
Fluid loading during the hemodynamic evaluation of pulmonary hypertension: a cross-sectional study
Highlight box
Key findings
• In patients who underwent right heart catheterization (RHC) for pulmonary hypertension (PH) evaluation and had a fluid challenge, about 80% of those with pulmonary artery wedge pressure (PAWP) 13–15 mmHg achieved a PAWP >18 mmHg, the current cut-off for a positive test.
What is known and what is new?
• Fluid challenge during RHC is used to unmask occult PH due to left heart disease (PH-LHD);
• Pulmonary hemodynamic determinations during fluid challenge had weak associations with parameters supportive of PH-LHD.
What is the implication, and what should change now?
• The change in PAWP and a PAWP >18 mmHg with fluid challenge are weakly associated with the pretest probability of PH-LHD. Patients with PAWP between 13–15 mmHg commonly have PAWP >18 mmHg with fluids, questioning the use of fluid challenge in this group of patients, and supporting a PAWP of >12 mmHg as abnormal.
Introduction
Pulmonary hypertension (PH) is a condition characterized by a mean pulmonary artery pressure (mPAP) >20 mmHg during right heart catheterization (RHC) (1). PH is classified into five different clinical categories based on the underlying cause of disease: group 1—pulmonary arterial hypertension (PAH); group 2—PH due to left heart disease (PH-LHD); group 3—PH due to lung disease or hypoxia; group 4—PH due to chronic thromboembolic disease; and group 5—PH due to unclear or multifactorial causes (2,3). PH-LHD is the most common type of PH (4) and is characterized by elevated left-sided filling pressures (5). The 6th World Symposium on PH (WSPH) categorized the hemodynamic phenotypes of PH-LHD as: (I) isolated postcapillary PH: pulmonary artery wedge pressure (PAWP) >15 mmHg and pulmonary vascular resistance (PVR) <3 Wood units (WU); and (II) combined pre- and post-capillary PH (CpcPH): PAWP >15 mmHg and PVR ≥3 WU (2).
Provocative testing such as exercise and fluid challenge may be used to unmask occult PH-LHD (5,6). Even though exercise is more physiologic, fluid challenge is more practical (5,7). In fact, the recent PH proceedings recommend fluid challenge over exercise for technical reasons (5). An increase in PAWP to >18 mmHg during fluid challenge supports the diagnosis of postcapillary PH (5). There is still uncertainty about the clinical value of fluid challenge, particularly in patients with PAWP between 13–15 mmHg, and the specific hemodynamic determinations and thresholds that support the diagnosis of PH-LHD.
In the present study, we hypothesized that other hemodynamic variables are better associated with parameters indicating PH-LHD rather than the currently used PAWP cutoff of >18 mmHg (5). Our objective was to test several hemodynamic variables during fluid challenge to assess whether they have a better association with the pretest probability of PH-LHD; and the value of fluid challenge in patients with PAWP between 13–15 mmHg, which is a pressure range above normal but below the threshold of >15 mmHg to define postcapillary PH (5). The pre-specified variables that we tested were PAWP, PAWP/cardiac output (CO) and right atrial pressure to pulmonary arterial wedge pressure ratio (RAP/PAWP) (8) during the fluid challenge or their absolute change from baseline. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-59/rc).
Methods
Study subjects and design
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This single-center cross-sectional study with the incorporation of retrospective data was approved by Cleveland Clinic Institutional Review Board (No. 19-104) and written informed consent was waived due to the retrospective analysis. Between April 2013 and January 2019, we included all consecutive patients who underwent rapid fluid challenge testing during RHC and had: (I) normal pulmonary pressures; (II) PH (mPAP >20 mmHg) with intermediate or high pretest probability of having PH-LHD (to determine whether they had occult postcapillary PH) (5); (III) PH with PAWP between 13–15 mmHg (regardless of the number of risk factors for PH-LHD; (IV) PAWP >15 mmHg (usually 16–18 mmHg) with limited risk factors for left heart disease (LHD); or (V) patients with inadequate response to PAH medications with suspicion of PH-LHD. Patients who failed to show improvement in World Health Organization (WHO) functional class and/or had clinical worsening after treatment with PAH-specific therapies were deemed to have inadequate treatment response (9).
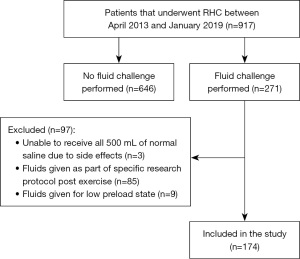
The rationale for including patients with PAWP >15 mmHg, in the absence of strong clinical suspicion of PH-LHD, was due to the frequent overestimation of PAWP in the setting of elevated intrathoracic pressure in patients with obesity (10). In fact, we previously showed that when adjusting for esophageal pressure there was a reduction in isolated postcapillary PH from 60% to 8% and CpcPH from 34% to 11% (10). We excluded patients who could not tolerate a total of 500 mL of normal saline due to the development of symptoms, or underwent fluid challenge for other reasons: e.g., to assess the effect on CO in patients with a low preload state, or post-exercise as part of a specific clinical research study protocol (11).
RHC and fluid challenge
RHC was performed in the outpatient setting by a single operator (Tonelli AR) (10,12). The mPAP and PAWP were measured at end-expiration, using electronic calipers and waveform tracings of three respiratory cycles. CO was measured by thermodilution.
After baseline measurements were obtained, 500 mL of normal saline (0.9%) at room temperature were administered over the course of 5 minutes through the side port of an 8.5-French introducer, with the tip located in the superior vena cava. The fluid bag was pressurized at 200 mmHg to facilitate a rapid infusion. Immediately after the end of the fluid administration we recorded a full set of hemodynamic measurements (6).
Other data reported
We collected data from our electronical medical records on clinical characteristics, cardiovascular risk factors, N-terminal pro-B type natriuretic peptide (NT-proBNP), six-minute walk test, and echocardiographic determinations.
Statistical analysis
Continuous data are presented as mean ± standard deviation (SD), or median [interquartile range (IQR)] as appropriate. Normality was tested visually with Q-Q plot and by the Kolmogorov-Smirnov test. Categorical data are summarized as discrete values and percentages [n (%)]. The PAWP and PAWP/CO change was calculated as the difference between the values obtained at the end of fluid administration and baseline determinations. Continuous and categorical variables were compared across the groups using t-test or analysis of variance (ANOVA) and Chi-square, respectively. Paired data were contrasted with paired t-test or Wilcoxon signed-rank test based on normality. Associations between variables were tested using linear regression. We included consecutive patients that met inclusion/exclusion criteria during the study period (convenience sample). All P values are two-tailed and a value of <0.05 was considered significant. All the hemodynamic data was available. In specific analyses like correlation, we excluded cases with a missing variable used in the comparison. Additionally, sensitivity analyses were performed removing patients with PAWP >15 mmHg. The statistical analyses were performed using the statistical package IBM SPSS, version 22 (IBM; Armonk, NY, USA).
Results
Patient characteristics
During the study period, 917 patients underwent RHC while 174 consecutive patients with a qualifying fluid challenge were included (Table 1 and Figure 1). Mean ± SD age was 63.7±13.0 years, of whom 123 (71%) were women. RHC was done due to abnormal echocardiogram (n=90, 52%), unexplained dyspnea with suspicion of PH (n=42, 24%), suggestion of worsening PH (n=22, 13%), hemodynamic assessment before PAH treatment changes (n=14, 8%), and abnormal echocardiogram during liver transplant evaluation (n=6, 3%).
Table 1
| Variables | Values (n=174) |
|---|---|
| Age (years) | 63.7±13.0 |
| Gender (female) | 123 [71] |
| Race | |
| White | 138 [79] |
| Black | 30 [17] |
| Other | 6 [3] |
| BMI (kg/m2) | 31±8 |
| WHO functional class | |
| I | 26 [15] |
| II | 78 [45] |
| III | 62 [36] |
| IV | 8 [5] |
| Hypertension | 105 [60] |
| Diabetes mellitus | 37 [21] |
| Obstructive sleep apnea | 54 [31] |
| Coronary artery disease | 42 [24] |
| History of venous thromboembolism | 32 [18] |
| NT-proBNP (pg/mL) (n=122) | 241 [104–734] |
| Six-minute walk test (m) (n=133) | 312±116 |
| LVEF (%) (n=169) | 63±7 |
| LA volume index (mL/m2) (n=158) | 32±12 |
| LV mass index (g/m2) (n=134) | 85±24 |
| RVSP (mmHg) (n=155) | 52±18 |
| SvO2 (%) | 70.3±6.4 |
| LV diastolic dysfunction (n=131) | |
| None | 39 [22] |
| Grade I | 64 [37] |
| Grade II | 28 [16] |
| RV dysfunction (n=165) | |
| Normal | 135 [78] |
| Mild | 17 [10] |
| Moderate to severe | 13 [7] |
Data are presented as mean ± SD, median [IQR] or n [%]. BMI, body mass index; WHO, World Health Organization; NT-proBNP, N-terminal pro-B type natriuretic peptide; LVEF, left ventricular ejection fraction; LA, left atrial; LV, left ventricle; RVSP, right ventricular systolic pressure; SvO2, mixed venous oxygen saturation; RV, right ventricle; SD, standard deviation; IQR, interquartile range.
Baseline hemodynamic determinations
At baseline, the mean ± SD PAWP was 11±5 mmHg, with a PAWP/CO ratio of 2.1±1.1 WU and a RAP/PAWP ratio of 0.54±0.27 (Table 2). The baseline PAWP was <13, 13–15 and >15 mmHg in 103 (59%), 37 (21%) and 34 (20%) patients, respectively. Only 15 (9%) patients had a PAWP >18 mmHg.
Table 2
| Variables | Baseline (n=174) | Fluid loading (n=174) | Mean difference (95% CI) | P value |
|---|---|---|---|---|
| Systolic BP (mmHg) | 144±26 | 144±25 | 0.0 (−2.4, 2.4) | >0.99† |
| Diastolic BP (mmHg) | 74±12 | 75±12 | 0.3 (−1.0, 1.6) | 0.64† |
| Heart rate (bpm) | 75±14 | 74±12 | −1.2 (−2.2, −0.2) | 0.02† |
| RAP (mmHg) | 6±4 | 11±5 | 4.5 (4.0, 5.0) | <0.001† |
| Systolic PAP (mmHg) | 38±15 | 45±14 | 7.2 (6.4, 8.1) | <0.001† |
| Mean PAP (mmHg) | 26±10 | 34±9 | 7.1 (6.4, 7.7) | <0.001† |
| Diastolic PAP (mmHg) | 18±8 | 24±7 | 5.7 (5.0, 6.5) | <0.001† |
| PAWP (mmHg) | 11±5 | 18±5 | 6.9 (6.3, 7.4) | <0.001† |
| RAP/PAWP | 0.54±0.27 | 0.58±0.23 | 0.05 (0.01, 0.09) | 0.02† |
| TPG (mmHg) | 13 [9, 19] | 13 [9, 19] | 0.3 (−0.2, 0.8) | 0.24‡ |
| DPG (mmHg) | 5 [2, 10] | 4 [2, 8] | −1.1 (−1.9, −0.4) | <0.001‡ |
| PAPi | 3.3 [2.0, 5.0] | 2.0 [1.3, 3.0] | −2.1 (−2.7, −1.4) | <0.001‡ |
| CO thermo (L/min) | 6.0±2.0 | 6.6±2.1 | 0.6 (0.5, 0.7) | <0.001† |
| CI thermo (L/min/m2) | 3.1±0.9 | 3.4±1.0 | 0.3 (0.2, 0.4) | <0.001† |
| SVI (mL/min/m2) | 42.4±13.5 | 45.9±12.6 | 3.6 (2.0, 5.2) | <0.001† |
| PAWP/CO (WU) | 2.1±1.1 | 3.1±1.5 | 1.1 (0.9, 1.2) | <0.001† |
| PVR (WU) | 2.2 [1.5, 3.4] | 2.2 [1.4, 3.3] | −0.2 (−0.4, 0.0) | 0.003‡ |
| SVR (dynes·seconds/cm5) | 1,354±515 | 1,187±479 | −167 (−205, −129) | <0.001† |
| PAC (mL/mmHg) | 4.3 [2.9, 6.3] | 4.4 [3.0, 6.6] | −0.2 (−1.1, 0.7) | 0.15‡ |
| SAC (mL/mmHg) | 1.2 [0.9, 1.6] | 1.3 [0.9, 1.7] | 0.1 (0.1, 0.2) | <0.001‡ |
| pEA (mmHg/mL) | 0.4 [0.3, 0.7] | 0.49 [0.4, 0.7] | 0.04 (0.02, 0.06) | <0.001‡ |
| sEA (mmHg/mL) | 1.6 [1.3, 2.1] | 1.5 [1.2, 1.9] | −0.2 (−0.2, −0.1) | <0.001‡ |
Data are presented as mean ± SD or median [IQR] if not otherwise specified. †, paired t-test; ‡, Wilcoxon signed-rank test. 95% CI, 95% confidence interval; BP, blood pressure; bpm, beats per minute; RAP, right atrial pressure; PAP, pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; TPG, transpulmonary gradient; DPG, diastolic pulmonary gradient; PAPi, pulmonary artery pulsatility index; CO, cardiac output; thermo, thermodilution; CI, cardiac index; SVI, stroke volume index; WU, Wood units; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance; PAC, pulmonary arterial compliance; SAC, systemic arterial compliance; pEA, pulmonary effective arterial elastance; sEA, systemic effective arterial elastance; SD, standard deviation; IQR, interquartile range.
Effect of fluid loading on hemodynamic determinations
The 500 mL of normal saline administered resulted in 5.8±1.3 mL/kg of fluid. Fluid loading resulted in significant changes in several hemodynamic determinations (Table 2). The absolute increase in PAWP was 6.9±3.6 mmHg. The PAWP change was inversely associated with baseline PAWP (unit: mmHg, constant: 9.2, β: −0.20, R2: 0.09, P<0.001). In fact, the increase in PAWP was 7.5±3.5, 6.9±3.3 and 4.8±3.6 mmHg, for patients with a baseline PAWP of <13, 13–15 and >15 mmHg, respectively (P=0.001). Remarkably, the PAWP with fluids was >18 mmHg in 24 (23%) patients with baseline PAWP <13 mmHg and 30 (81%) patients with baseline PAWP 13–15 mmHg. All patients (n=19) with baseline PAWP between 16 and 18 mmHg had a PAWP >18 mmHg during fluid administration.
The absolute change in PAWP with fluids was directly but weakly associated with age (unit: year, constant: 3.4, β: 0.05, R2: 0.04, P=0.01), female gender (female: 1, constant: 5.5, β: 2.0, R2: 0.06, P=0.001), and systolic blood pressure (unit: mmHg, constant: 2.4, β: 0.03, R2: 0.05, P=0.003), while it was inversely associated with body mass index (BMI) (unit: kg/m2, constant: 9.2, β: −0.07, R2: 0.03, P=0.04). No significant association was noted between change in PAWP with fluids and left atrial volume index (P=0.86), left ventricular (LV) mass index (P=0.75), right ventricular function (P=0.17), or mitral regurgitation (MR) severity (P=0.12).
The PAWP/CO had an absolute increase of 1.06±0.91 WU. The absolute change in PAWP/CO with fluids was predominantly driven by the absolute change in PAWP (unit: WU, constant: 3.8, β: 2.9, R2: 0.52, P<0.001) and less by the change in CO (unit: L/min, constant: 1.28, β: −0.37, R2: 0.13, P<0.001). The PAWP/CO slopes are not provided because as we have only two time points (baseline and with fluids) and since the CO minimally and inconsistently increased with fluids, the values are not reliable. The RAP/PAWP ratio was 0.58±0.23 with fluids, with an absolute increase of 0.05±0.28 when compared to baseline.
Effect of fluid loading in the hemodynamic classification of PH
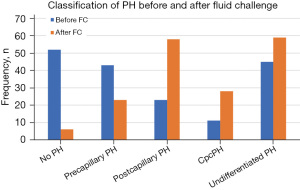
At baseline, 52 (30%) patients had no PH, 43 (25%) had precapillary PH, 23 (13%) had postcapillary PH, 11 (6%) had combined pre and postcapillary PH and 45 (26%) had undifferentiated PH (mPAP >20 mmHg, PAWP ≤15 mmHg and PVR <3 WU). With fluid challenge and using a cutoff of >18 mmHg to identify the presence of postcapillary PH, 6 (3%) patients had no PH, 23 (13%) had precapillary PH, 58 (33%) had postcapillary PH, 28 (16%) had combined pre and postcapillary PH and 59 (34%) had undifferentiated PH (mPAP >20 mmHg, PAWP ≤18 mmHg and PVR <3 WU) (Figure 2).
Fluid loading and pretest probability of PH-LHD
The association between PAWP and PAWP/CO response to fluid loading and the pretest probability of PH-LHD (5,13) is shown in Table 3. The PAWP at baseline, with fluids, its absolute change or the percentage of patients that achieve a value >18 mmHg, were not associated with age, number of cardiovascular risk factors, left atrial enlargement or degree of LV diastolic dysfunction on echocardiogram. All PAWP determinations were higher in patients with any degree of MR. The PAWP with fluids and its absolute change from baseline were higher in patients with ≥ mild increase in LV mass index. The PAWP/CO with fluids and its absolute change from baseline were higher with increasing age groups; while PAWP/CO at baseline and with fluids were higher in the presence of MR and LV diastolic dysfunction. The RAP/PAWP with fluids (but not the absolute change) was only higher in patients with mild to moderate MR, compared to no MR, but there was no statistical association with other markers of pretest probability of PH-LHD.
Table 3
| Features | High probability of PH-LHD | Intermediate probability of PH-LHD | Low probability of PH-LHD | P (ANOVA, Chi-square) |
|---|---|---|---|---|
| Age (years) | >70 | 60–70 | <60 | – |
| Number | 56 | 59 | 59 | – |
| ΔPAWP (mmHg) | 6.0±3.9 | 6.9±3.1 | 7.6±3.7 | 0.06 |
| ΔPAWP/CO (WU) | 1.4±1.1 | 1.0±0.8 | 0.8±0.6 | 0.001 |
| RAP/PAWP with fluids | 0.58±0.28 | 0.58±0.19 | 0.58±0.21 | 0.97 |
| PAWP >18 mmHg with fluids (n=140) | 21/45 [47] | 19/46 [41] | 14/49 [29] | 0.18 |
| Cardiovascular risk factors† | >2 | 1–2 | 0 | – |
| Number | 40 | 115 | 19 | – |
| ΔPAWP (mmHg) | 6.8±3.3 | 7.0±3.8 | 6.1±3.4 | 0.56 |
| ΔPAWP/CO (WU) | 1.0±0.9 | 1.1±0.9 | 0.8±0.7 | 0.40 |
| RAP/PAWP with fluids | 0.60±0.27 | 0.57±0.22 | 0.67±0.22 | 0.19 |
| PAWP >18 fluids (n=140) | 17/32 [53] | 34/92 [37] | 3/16 [19] | 0.06 |
| LA enlargement (LAVi, mL/m2) | >34 | 28–34 | <28 | – |
| Number‡ | 56 | 35 | 67 | – |
| ΔPAWP (mmHg) | 7.0±3.6 | 6.4±3.9 | 6.8±3.5 | 0.73 |
| ΔPAWP/CO (WU) | 0.9±0.6 | 1.3±1.5 | 1.0±0.7 | 0.19 |
| RAP/PAWP with fluids | 0.55±0.21 | 0.63±0.20 | 0.59±0.26 | 0.27 |
| PAWP >18 mmHg with fluids (n=127) | 19/42 [45] | 13/27 [48] | 20/58 [34] | 0.39 |
| MR severity | Moderate, 2+ | Mild, 1+ | None | – |
| Number | 63 | 43 | 63 | – |
| ΔPAWP (mmHg) | 9.1±2.9 | 6.3±3.8 | 7.2±3.3 | 0.03 |
| ΔPAWP/CO (WU) | 1.6±0.7 | 1.0±1.0 | 1.0±0.7 | 0.16 |
| RAP/PAWP with fluids | 0.58±0.17 | 0.64±0.23 | 0.51±0.23 | 0.003 |
| PAWP >18 mmHg with fluids (n=137) | 2/6 [33] | 37/71 [52] | 14/60 [23] | 0.003 |
| LV diastolic dysfunction | Grade II | Grade I | None | – |
| Number§ | 28 | 64 | 39 | – |
| ΔPAWP (mmHg) | 6.3±3.7 | 7.1±3.3 | 6.3±3.9 | 0.40 |
| ΔPAWP/CO (WU) | 1.2±1.4 | 1.0±0.7 | 0.9±0.8 | 0.58 |
| RAP/PAWP with fluids | 0.50±0.17 | 0.50±0.11 | 0.61±0.24 | 0.19 |
| PAWP >18 mmHg with fluids (n=107) | 11/20 [55] | 20/54 [37] | 11/33 [33] | 0.26 |
| LV mass index¶ | Moderate/severe | Mild | Normal | – |
| Number# | 18 | 19 | 114 | – |
| ΔPAWP (mmHg) | 7.0±3.5 | 9.8±3.8 | 6.3±3.5 | 0.001 |
| ΔPAWP/CO (WU) | 1.0±0.7 | 1.6±1.2 | 1.0±0.9 | 0.04 |
| RAP/PAWP with fluids | 0.58±0.21 | 0.58±0.21 | 0.58±0.23 | 0.99 |
| PAWP >18 mmHg with fluids (n=105) | 6/11 [55] | 9/13 [69] | 31/81 [38] | 0.09 |
Variable cutoffs were based on Vachiéry et al. (5) and Lang et al. (13). Data are presented as mean ± SD or n/total [%]. †, including diabetes mellitus, hyperlipidemia, obesity (BMI ≥30 kg/m2) and arterial hypertension; ‡, available in 158 patients; §, reported in 131 patients; ¶, normal: ≤95 g/m2 for female and ≤115 g/m2 for males, mild: 96–108 g/m2 for females and 116–131 g/m2 for males, moderate to severe: ≥109 g/m2 for females and ≥132 g/m2 for males. The number of patients in whom we assessed whether the PAWP increased >18 mmHg with fluids is lower than the total number of patients in the study (n=174), since only 140 had a baseline PAWP ≤15 mmHg; #, reported in 151 patients. PH-LHD, pulmonary hypertension due to left heart disease; ANOVA, analysis of variance; PAWP, pulmonary artery wedge pressure; CO, cardiac output; WU, Wood units; RAP, right atrial pressure; LA, left atrial; LAVi, left atrial volume index; MR, mitral regurgitation; LV, left ventricular; SD, standard deviation; BMI, body mass index.
Sensitivity analyses
At total of 140 patients had a PAWP ≤15 mmHg, age 63.1±13.0 years and 96 (69%) were women. In this cohort, the PAWP was 9.6±3.5 mmHg at baseline and 17.0±4.6 mmHg after fluids with an increase of 7.4±3.4 mmHg. Similarly, the absolute change in PAWP with fluids was directly but weakly associated with age (unit: year, constant: 3.8, β: 0.06, R2: 0.05, P=0.01), female gender (female: 1, constant: 5.8, β: 2.2, R2: 0.09, P<0.001), and systolic blood pressure (unit: mmHg, constant: 3.3, β: 0.03, R2: 0.04, P=0.01), while it was not significantly associated with BMI (P=0.59) or echocardiographic variables tested. The effect of fluid loading on hemodynamics (Table S1) or the associations of hemodynamic changes with fluids and the pretest probability of PH-LHD (Table S2) were similar for the group of patients with PAWP ≤15 mmHg that for the entire cohort.
Discussion
In the present study, we included a relatively large number of patients who underwent RHC for PH evaluation and had a fluid challenge. We noted a median absolute increase in PAWP of 7 mmHg, with a change inversely associated with baseline PAWP. About a quarter of patients with baseline PAWP <13 mmHg and about 80% of those with PAWP 13–15 mmHg achieved a PAWP >18 mmHg with fluids. Patients that achieved a PAWP >18 mmHg with fluids, or the change in PAWP, PAWP/CO or RAP/PAWP with fluids had limited associations with parameters related with PH-LHD (5).
Diagnosing PH-LHD in compensated patients can be challenging, since the PAWP could be temporarily ≤15 mmHg when there is adequate blood volume. Hence, compensated PH-LHD patients may show normal hemodynamics, isolated precapillary PH (in those with combined pre and postcapillary PH) or undifferentiated PH. In this context, a hemodynamic challenge such as exercise (14-16) or rapid fluid infusion (5) is recommended. In patients with heart failure with preserved ejection fraction there is a steeper rise in PAWP with fluids (17) and a PAWP ≥18 mmHg is considered abnormal (5,18).
Since there is no gold-standard to determine the presence of PH-LHD, we incorporated known variables associated with this condition and supported by the 6th WSPH proceedings (5). Overall, the value of the fluid challenge appears limited with unclear hemodynamic determinations to track or thresholds to consider. From our data, it is evident that most of the patients (80%) who have a baseline PAWP between 13 and 15 mmHg have an increase in PAWP with fluids to >18 mmHg (a positive test by current recommendations), questioning the utility of challenging this group of patients and introducing the observation of whether we should be using a PAWP of >12 mmHg during baseline hemodynamic determinations to identify postcapillary PH instead of the current cut-off of >15 mmHg (19). One of the benefits of fluid challenge could potentially be in identifying patients who might experience harm from PAH treatment since it can estimate the effect of an eventual increase in LV preload, that could occur with pulmonary vasodilator therapies, rather than strictly categorizing patients into PH groups 1 and/or 2.
Moghaddam et al. described that a positive fluid challenge led to reclassification of 20% of patients to PH-LHD, and affected treatment decisions in 6.5% of the cases (20). Similarly, Robbins et al. showed that 22% of 207 patients previously diagnosed as PAH, were reclassified as PH-LHD after fluid loading, using a PAWP threshold of >15 mmHg to define a positive fluid challenge (21). Our current data questions the value of fluid challenge to define PH-LHD since it predominantly identified subjects with already high PAWP at baseline (>12 mmHg). The degree of increase in PAWP with fluids was only weakly associated with a few clinical and echocardiography variables associated with LHD. The PAWP/CO change did not substantially outperform the absolute PAWP change, probably because of the relatively small increase in CO with fluids (mean increase of 0.6 L/min).
We acknowledge that our study has limitations. It is a single-center, retrospective cohort study at a tertiary care center that may have overestimated the prevalence of occult PH-LHD. Although the protocol for fluid administration is generally similar among studies, the actual flow may vary based on the relation between the introducer and pulmonary arterial compliance (PAC) diameters, administration site and use of pressurized system, etc. In addition, the determination of PAWP may be subject to errors; however, we followed a strict protocol to establish a valid PAWP measurement (22).
Conclusions
The absolute change in PAWP, PAWP/CO or achieving a PAWP >18 mmHg with rapid fluid loading do not have a robust association with the pretest probability of PH-LHD. Patients with PAWP between 13–15 mmHg commonly have PAWP elevations >18 mmHg with rapid fluid loading, questioning the use of fluid challenge in this group of patients, and raising the observation of whether we should be using a PAWP of >12 mmHg during baseline hemodynamic determinations to identify postcapillary PH, instead of the current cut-off of >15 mmHg.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-59/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-59/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-59/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-59/coif). ART serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2021 to August 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Cleveland Clinic Institutional Review Board (No. 19-104). The consent to participate was waived due to the retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [Crossref] [PubMed]
- Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J 2019;53:1802148. [Crossref] [PubMed]
- Goldberg AB, Mazur W, Kalra DK. Pulmonary hypertension: diagnosis, imaging techniques, and novel therapies. Cardiovasc Diagn Ther 2017;7:405-17. [Crossref] [PubMed]
- Strange G, Playford D, Stewart S, et al. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart 2012;98:1805-11. [Crossref] [PubMed]
- Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019;53:1801897. [Crossref] [PubMed]
- Arunachalam A, Chaisson NF, Tonelli AR. Methods to improve the yield of right heart catheterization in pulmonary hypertension. Respiratory Medicine: X. 2020;2:100015. [Crossref]
- D'Alto M, Badesch D, Bossone E, et al. A Fluid Challenge Test for the Diagnosis of Occult Heart Failure. Chest 2021;159:791-7. [Crossref] [PubMed]
- Fares WH, Bellumkonda L, Tonelli AR, et al. Right atrial pressure/pulmonary artery wedge pressure ratio: A more specific predictor of survival in pulmonary arterial hypertension. J Heart Lung Transplant 2016;35:760-7. [Crossref] [PubMed]
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC), European Respiratory Society (ERS), International Society of Heart and Lung Transplantation (ISHLT), et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219-63. [Crossref] [PubMed]
- Khirfan G, Melillo CA, Al Abdi S, et al. Impact of Esophageal Pressure Measurement on Pulmonary Hypertension Diagnosis in Obese Patients. Chest 2022;162:684-92. [Crossref] [PubMed]
- Montané B, Tonelli AR, Arunachalam A, et al. Hemodynamic Responses to Provocative Maneuvers during Right Heart Catheterization. Ann Am Thorac Soc 2022;19:1977-85. [Crossref] [PubMed]
- Ennala S, Melillo CA, Lane JE, et al. Effect of pulmonary artery catheter balloon inflation on pulmonary hemodynamics. Cardiovasc Diagn Ther 2022;12:37-41. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Borlaug BA, Nishimura RA, Sorajja P, et al. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588-95. [Crossref] [PubMed]
- Sorajja P, Borlaug BA, Dimas V, et al. Executive summary of the SCAI/HFSA clinical expert consensus document on the use of invasive hemodynamics for the diagnosis and management of cardiovascular disease. Catheter Cardiovasc Interv 2017;89:1294-9. [Crossref] [PubMed]
- Reddy YNV, Carter RE, Obokata M, et al. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation 2018;138:861-70. [Crossref] [PubMed]
- Fujimoto N, Borlaug BA, Lewis GD, et al. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation 2013;127:55-62. [Crossref] [PubMed]
- D'Alto M, Romeo E, Argiento P, et al. Clinical Relevance of Fluid Challenge in Patients Evaluated for Pulmonary Hypertension. Chest 2017;151:119-26. [Crossref] [PubMed]
- Kovacs G, Avian A, Pienn M, et al. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med 2014;190:252-7. [Crossref] [PubMed]
- Moghaddam N, Swiston JR, Weatherald J, et al. Impact of saline loading at cardiac catheterization on the classification and management of patients evaluated for pulmonary hypertension. Int J Cardiol 2020;306:181-6. [Crossref] [PubMed]
- Robbins IM, Hemnes AR, Pugh ME, et al. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail 2014;7:116-22. [Crossref] [PubMed]
- Tonelli AR, Mubarak KK, Li N, et al. Effect of balloon inflation volume on pulmonary artery occlusion pressure in patients with and without pulmonary hypertension. Chest 2011;139:115-21. [Crossref] [PubMed]


