophage polarization and pathological intimal hyperplasia. According to Ying et al., miR-127 can promote the development of M1 macrophages and inhibit the transcription of M2 macrophage genes while simultaneously acting on downstream TLR4 receptors to encourage inflammation (23). In addition, the upregulation of miR-127 increased Fe2+ content and inhibited the expression of GPX4 protein (24), overload of Fe2+ will aggravate myocardial cell injury (25-26). In contrast, miR-150 is involved in apoptosis (27), with one of its pathways involving the regulation of Akt2 phenotypes (28). Finally, miR-150 is also involved in the oxidative metabolism of mitochondria in endothelial cells through protein tyrosine phosphatase, mitochondrial 1 (PTPMT1)-cardiolipin signal pathways; insufficient mitochondrial oxidation is the main cause of endothelial cell apoptosis. It can also act on intermediary factors, such as c-MYB, NOTCH3, and transforming growth factor-β, thereby becoming involved in the occurrence of cell inflammation (29). And miR-150 regulates endothelial progenitor cell differentiation via Akt and promotes thrombus resolution (30). Interestingly, miR-145 is the most abundant miRNA in vascular walls (31) and a phenotype marker of vascular smooth muscle cells (VSMCs) (32). It can also control cytoskeletal reorganization and phenotypic transformation of smooth muscle cells during vascular injury through serum response factors (33). Moreover, vascular injury can decrease miR-145 expression in VSMCs, and miR-145, through negative feedback, acts on the target gene KLF5, which reduces VSMC differentiation through its downstream molecule myocardin. Additionally, overexpression of miR-145 inhibited the mtDNA copy number, ATP production, and mitochondrial activity, which happen when myocardial ischemia (34). And finally,miR-145 is involved in neointima hyperplasia (32).
Implications and actions needed
Furthermore, because miRNAs play a critical role in pharmacology owing to their downregulated genes and, therefore, affect drug function, greater attention should be paid to the functions of miR-127, miR-150, and miR-145 in treating UA.
Conclusions
MiR-127, miR-150, and miR-145 in serum EVs are closely linked with UA and could serve as novel diagnostic biomarkers.
Acknowledgments
We would like to express our gratitude to all those who helped us during the progress of the research and writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions. We would like to thank Editage for its help in polishing our paper.
Funding: This paper received support from the General Program of the National Natural Science Foundation of China (No. 81774229); The Second Batch of TCM Leading Talents Training Project in Jiangsu Province (Jiangsu TCM Education [2018] No. 4); General Program of Natural Science Foundation in Jiangsu (No. BK20161115); Specific Program of TCM Science and Technology in Nanjing (Nanjing Health Finance No. 2022, 79); and Scientific Research Program of Nanjing Hospital of Traditional Chinese Medicine (TCM Scientific Research of Nanjing No. 2019, 4).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-575/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-575/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-575/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-575/coif). All authors report that they received consulting fees from AMCA and the study was supported by General Program of National Natural Science Foundation of China (No. 81774229), the Second Batch of TCM Leading Talents Training Project in Jiangsu Province (Jiangsu TCM Education [2018] No. 4), General Program of Natural Science Foundation in Jiangsu (No. BK20161115), Specific Program of TCM Science and Technology in Nanjing (Nanjing Health Finance No. 2022, 79), Scientific Research Program of Nanjing Hospital of Traditional Chinese Medicine (TCM Scientific Research of Nanjing No. 2019, 4). NG participated on a Data Safety Monitoring Board for the Science and Technology Department of Nanjing Hospital of Traditional Chinese Medicine. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Interventions were not given to patients during the treatment. Therefore, we did not register this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Nanjing Hospital of Traditional Chinese Medicine affiliated with Nanjing University of Chinese Medicine (No. KY2019067) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289-367. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Mendis S, Thygesen K, Kuulasmaa K, et al. World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol 2011;40:139-46. [Crossref] [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-94. [Crossref] [PubMed]
- Hunter MP, Ismail N, Zhang X, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One 2008;3:e3694. [Crossref] [PubMed]
- Jansen F, Yang X, Proebsting S, et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc 2014;3:e001249. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. [Crossref] [PubMed]
- Hinterdobler J, Schott S, Jin H, et al. Acute mental stress drives vascular inflammation and promotes plaque destabilization in mouse atherosclerosis. Eur Heart J 2021;42:4077-88. [Crossref] [PubMed]
- Zeller T, Keller T, Ojeda F, et al. Assessment of microRNAs in patients with unstable angina pectoris. Eur Heart J 2014;35:2106-14. [Crossref] [PubMed]
- Ali Sheikh MS. Diagnostic Role of Plasma MicroRNA-21 in Stable and Unstable Angina Patients and Association with Aging. Cardiol Res Pract 2020;2020:9093151. [Crossref] [PubMed]
- Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2000;36:970-1062. [Crossref] [PubMed]
- Bonauer A, Carmona G, Iwasaki M, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009;324:1710-3. [Crossref] [PubMed]
- da Costa Martins PA, Salic K, Gladka MM, et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol 2010;12:1220-7. [Crossref] [PubMed]
- Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980-4. [Crossref] [PubMed]
- Gupta SK, Bang C, Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet 2010;3:484-8. [Crossref] [PubMed]
- Gacoń J, Kabłak-Ziembicka A, Stępień E, et al. Decision-making microRNAs (miR-124, -133a/b, -34a and -134) in patients with occluded target vessel in acute coronary syndrome. Kardiol Pol 2016;74:280-8. [Crossref] [PubMed]
- Oerlemans MI, Mosterd A, Dekker MS, et al. Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol Med 2012;4:1176-85. [Crossref] [PubMed]
- Kalayinia S, Arjmand F, Maleki M, et al. MicroRNAs: roles in cardiovascular development and disease. Cardiovasc Pathol 2021;50:107296. [Crossref] [PubMed]
- Leistner DM, Boeckel JN, Reis SM, et al. Transcoronary gradients of vascular miRNAs and coronary atherosclerotic plaque characteristics. Eur Heart J 2016;37:1738-49. [Crossref] [PubMed]
- Cipollone F, Felicioni L, Sarzani R, et al. A unique microRNA signature associated with plaque instability in humans. Stroke 2011;42:2556-63. [Crossref] [PubMed]
- Chen BL, Li J, Li XB, et al. Pathological characteristics of unstable carotid plaques. Journal of Apoplexy and Nervous Diseases 2014;31:619-21.
- Soeki T, Yamaguchi K, Niki T, et al. Plasma microRNA-100 is associated with coronary plaque vulnerability. Circ J 2015;79:413-8. [Crossref] [PubMed]
- Ying H, Kang Y, Zhang H, et al. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J Immunol 2015;194:1239-51. [Crossref] [PubMed]
- Zhang J, Liu Z, Dong Y. miR-127-5p Targets JAM3 to Regulate Ferroptosis, Proliferation, and Metastasis in Malignant Meningioma Cells. Dis Markers 2022;2022:6423237. [Crossref] [PubMed]
- Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 2021;22:266-82. [Crossref] [PubMed]
- Zhang Y, Xin L, Xiang M, et al. The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed Pharmacother 2022;145:112423. [Crossref] [PubMed]
- Zhu XG, Zhang TN, Wen R, et al. Overexpression of miR-150-5p Alleviates Apoptosis in Sepsis-Induced Myocardial Depression. Biomed Res Int 2020;2020:3023186. [Crossref] [PubMed]
- Russomanno G, Jo KB, Abdul-Salam VB, et al. miR-150-PTPMT1-cardiolipin signaling in pulmonary arterial hypertension. Mol Ther Nucleic Acids 2020;23:142-53. [Crossref] [PubMed]
- Ji R, Cheng Y, Yue J, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 2007;100:1579-88. [Crossref] [PubMed]
- Du X, Hu N, Yu H, et al. miR-150 regulates endothelial progenitor cell differentiation via Akt and promotes thrombus resolution. Stem Cell Res Ther 2020;11:354. [Crossref] [PubMed]
- Cheng Y, Liu X, Yang J, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 2009;105:158-66. [Crossref] [PubMed]
- Xin M, Small EM, Sutherland LB, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 2009;23:2166-78. [Crossref] [PubMed]
- Kaudewitz D, Zampetaki A, Mayr M. MicroRNA Biomarkers for Coronary Artery Disease? Curr Atheroscler Rep 2015;17:70. [Crossref] [PubMed]
- Zhao S, Zhang Y, Pei M, et al. miR-145 inhibits mitochondrial function of ovarian cancer by targeting ARL5B. J Ovarian Res 2021;14:8. [Crossref] [PubMed]


