
Left ventricular mass and valve performance after surgical and transcatheter aortic valve replacement: a single-center experience from Japan
Highlight box
Key findings
• The aortic valve pressure gradient (PG), aortic valve area (AVA), and stroke volume (SV) were better in the transcatheter aortic valve replacement (TAVR) group. The surgical aortic valve replacement (SAVR) group had less paravalvular leakage and new pacemaker implantation.
• Left ventricular mass (LVM) regression and postoperative outcomes were also better in the SAVR group.
What is known and what is new?
• The LVM after SAVR and TAVR correlated with postoperative outcomes.
• We discussed the LVM, aortic valve PG, AVA, and SV in the context of postoperative outcomes after SAVR and TAVR from Japan.
What is the implication, and what should change now?
• LVM regression can be used not only as an index of postoperative left ventricular remodeling, but also as an index of the appropriate timing and selection of procedures (SAVR or TAVR).
Introduction
Since the study by Cribier et al. (1) in 2002, the number of transcatheter aortic valve replacement (TAVR) procedures as an alternative option for treating aortic valve stenosis (AS) has been increasing (2-5). Recently, good outcomes after TAVR have been reported in Japan as well (6,7). The latest Japanese Circulation Society (JCS) guidelines on the management of valvular heart disease generally recommend surgical aortic valve replacement (SAVR) for patients under the age of 75 years and TAVR for those over the age of 80 years as a treatment for AS (8). Reportedly, the left ventricular mass (LVM) after SAVR and TAVR correlates with operative outcome (9-12). In addition, the aortic valve area (AVA) and pressure gradient (PG) reportedly measure the durability and performance of prosthetic valves after SAVR and TAVR (3,5,13). Therefore, the LVM, AVA, and PG are considered as important factors when discussing the outcomes of SAVR and TAVR. However, there are few reports that discuss the LVM, AVA, and PG in the context of postoperative outcomes after SAVR and TAVR. Further, there are a few reports from Japan on valve performance after TAVR (14).
In the present study, we aimed to compare the postoperative changes in the LVM, AVA, stoke volume (SV) and PG between SAVR and TAVR patients from a single center in Japan. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-119/rc).
Methods
All surgical and clinical data were collected at the Ise Red Cross Hospital, Ise, Japan. Clinical outcome data were obtained from the hospital patient records and were confirmed either by information provided by the patient’s family physician or a telephone survey with the patient’s family.
This study was approved by the institutional review board of the Ise Red Cross Hospital (5/6/2022, approval No. ER2022-11) and was conducted according to the principles of the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice guidelines. The need for informed consent was waived because of the retrospective nature of the study. All study activities were carried out in accordance with the relevant guidelines and regulations.
Study design and patients
Between January 2012 and May 2022, 257 patients with severe AS underwent SAVR at Ise Red Cross Hospital, and 107 of these were included in the SAVR group. All patients with concomitant mitral valve and/or tricuspid valve surgery, aortic aneurysm repair, coronary artery bypass grafting (CABG) and implanted mechanical prosthetic valves were excluded. In comparison, between January 2016 and May 2022, 278 patients with severe AS underwent TAVR at our institution. Of these, 274 patients were included in the TAVR group, while valve-in-valve patients were excluded. The decision to choose between SAVR and TAVR was made by the institution’s heart team, consisting of cardiothoracic surgeons, cardiologists, radiologists, anesthesiologists, and other related medical professionals.
Operative technique
SAVR was performed via a median sternotomy using a moderately hypothermic cardiopulmonary bypass. Myocardial protection was performed with cold and warm blood cardioplegia using a combination of antegrade and retrograde methods. Antithrombotic therapy in SAVR patients consisted of oral warfarin for three months after replacement with biological valves. TAVR was performed under general or local anesthesia in the hybrid catheterization laboratory. Antithrombotic therapy following TAVR consisted of dual antiplatelet agents from 2016 to 2019 and a single antiplatelet agent since 2020. SAVR was performed by two cardiovascular surgeons with more than 20 years of experience. TAVR was performed by three cardiologists with more than 15 years of experience.
Echocardiographic data
Echocardiographic variables were measured by five experienced echocardiographers in all patients. Echocardiograms were obtained at preoperatively, at discharge and at 1 and 3 years after the procedures. The valve performance was evaluated by a serial assessment of the aortic valve (AV) peak velocity, AV peak PG, AV mean PG, AVA, aortic valve area index (AVAI), stroke volume (SV) and stroke volume index (SVI). Left ventricular (LV) volumes in cubic centimeters were measured from apical 4-chamber views using the Simpson’s rule. In patients in whom the Simpson’s rule could not be used, the area-length method was used. The LVM was calculated using the formula recommended by the American Society of Echocardiography and indexed to the body surface area (15,16). LV mass was calculated using values obtained for the external (EDVe) and internal (EDVi) end-diastolic volume using the following equation: LVM = 1.05 (EDVe − EDVi) (g).
Primary outcomes and secondary outcomes
Primary outcomes were AV mean PG, AVAI, SVI and LVM in the SAVR and TAVR patients. Secondary outcomes were all cause of death at 5 years, heart failure rehospitalization at 5 years and major adverse cerebral and cardiovascular events (MACCEs) at 5 years in the SAVR and TAVR patients.
Statistical analysis
All statistical analyses were performed using the statistical software EZR (Easy R) on R commander (17). Continuous variables are expressed as mean ± standard deviation. Non-parametric data were compared using an unpaired Mann-Whitney U test, and parametric data were compared using an unpaired Student’s t-test. Categorical variables are expressed as counts and percentages and were compared using Fisher’s exact test. In comparisons between the SAVR and TAVR groups, cumulative incidences were estimated using Kaplan-Meier curves, and differences were evaluated using the log-rank test. The effects of SAVR and TAVR on change over time in AV mean PG, AVAI, SVI and LVM index (LVMI) were evaluated using the two-way repeated-measured analysis of variance (ANOVA). Statistical significance was set at P<0.05 for all analyses.
Results
Preoperative characteristics
Overall, 257 patients with severe AS underwent SAVR at our institution, and 107 of these were included in the SAVR group. All patients with concomitant mitral valve and/or tricuspid valve surgery, aortic aneurysm repair, CABG and implanted mechanical prosthetic valves were excluded. In comparison, 278 patients with severe AS underwent TAVR at our institution. Of these, 274 patients were included in the TAVR group, while valve-in-valve patients were excluded. The preoperative characteristics of patients in the TAVR and SAVR groups are listed in Table 1. Patients in the TAVR group were significantly older (P<0.001) and comprised more females (P=0.003). The body mass index (BMI) and body surface area (BSA) were significantly lower in the TAVR group (P<0.001). The serum creatinine level was significantly higher in the SAVR group (P<0.001), and hemoglobin and albumin levels were significantly lower in the TAVR group (P<0.001 and P=0.01, respectively). The number of patients undergoing hemodialysis was significantly higher in the SAVR group (P<0.001). The AVAI was significantly smaller in the TAVR group (P<0.001). There was no difference in the LVMI between the two groups (P=0.18). The incidences of mitral regurgitation (MR) and tricuspid regurgitation (TR) were significantly higher in the TAVR group (P=0.01 and P=0.02, respectively). The Society of Thoracic Surgeons (STS) score and European System for Cardiac Operative Risk Evaluation (EuroSCORE) II score were significantly higher in the TAVR group (P<0.001).
Table 1
| Characteristics | TAVR group (n=274) | SAVR group (n=107) | P value |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 84.8±4.5 | 74.7±7.0 | <0.001 |
| Female gender | 178 (65.0) | 52 (48.6) | 0.003 |
| BMI (kg/m2) | 21.7±3.6 | 23.4±4.1 | <0.001 |
| BSA (m2) | 1.45±0.1 | 1.57±0.1 | <0.001 |
| NYHA class III or IV | 90 (32.8) | 41 (38.3) | 0.33 |
| Past medical history | |||
| Hypertension | 199 (72.6) | 98 (91.6) | <0.001 |
| Dyslipidemia | 99 (36.1) | 70 (65.4) | <0.001 |
| Diabetes mellitus | 79 (28.8) | 41 (38.3) | 0.08 |
| Diabetes mellitus on insulin therapy | 15 (5.5) | 6 (5.6) | 1 |
| COPD | 21 (7.7) | 10 (9.3) | 0.67 |
| Malignancy | 55 (20.1) | 26 (24.3) | 0.4 |
| Peripheral vascular disease | 30 (10.9) | 9 (8.4) | 0.57 |
| Cerebral vascular disease | 52 (19.0) | 12 (11.2) | 0.09 |
| Prior PCI | 49 (17.9) | 12 (11.2) | 0.12 |
| Prior CABG | 8 (2.9) | 0 | 0.11 |
| Prior cardiac surgery | 0 | 0 | – |
| Atrial fibrillation or flutter | 31 (11.3) | 3 (2.8) | 0.008 |
| Pacemaker implantation | 11 (4.0) | 3 (2.8) | 0.76 |
| Laboratory data | |||
| Serum creatinine (mg/dL) | 1.04±0.7 | 1.9±2.5 | <0.001 |
| Hemoglobin (g/dL) | 11.5±1.6 | 12.1±1.9 | <0.001 |
| Albumin (g/dL) | 3.7±0.4 | 3.8±0.4 | 0.01 |
| Hemodialysis | 1 (0.4) | 17 (15.9) | <0.001 |
| Echocardiographic variables | |||
| AV peak velocity (m/s) | 4.6±0.6 | 4.5±0.6 | 0.21 |
| AV peak PG (mmHg) | 86.5±23.7 | 83.7±24.1 | 0.3 |
| AV mean PG (mmHg) | 51.2±15.2 | 48.5±15.8 | 0.11 |
| AVAI (cm2/m2) | 0.40±0.10 | 0.46±0.14 | <0.001 |
| LVDd (mm) | 47.5±5.0 | 49.5±5.5 | <0.001 |
| LVDs (mm) | 30.4±6.0 | 31.1±6.5 | 0.46 |
| LVEF (%) | 64.6±10.8 | 66.6±9.7 | 0.1 |
| SVI (mL/m2) | 47.1±10.0 | 49.2±10.2 | 0.06 |
| LVMI (g/m2) | 157.0±30.1 | 162.4±45.0 | 0.18 |
| AR ≥ moderate | 35 (12.8) | 21 (19.6) | 0.1 |
| MR ≥ moderate | 59 (21.5) | 12 (11.2) | 0.01 |
| TR ≥ moderate | 27 (9.9) | 3 (2.8) | 0.02 |
| STS score | 5.6±3.4 | 3.3±2.3 | <0.001 |
| EuroSCORE II | 3.9±3.0 | 2.1±1.2 | <0.001 |
Data are presented as mean ± standard deviation or n (%). TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement; BMI, body mass index; BSA, body surface area; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; AV, aortic valve; PG, pressure gradient; AVAI, aortic valve area index; LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; LVEF, left ventricular ejection fraction; SVI, stroke volume index; LVMI, left ventricular mass index; AR, aortic regurgitation; MR, mitral regurgitation; TR, tricuspid regurgitation; STS, Society of Thoracic Surgeons; EuroSCORE, European system for cardiac operative risk evaluation.
Intraoperative outcomes
The intraoperative outcomes of the patients in the TAVR and SAVR groups are listed in Table 2. All patients in the SAVR group had their procedures under general anesthesia, while, 213 patients (77.7%) in the TAVR group had their procedures under local anesthesia. A transfemoral approach was used in 264 patients (96.4%) in the TAVR group. The operation time was significantly shorter in the TAVR group (67.8±41.4 vs. 300.2±54.2 min, respectively, P<0.001). The SAPIEN valve series was used for TAVR in 191 patients (69.7%), whereas TAVR using the CoreValve series was performed in 83 patients (30.3%). In the SAVR group, the Carpentier Edwards Perimount (CEP) valve series and Inspiris Resilia valve series were performed in 94 patients (87.9%).
Table 2
| Characteristics | TAVR group (n=274) | SAVR group (n=107) | P value |
|---|---|---|---|
| Procedural characteristics | |||
| Anesthesia | |||
| Local | 213 (77.7) | 0 | <0.001 |
| General | 61 (22.3) | 107 (100.0) | <0.001 |
| Approach | – | ||
| Transfemoral approach | 264 (96.4) | ||
| Transaortic approach | 6 (2.2) | ||
| Transsubclavian approach | 3 (1.1) | ||
| Transapical approach | 1 (0.4) | ||
| Operation time (min) | 67.8±41.4 | 300.2±54.2 | <0.001 |
| Concomitant procedure | – | ||
| PCI | 2 | ||
| Left atrial appendage resection | 16 | ||
| Pulmonary vein ablation | 7 | ||
| Transcatheter heart valve type | – | ||
| SAPIEN XT | 12 (4.4) | ||
| SAPIEN 3 | 179 (65.3) | ||
| CoreValve Evolut R | 18 (6.6) | ||
| CoreValve Evolut PRO | 33 (12.0) | ||
| CoreValve Evolut PRO+ | 32 (11.7) | ||
| Transcatheter heart valve size | – | ||
| SAPIEN series | |||
| 20 mm | 8 | ||
| 23 mm | 76 | ||
| 26 mm | 86 | ||
| 29 mm | 21 | ||
| CoreValve Evolut series | |||
| 23 mm | 6 | ||
| 26 mm | 30 | ||
| 29 mm | 45 | ||
| 34 mm | 2 | ||
| Prosthetic valve type | – | ||
| CEP Magna | 5 (4.7) | ||
| CEP Magna Ease | 62 (57.9) | ||
| Inspiris Resilia | 27 (25.2) | ||
| Trifecta | 8 (7.5) | ||
| Mitroflow | 5 (4.7) | ||
| Prosthetic valve size | – | ||
| 19 mm | 34 | ||
| 21 mm | 42 | ||
| 23 mm | 28 | ||
| 25 mm | 3 |
Concomitant procedure: no other than the procedures listed. Data are presented as mean ± standard deviation, n (%) or n. TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement; PCI, percutaneous coronary intervention; CEP, Carpentier Edwards Perimount.
Postoperative outcomes
The postoperative outcomes of the patients in the TAVR and SAVR groups are listed in Table 3. New pacemaker implantations were observed in 19 patients (6.9%) in the TAVR group, but not in the SAVR group. The incidence of postoperative atrial fibrillation (POAF) was significantly higher in the SAVR group (4.4% vs. 38.3%, respectively; P<0.001). The intensive care unit (ICU) length of stay (1.0±0.4 vs. 3.2±1.7 days, respectively; P<0.001) and hospital length of stay (6.0±4.7 vs. 18.0±13.0 days, respectively; P<0.001) were significantly shorter in the TAVR group. There was no difference in hospital mortality between the two groups (0.4% vs. 1.9%, respectively; P=0.19). One death in the TAVR group was due to an intraoperative aortic perforation. Two deaths in the SAVR group were due to an intraoperative right coronary artery occlusion, resulting in low cardiac output syndrome and non-occlusive mesenteric ischemia (NOMI) postoperatively. At discharge, the AV peak velocity (2.3±0.4 vs. 2.5±0.4 m/s, respectively; P<0.001), AV peak PG (22.7±7.9 vs. 27.0±9.8 mmHg, respectively; P<0.001) and AV mean PG (11.2±4.0 vs. 13.8±5.4 mmHg, respectively; P<0.001) were significantly lower in the TAVR group. The AVAI (0.99±0.23 vs. 0.88±0.21 cm2/m2, respectively; P<0.001), LVEF (66.8%±8.9% vs. 64.5%±7.7%, respectively; P=0.01), and SVI (47.9±10.5 vs. 42.7±9.5 mL/m2, respectively; P<0.001) were significantly lower in the SAVR group. The LVMI at discharge was significantly lower in the SAVR group (155.1±32.8 vs. 143.6±37.3 g/m2, respectively; P=0.003). The incidence of mild or higher paravalvular leak (PVL) at discharge was significantly higher in the TAVR group than in the SAVR group (32.5% vs. 3.7%, respectively; P<0.001).
Table 3
| Characteristics | TAVR group (n=274) | SAVR group (n=107) | P value |
|---|---|---|---|
| Etiology of aortic valve stenosis | – | ||
| Degenerative | 272 (99.3) | 100 (93.5) | |
| Bicuspid | 2 (0.7) | 7 (6.5) | |
| Intraoperative complications | – | ||
| Iliac or femoral artery injury | 7 (2.6) | ||
| Right coronary artery occlusion | 1 (0.4) | 1 (0.9) | |
| Aortic rupture | 1 (0.4) | ||
| Postoperative complications | |||
| NOMI | – | 1 (0.9) | – |
| New pacemaker implantation | 19 (6.9) | 0 | 0.002 |
| Cerebrovascular events | 6 (2.2) | 4 (3.7) | 0.47 |
| Delirium | 37 (13.5) | 15 (14.0) | 0.87 |
| POAF | 12 (4.4) | 41 (38.3) | <0.001 |
| RRT | 0 | 13 (12.1) | <0.001 |
| ICU length of stay (days) | 1.0±0.4 | 3.2±1.7 | <0.001 |
| Hospital length of stay (days) | 6.0±4.7 | 18.0±13.0 | <0.001 |
| Hospital death | 1 (0.4) | 2 (1.9) | 0.19 |
| Postoperative echocardiographic variables (at discharge) | |||
| AV peak velocity (m/s) | 2.3±0.4 | 2.5±0.4 | <0.001 |
| AV peak PG (mmHg) | 22.7±7.9 | 27.0±9.8 | <0.001 |
| AV mean PG (mmHg) | 11.2±4.0 | 13.8±5.4 | <0.001 |
| AVAI (cm2/m2) | 0.99±0.23 | 0.88±0.21 | <0.001 |
| LVDd (mm) | 47.1±4.8 | 47.2±4.9 | 0.82 |
| LVDs (mm) | 29.3±5.1 | 30.3±5.0 | 0.11 |
| LVEF (%) | 66.8±8.9 | 64.5±7.7 | 0.01 |
| SVI (mL/m2) | 47.9±10.5 | 42.7±9.5 | <0.001 |
| LVMI (g/m2) | 155.1±32.8 | 143.6±37.3 | 0.003 |
| PVL ≥ mild | 89 (32.5) | 4 (3.7) | <0.001 |
Intraoperative complications: no complication other than those listed. Data are presented as mean ± standard deviation or n (%). TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement; NOMI, non-occlusive mesenteric ischemia; POAF, postoperative atrial fibrillation; RRT, renal replacement therapy; ICU, intensive care unit; AV, aortic valve; PG, pressure gradient; AVAI, aortic valve area index; LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; LVEF, left ventricular ejection fraction; SVI, stroke volume index; LVMI, left ventricular mass index; PVL, paravalvular leak.
Postoperative valve performance
Figure 1 shows the changes in PVL over time. Approximately 30% of mild or higher PVL in the TAVR group persisted for 3 years after the procedure. Figure 2A shows the changes in the AV mean PG over time. There was a significant difference between SAVR and TAVR in the change of AV mean PG over time (P<0.001). Figure 2B shows the changes in the AVA index over time. There was a significant difference between SAVR and TAVR in the change of AVA index over time (P<0.001). Figure 2C shows the changes in the SVI over time. There was a significant difference between SAVR and TAVR in the change of SVI over time (P=0.02). Figure 2D shows the changes in the LVMI over time. There was a significant difference between SAVR and TAVR in the change of LVMI over time (P<0.001). Table 4 shows preoperative and postoperative echocardiographic variables.
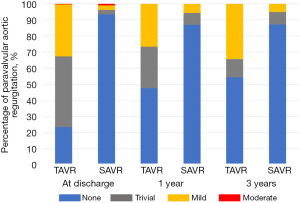
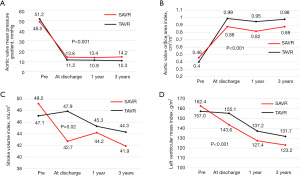
Table 4
| Variables | TAVR group (n=274) | SAVR group (n=107) | P value |
|---|---|---|---|
| Preoperative echocardiographic variables | |||
| LVDd (mm) | 47.5±5.0 | 49.5±5.5 | <0.001 |
| LVDs (mm) | 30.4±6.0 | 31.1±6.4 | 0.36 |
| LVEF (%) | 64.6±10.8 | 66.6±9.7 | 0.1 |
| SV (mL) | 67.9±15.4 | 76.7±15.8 | <0.001 |
| SVI (mL/m2) | 47.1±10.0 | 49.2±10.2 | 0.06 |
| AVA (cm2) | 0.58±0.16 | 0.72±0.21 | <0.001 |
| AVAI (cm2/m2) | 0.40±0.10 | 0.46±0.14 | <0.001 |
| LVMI (g/m2) | 157.0±30.1 | 162.4±45.0 | 0.18 |
| Postoperative echocardiographic variables (at discharge) | |||
| LVDd (mm) | 47.1±4.8 | 47.2±4.9 | 0.82 |
| LVDs (mm) | 29.3±5.1 | 30.3±5.0 | 0.11 |
| LVEF (%) | 66.8±8.9 | 64.5±7.7 | 0.01 |
| SV (mL) | 69.0±15.1 | 66.7±14.8 | 0.2 |
| SVI (mL/m2) | 47.9±10.5 | 42.7±9.5 | <0.001 |
| AVA (cm2) | 1.43±0.36 | 1.40±0.37 | 0.41 |
| AVAI (cm2/m2) | 0.99±0.23 | 0.88±0.21 | <0.001 |
| LVMI (g/m2) | 155.1±32.8 | 143.6±37.3 | 0.003 |
| Postoperative echocardiographic variables (one year postoperatively) | |||
| LVDd (mm) | 45.5±4.4 | 46.5±3.8 | 0.1 |
| LVDs (mm) | 27.9±4.0 | 28.2±3.2 | 0.6 |
| LVEF (%) | 68.4±6.2 | 69.2±4.7 | 0.3 |
| SV (mL) | 65.5±14.3 | 69.6±12.8 | 0.03 |
| SVI (mL/m2) | 45.3±9.2 | 44.2±9.2 | 0.39 |
| AVA (cm2) | 1.38±0.33 | 1.30±0.33 | 0.1 |
| AVAI (cm2/m2) | 0.95±0.21 | 0.82±0.19 | <0.001 |
| LVMI (g/m2) | 137.2±26.2 | 127.4±30.4 | 0.009 |
Data are presented as mean ± standard deviation. TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement; LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; LVEF, left ventricular ejection fraction; SV, stroke volume; SVI, stroke volume index; AVA, aortic valve area; AVAI, aortic valve area index; LVMI, left ventricular mass index.
Long-term results
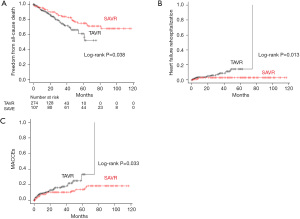
The overall mean follow-up period was 28.8±25.9 months (median: 24 months; range, 0.03–117 months). In the TAVR group, the mean follow-up period was 20.9±17.0 months (median: 16.5 months; range, 0.03–75 months), while in the SAVR group, it was 49.0±33.0 months (median: 47 months; range, 0.16–117 months). The cumulative 5-year freedom from all-cause death was significantly better in the SAVR group when compared with the TAVR group [74.9%, 95% confidence interval (CI): 63.9–83.0% vs. 60.9%, 95% CI: 46.6–72.4%, respectively; log-rank P=0.038; Figure 3A]. The cumulative 5-year heart failure rehospitalization rate was significantly lower in the SAVR group than that in the TAVR group (3.1%, 95% CI: 1.0–9.3% vs. 14.6%, 95% CI: 7.7–26.4%, respectively; log-rank P=0.013; Figure 3B). The cumulative 5-year proportion of MACCE was significantly lower in the SAVR group than that in the TAVR group (14.0%, 95% CI: 8.0–23.7% vs. 33.5%, 95% CI: 19.1–54.3%, respectively; log-rank P=0.033; Figure 3C).
Discussion
TAVR was initiated in our institution in 2016. As shown in Figure 4, TAVR increased over time, whereas, SAVR without concomitant procedures was decreased over time.
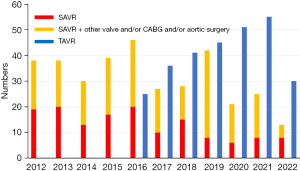
Kamon et al. (7) reported that the number of TAVR procedures was increased over time in all age categories; however, that of SAVR procedures was not decreased after the introduction of TAVR. In addition, according to the Japan Cardiovascular Surgery Database (18), despite the dramatic increase in the number of TAVR procedures, that of SAVR has also increased. However, these reports include data up to 2018 only, and SAVR may decrease with the increase in TAVR in the future, as in our institution.
Although there are various reports on the hemodynamic and long-term performance of SAVR valves (19-24), the same cannot be said for TAVR valves. The number of TAVR valves is expected to increase dramatically in the future, which makes it essential to elucidate the hemodynamic and long-term performance of TAVR valves too.
In the present study, we compared the hemodynamic performance of both SAVR and TAVR valves. The postoperative aortic valve mean PG was significantly smaller in the TAVR valves than in the SAVR valves. The postoperative AVA index was significantly larger in the TAVR valves than in the SAVR valves. In contrast, the SVI was significantly smaller in the SAVR group than in the TAVR group. The LVMI was significantly smaller in the SAVR group than in the TAVR group. A study reported (2) no difference in the postoperative aortic valve PG and AVA between the TAVR and SAVR cohorts, which contradicts many other reports (3,5,13,25) showing that both the aortic valve PG and AVA were favorable in the TAVR cohort, as it was shown in the present study. TAVR valves have the ability to expand to the anatomical annulus size, whereas SAVR valves do not have this ability because of a fixed-size surgical ring (3). This may be why TAVR valves perform well postoperatively although it should be noted that self-expanding bioprosthetic valves also have this ability to a large extent (13).
Considering the effects of the postoperative aortic valve PG and AVA, the LVMI—an index of postoperative left ventricular remodeling—was expected to be significantly reduced in the TAVR group. However, in the present study, the LVM regression was significant in the SAVR group. Similar to the present study, Patel et al. (12) reported that the LVMI was significantly reduced in their SAVR cohort. A lower LVM regression was associated with postoperative aortic regurgitation, mitral regurgitation, PVL, and pacemaker implantation, all of which were more prevalent in the TAVR cohort. In the present study, the preoperative MR and TR of moderate or higher grade were significantly higher in the TAVR group and the postoperative PVL of mild or higher grade as well as pacemaker implantation were significantly higher in the TAVR group. These factors are thought to have contributed to the LVM regression in the SAVR group.
LVM regression has been associated with improved long-term survival and rehospitalization (9,11,12). In the present study, long-term outcomes were better in the SAVR group, where the LVM regression was marked. In addition, Patel et al. (12) reported that a better understanding of LVM regression may allow us to better define the appropriate timing of intervention and thus determine which patients could benefit more from SAVR than TAVR.
Sá et al. (26) reported that PVL after TAVR is a risk factor for mortality and rehospitalization. In this study, PVL was more common and postoperative mortality was significantly higher in the TAVR group. In addition, postoperative pacemaker implantation may cause pacemaker-induced ventricular dyssynchrony and affect the postoperative outcomes.
Patient-prosthetic mismatch (PPM) was a risk factor for early and long-term mortality after TAVR and SAVR (27,28). In this study, severe PPM with an effective orifice area index (EOAI) <0.65 was observed in 13 patients (4.7%) in the TAVR group and 10 patients (9.3%) in the SAVR group. A moderate PPM with an EOAI of 0.65 to 0.85 was observed in 66 patients (24.1%) in the TAVR group and 36 patients (33.6%) in the SAVR group. Some reports (29,30) recommend aortic annulus enlargement (AAE) to avoid PPM, but we do not actively use AAE at our institution, because we think that AAE is invasive and affects postoperative outcomes. Based on the BSA of the patient, we selected and implanted prosthetic valves with an EOAI of 0.85 or higher. However, echocardiography at discharge revealed a measured EOAI of <0.85 PPM, as described above. Further studies on PPM and postoperative outcomes are needed at our institution.
Based on the above, an ideal prosthetic valve should have as large an effective AVA as possible and the ability to expand with the aortic annulus. It should also have a small aortic valve PG, a large AVA, less PVL, and less pacemaker implantation. Consequently, LVM regression will be more likely. Furthermore, the simplification of the valve-in-valve procedure in the future will be an important factor in designing the ideal prosthetic valve.
Malnutrition is common in older patients undergoing TAVR and is associated with increased mortality (31). In this study, the patients in the TAVR group were older than those in the SAVR group. It is highly likely that the frailty and sarcopenia in the TAVR group may have played an important role in the postoperative outcomes. We are currently conducting a study to assess postoperative activities of daily living in the TAVR and SAVR groups.
The present study had some limitations. First, the TAVR group was significantly older than the SAVR group, and it had significantly higher STS scores and EuroSCORE II values. In addition, the preoperative MR and TR of moderate or higher degree were significantly more common in the TAVR group. These findings suggest that the patients who were treated with TAVR had more advanced left ventricular hypertrophy than SAVR patients. In contrast, there were significantly more patients in the SAVR group with a history of hypertension and renal dysfunction. These situations may have affected the long-term outcomes. However, it is unclear how these factors affected the LVM regression. From this perspective, age matching is necessary. However, the candidate patients for TAVR are older, and we think that it would have been inappropriate to match the cohort of this study by age. Second, both the SAVR and TAVR groups used multiple types of prosthetic valves. In the TAVR group, most of the prosthetic valves were from the SAPIEN series, but the self-expanding CoreValve was also used. Additionally, more than half of the prosthetic valves used in the SAVR group were 19 or 21 mm in size. In these situations, comparing the valve performance between both groups can be biased. Further, a longer follow-up period is required to establish the durability and long-term performance of TAVR valves. Finally, the present study was retrospective, with a small sample size from a single center. This may not be representative of the wider TAVR and SAVR population: hence, findings should be taken in the context of these limitations.
Conclusions
The postoperative aortic valve PG, AVA, and SV were better in the TAVR group than in the SAVR group. The postoperative PVL and pacemaker implantation rates were lower in the SAVR group; therefore, the LVM regression and postoperative outcomes were better in the SAVR group. LVM regression can be used not only as an index of postoperative left ventricular remodeling, but also a criterion for the appropriate timing and selection of procedures (SAVR or TAVR).
Acknowledgments
We are grateful to Dr. Noriyuki Kato (Department of Radiology, Mie University Hospital) for establishing a hybrid catheterization laboratory for performing TAVR.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-119/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-119/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-119/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-119/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice guidelines. The study was approved by institutional ethics review board of the Ise Red Cross Hospital (5/6/2022, approval No. ER2022-11) and the need for individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomized controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 2019;380:1695-705. [Crossref] [PubMed]
- Makkar RR, Thourani VH, Mack MJ, et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med 2020;382:799-809. [Crossref] [PubMed]
- Takeji Y, Taniguchi T, Morimoto T, et al. Transcatheter Aortic Valve Implantation vs. Surgical Aortic Valve Replacement for Severe Aortic Stenosis in Real-World Clinical Practice. Circ J 2020;84:806-14. [Crossref] [PubMed]
- Kamon T, Kaneko H, Kiriyama H, et al. Transcatheter Aortic Valve Implantation and Surgical Aortic Valve Replacement for Aortic Stenosis in Japan – Analysis of a Nationwide Inpatient Database. Circ Rep 2020;2:753-8. [Crossref] [PubMed]
- Izumi C, Eishi K, Ashihara K, et al. JCS/JSCS/JATS/JSVS 2020 Guidelines on the Management of Valvular Heart Disease. Circ J 2020;84:2037-119. [Crossref] [PubMed]
- Ali A, Patel A, Ali Z, et al. Enhanced left ventricular mass regression after aortic valve replacement in patients with aortic stenosis is associated with improved long-term survival. J Thorac Cardiovasc Surg 2011;142:285-91. [Crossref] [PubMed]
- Lindman BR, Stewart WJ, Pibarot P, et al. Early regression of severe left ventricular hypertrophy after transcatheter aortic valve replacement is associated with decreased hospitalizations. JACC Cardiovasc Interv 2014;7:662-73. [Crossref] [PubMed]
- Chau KH, Douglas PS, Pibarot P, et al. Regression of Left Ventricular Mass After Transcatheter Aortic Valve Replacement: The PARTNER Trials and Registries. J Am Coll Cardiol 2020;75:2446-58. [Crossref] [PubMed]
- Patel V, Jneid H, Cornwell L, et al. Left Ventricle Mass Regression After Surgical or Transcatheter Aortic Valve Replacement in Veterans. Ann Thorac Surg 2022;114:77-83. [Crossref] [PubMed]
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 2019;380:1706-15. [Crossref] [PubMed]
- Nishigawa K, Onga Y, Uemura K, et al. Surgical aortic valve replacement provides better left ventricular mass regression than transcatheter aortic valve replacement in patients with small aortic annulus. Gen Thorac Cardiovasc Surg 2023;71:167-74. [Crossref] [PubMed]
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450-8. [Crossref] [PubMed]
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440-63. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Abe T, Kumamura H, Nakano K, et al. Current status of cardiovascular surgery in Japan: a report based on the Japan Cardiovascular Surgery Database in 2017, 2018 3. Valvular Heart Surgery. Japanese Journal of Cardiovascular Surgery 2020;49:160-8. [Crossref]
- Nishioka N, Yamada A, Ujihira K, et al. Outcomes of surgical aortic valve replacement using Carpentier-Edwards PERIMOUNT bioprosthesis series in elderly patients with severe aortic valve stenosis: a retrospective cohort study. Gen Thorac Cardiovasc Surg 2016;64:728-34. [Crossref] [PubMed]
- Goldman S, Cheung A, Bavaria JE, et al. Midterm, multicenter clinical and hemodynamic results for the Trifecta aortic pericardial valve. J Thorac Cardiovasc Surg 2017;153:561-9.e2. [Crossref] [PubMed]
- Yoshikawa Y, Okada Y, Okita Y, et al. Long-Term Outcomes of the Mosaic Aortic Porcine Bioprosthesis in Japan – Results From the Japan Mosaic Valve Long-Term Multicenter Study. Circ J 2020;84:1261-70. [Crossref] [PubMed]
- Yongue C, Lopez DC, Soltesz EG, et al. Durability and Performance of 2298 Trifecta Aortic Valve Prostheses: A Propensity-Matched Analysis. Ann Thorac Surg 2021;111:1198-205. [Crossref] [PubMed]
- Bartus K, Litwinowicz R, Bilewska A, et al. Final 5-year outcomes following aortic valve replacement with a RESILIA™ tissue bioprosthesis. Eur J Cardiothorac Surg 2021;59:434-41. [Crossref] [PubMed]
- Persson M, Glaser N, Nilsson J, et al. Comparison of Long-term Performance of Bioprosthetic Aortic Valves in Sweden From 2003 to 2018. JAMA Netw Open 2022;5:e220962. [Crossref] [PubMed]
- Søndergaard L, Ihlemann N, Capodanno D, et al. Durability of Transcatheter and Surgical Bioprosthetic Aortic Valves in Patients at Lower Surgical Risk. J Am Coll Cardiol 2019;73:546-53. [Crossref] [PubMed]
- Sá MP, Jacquemyn X, Van den Eynde J, et al. Impact of Paravalvular Leak on Outcomes After Transcatheter Aortic Valve Implantation: Meta-Analysis of Kaplan-Meier-derived Individual Patient Data. Struct Heart 2022;7:100118. [Crossref] [PubMed]
- Sá MPBO, de Carvalho MMB, Sobral Filho DC, et al. Surgical aortic valve replacement and patient-prosthesis mismatch: a meta-analysis of 108 182 patients. Eur J Cardiothorac Surg 2019;56:44-54. [Crossref] [PubMed]
- Sá MP, Jacquemyn X, Van den Eynde J, et al. Impact of Prosthesis-Patient Mismatch After Transcatheter Aortic Valve Replacement: Meta-Analysis of Kaplan-Meier-Derived Individual Patient Data. JACC Cardiovasc Imaging 2023;16:298-310. [Crossref] [PubMed]
- Sá MPBO, Zhigalov K, Cavalcanti LRP, et al. Impact of Aortic Annulus Enlargement on the Outcomes of Aortic Valve Replacement: A Meta-analysis. Semin Thorac Cardiovasc Surg 2021;33:316-25. [Crossref] [PubMed]
- Penaranda JG, Greason KL, Pislaru SV, et al. Aortic root enlargement in octogenarian patients results in less patient prosthesis mismatch. Ann Thorac Surg 2014;97:1533-8. [Crossref] [PubMed]
- Ishizu K, Shirai S, Tashiro H, et al. Prevalence and Prognostic Significance of Malnutrition in Older Japanese Adults at High Surgical Risk Undergoing Transcatheter Aortic Valve Implantation. J Am Heart Assoc 2022;11:e026294. [Crossref] [PubMed]


