
Very long-term efficacy and safety of paclitaxel-eluting balloon after a bare-metal stent for the treatment of ST-elevation myocardial infarction: 8-year results of a randomized clinical trial (PEBSI study)
Highlight box
Key findings
• Paclitaxel-drug-coated balloon after a bare-metal stent implantation (DCB-combined strategy) is associated, after 8 years of follow-up, with a very low rate of target lesion (2.8%) and target vessel revascularization (3.7%) with no stent thrombosis after the first year.
• These numbers represent the lowest revascularizations and stent thrombosis ever reported in a ST-segment elevation myocardial infarction (STEMI) trial.
What is known and what is new?
• Despite drug-eluting stents (DES) are the therapy of choice in STEMI, a low but persistent rate of revascularizations and stent thrombosis exist over the time.
• Our DCB-combined strategy offers excellent very long-term safety and efficacy results.
What is the implication, and what should change now?
• Our DCB-combined strategy might be an alternative, if not better, to DES in STEMI particularly when considering the very long-term clinical outcome.
• Development of this novel strategy on a large-scale trial is warranted to address the unmet need of the potential long-term risk associated to DES.
Introduction
Background
Drug-eluting stents (DES) are considered the therapy of choice in ST-segment elevation myocardial infarction (STEMI) (1). However, even with latest DES generation, long-term data shows that there is a low but persistent rate of revascularizations and stent thrombosis maintained over time (2,3). Target lesion revascularization (TLR) increased from the first to the fifth year, from 1.6% to 4.4% in the EXAMINATION trial (2) and from 1.6% to 4.4%, in the COMFORTABLE AMI trial (3). Similarly, the stent thrombosis rate rose from 2.2% in the first year to 3.9% at 5 years in the COMFORTABLE AMI trial and from 0.5% to 1.6% in the EXAMINATION trial.
Rationale and knowledge gap
The use of a paclitaxel (PTX)-drug-coated balloon (DCB) after a bare-metal stent (BMS) implantation (DCB-combined strategy) in STEMI has shown in a randomized trial an excellent efficacy and safety (4). This DCB-combined strategy obtained results comparable to the best results with DES in STEMI trials at 1 year of follow-up (5,6). Optical coherence tomography (OCT) studies also have suggested that the DCB-combined strategy provides superb strut coverage (99.5%) at 9 months (4) and superior healing at 3 months compared to new-generation DES (7). These favorable clinical, angiographic, and healing characteristics of the DCB-combined strategy might mitigate the problem of the persistent long-term risk associated to current DES.
Objective
We sought to investigate the very late clinical safety and efficacy of our DCB-combined strategy in STEMI patients by comparing the 8-year clinical outcomes of patients treated with the DCB-combined strategy vs. BMS only, in the PEBSI-1 randomized trial (NCT01839890) (4). We present this article in accordance with the CONSORT reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-623/rc).
Methods
Study design and participants
The PEBSI-1 trial (4) was a multicenter, parallel, patient-blinded, randomized controlled trial conducted in patients with STEMI. Primary endpoint was in-stent late luminal loss (LLL) as measured by quantitative coronary angiography (QCA) at 9-month follow-up. The trial involved the participation of seventeen tertiary university centers. To ensure data accuracy and reliability, independent on-site monitoring of all cases was conducted. Furthermore, an independent clinical events committee, unaware of the treatment allocation, carefully assessed and adjudicated all clinical events. The current follow-up study was adhered to the ethical principles outlined in the Declaration of Helsinki (revised in 2013). The study received approval from the local ethic committee of Puerta de Hierro University Hospital (No. 19/2019), and all participating hospitals were informed and agreed on the study. All patients included in the study provided signed informed consent.
The study’s inclusion criteria consisted of patients aged 18 years or older, presenting within the first 12 hours after STEMI onset, and with a clinical indication for primary percutaneous coronary intervention (PCI). STEMI was defined as ST-segment elevation of at least 1 mm in two or more contiguous electrocardiographic leads, presence of a new left bundle branch block, or evidence of a true posterior myocardial infarction with angiographic confirmation of a single culprit lesion in the target vessel.
Exclusion criteria encompassed cardiogenic shock, life expectancy of less than 12 months, and women of childbearing age. Procedural exclusion criteria included unprotected left main stenosis greater than 50%, bifurcations with a side branch diameter larger than 2.5 mm, stent thrombosis, lesion length exceeding 30 mm (beyond the longest available paclitaxel-balloon), reference vessel diameter less than 2.5 mm or greater than 4 mm, presence of more than one severe stenosis (>70% visually) in the same coronary artery, patients being considered for coronary artery bypass grafting (CABG) within 30 days post-STEMI, and overlapping stents required to treat the culprit segment.
Randomization and masking
A randomized allocation was employed in the study, with all recruited patients assigned in a 1:1 ratio to either the BMS (PRO-Kinetic Energy stent, Biotronik, Berlin, Germany) or the DCB-combined strategy (Pantera Lux, Biotronik, Berlin, Germany). The random assignment sequence was generated by a computer-generated randomization code. The block sizes for randomization were either four or six. The subjects were recruited by the interventional cardiologist responsible for the procedure. The interventions were assigned by using opaque sealed and sequentially numbered envelopes.
Procedure
The choice of vascular access (radial or femoral) was at the discretion of the interventionalist. Prior to the procedure, all patients received a loading dose of aspirin (250 to 500 mg) and one of the following antiplatelet medications: clopidogrel (600 mg), prasugrel (60 mg), or ticagrelor (180 mg). Heparin (70–100 U/kg) was administered before the procedure. The use of glycoprotein IIb/IIIa inhibitors and additional boluses of heparin were left to the operator’s discretion.
The length of the stent was selected to completely cover the stenosis, and the stent size was chosen to achieve a stent/distal artery ratio of 1–1.1/1. The decision to perform pre- and post-dilation before randomization was also left to the operator’s discretion. After successful implantation of the BMS [final thrombolysis in myocardial infarction (TIMI) flow 2–3, final residual stenosis <30%, and no post-implantation complications], patients were randomized in a 1:1 ratio to one of two groups: the DCB-combined strategy [post-dilation with paclitaxel-eluting balloon (PTX-B) for 45 s] or the BMS alone group (no further post-dilation).
In the DCB-combined strategy, only one PTX-B was allowed (i.e., the use of two 15 mm PTX-Bs to treat a 30 mm stent segment was not permitted), and a single 45-s PTX-B inflation was allowed. The diameter of the PTX-B was chosen to achieve a 1.1:1 ratio with the final BMS diameter based on the manufacturer’s pressure/diameter tables. The length of the PTX-B had to be equal to the length of the previously selected stent or slightly longer, with caution taken to avoid balloon protrusion of more than 2 mm from each edge of the stent.
Outcomes
Primary and secondary endpoints of the primary study have been reported elsewhere (4). Outcomes evaluated in this follow-up study adhered to the ARC-2 criteria (8) and are outlined below. Death: cardiovascular, non-cardiovascular, and undetermined. Cardiovascular death was defined as death resulting from cardiovascular causes (acute myocardial infarction, sudden cardiac and death resulting from heart failure). Cardiac deaths were assumed unless a definitive noncardiac cause could be established. Myocardial re-infarction, as defined by ARC-2 criteria, was characterized by a significant rise in cardiac troponin levels (≥35 times upper reference limit from baseline) along with one or more of the following criteria: (I) new significant Q waves or their equivalent; (II) flow-limiting complications observed during angiography; and (III) new substantial loss of myocardium detected through imaging techniques.
Ischemia-driven repeated revascularizations included TLR, encompassing the treated segment from 5 mm proximal to the stent to 5 mm distal to the stent, which involved either repeat percutaneous intervention or bypass surgery of the target vessel to address restenosis or other complications arising from the target lesion. Functional assessment using fractional flow reserve (FFR) or similar techniques was prioritized for demonstrating ischemia.
Target vessel revascularization (TVR) encompassed the upstream and downstream branches, as well as the target lesion itself. Stent thrombosis was classified as definite or probable. Definite stent thrombosis required angiographic confirmation either in the segment 5 mm proximal or distal to the stent or in a side branch originating from the stented segment, along with the presence of at least one of the following criteria: (I) acute onset of ischemic symptoms at rest; (II) new electrocardiographic changes indicative of acute ischemia; and (III) characteristic rise and fall in cardiac biomarkers. Probable stent thrombosis encompassed any myocardial infarction related to documented acute ischemia within the territory of the implanted stent, without angiographic confirmation of stent thrombosis and in the absence of any other obvious cause.
The device-oriented combined endpoint included cardiovascular death, target vessel myocardial infarction (excluding those clearly attributed to non-target vessels), and TLR. The patient-oriented combined endpoint encompassed all-cause mortality, any myocardial infarction (including those occurring in non-target vessel territories), and any revascularization (including both target and non-target vessels).
All the above endpoints were assessed by yearly contact up to the 8-year follow-up by a clinical visit, telephone contact or by electronic records and blindly adjudicated by the clinical events committee.
Statistical analysis
The 8 years follow-up analysis of outcomes was not specified in the study protocol and, therefore, represents a post hoc analysis. The initial study sample size of 220 patients was estimated to detect a significant reduction in late lumen loss. The BMS group aimed for a reduction of 0.8 mm compared to 0.4 mm in the PTX-B group. The study had a power of 80% and an alpha of 0.05. Data for this 8 years follow-up analysis were collected from clinical consultations, review of electronic records or telephone calls.
Continuous variables are presented as mean and standard deviation (SD), and categorical data are presented as counts and percentages. The Kolmogorov-Smirnov test was used to analyze the normal distribution of quantitative variables. Patients who were lost to follow-up were censored at their last known contact. For quantitative variables with normal distribution, the Levene test and the t-test were used; for quantitative variables with non-normal distribution, non-parametric tests were used (the Mann-Whitney test). For qualitative variables: chi-square test or, where appropriate, Fisher’s exact test, were used. A two-sided P value <0.05 was considered statistically significant.
Survival curves for time-to-event variables were constructed using Kaplan-Meier estimates. Hazard ratios (HRs) and their confidence intervals (CIs) were calculated using the Mantel-Cox method for comparisons of clinical outcomes between groups The log-rank test was used to calculate corresponding P values.
Subgroup analyses were the following: sex, age (>75 years), cardiovascular risk factors [hypertension, dyslipidemia, smoker, diabetes, peripheral vascular disease, previous myocardial infarction, previous revascularization, ischemia time (<3 hours), multivessel disease, TIMI flow post-PCI (<3), left anterior descending artery culprit vessel, use of aspiration thrombectomy catheters, Killip class (>1), and left ventricular ejection fraction <35%]. This trial was registered with ClinicalTrials.gov identifier, NCT01839890.
Results
A total of 223 patients with STEMI <12 hours from symptoms onset were initially randomized (112 to the BMS group and 111 to the DCB-combined strategy). Overall, clinical, angiographic and procedural characteristics and the hospital course in the initial study were similar in both groups (Appendices 1,2, Tables S1,S2).
From April 2012 to July 2021, complete 8 years clinical follow-up was obtained for 111 patients treated with BMS and 108 in the DCB-combined strategy (99.1% and 97.3% of follow-up respectively), Figure 1.
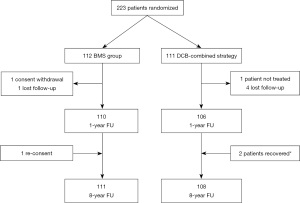
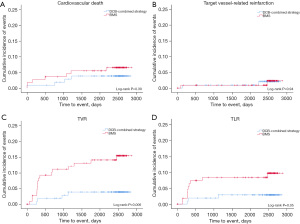
Follow-up of clinical events at 1, 5, and 8 years are summarized in Table 1. At 8 years there were a lower rate of TVR (3.7% vs. 14.3%; HR: 0.243; 95% CI: 0.081–0.727; P=0.006), and a trend towards lower TLR (2.8% vs. 8.9%; HR: 0.300; 95% CI: 0.083–1.090; P=0.052) in the DCB-combined strategy group. No statistical difference between the DCB-combined strategy and BMS groups were found for all causes of death, deaths from cardiovascular disease, reinfarctions or stent thrombosis. There was also a trend towards fewer events in the device-oriented endpoint occurring in the DCB-combined strategy as compared with BMS-only treated patients (7.4% vs. 14.3%; HR: 0.493; 95% CI: 0.211–1.152; P=0.095). There were no cardiovascular deaths, no TVR, TLR in the DCB-combined strategy beyond the 5-year follow-up. In contrast, during the period from year 5 to 8, the BMS group experienced an additional cardiovascular death, as well as one case of TVR, one case of TLR, and one case of stent thrombosis. Notably, in the DCB-combined strategy, there was no episode of very late stent thrombosis (VLST) during the entire follow-up. Antiplatelet and anticoagulant therapy at 8 years were comparable between the two groups (Appendix 3, Table S3).
Table 1
| Clinical outcomes | DCB-combined strategy (n=108), n (%) | BMS (n=111), n (%) | HR (95% CI) | P value |
|---|---|---|---|---|
| First year | ||||
| All cause of death | 1 (0.9) | 3 (2.7) | 0.344 (0.036–3.310) | 0.356 |
| Cardiovascular death | 1 (0.9) | 2 (1.8) | 0.344 (0.036–3.310) | 0.356 |
| Reinfarction | 2 (1.9) | 2 (1.8) | 1.038 (0.146–7.369) | 0.970 |
| Q-wave | 0 | 1 (0.9) | ||
| Non-Q-wave | 2 (1.9) | 1 (0.9) | 2.082 (0.189–22.964) | 0.549 |
| Target vessel-related reinfarction | 1 (0.9) | 1 (0.9) | 1.033 (0.065–16.515) | 0.982 |
| Q-wave | 0 | 0 | ||
| Non-Q-wave | 1 (0.9) | 1 (0.9) | 1.033 (0.065–16.515) | 0.982 |
| Any revascularization | 12 (11.1) | 19 (17.0) | 0.632 (0.307–1.301) | 0.209 |
| TVR | 2 (1.9) | 10 (8.9) | 0.197 (0.043–0.900) | 0.020 |
| No TVR | 10 (9.3) | 9 (8.0) | 1.146 (0.466–2.821) | 0.766 |
| TLR | 2 (1.9) | 8 (7.1) | 0.249 (0.053–1.174) | 0.057 |
| Definite stent thrombosis | 1 (0.9) | 0 | ||
| Patient oriented endpoint | 14 (13.0) | 23 (20.5) | 0.612 (0.315–1.189) | 0.143 |
| Device oriented endpoint | 3 (2.8) | 11 (9.8) | 0.273 (0.076–0.978) | 0.033 |
| At 5 years | ||||
| All cause of death | 10 (9.3) | 10 (9.0) | 1.023 (0.426–2.458) | 0.959 |
| Cardiovascular death | 4 (3.7) | 6 (5.4) | 0.683 (0.193–2.421) | 0.555 |
| Reinfarction | 4 (3.8) | 4 (3.6) | 1.024 (0.256–4.095) | 0.973 |
| Q-wave | 1 (0.9) | 2 (1.8) | 0.506 (0.046–5.583) | 0.578 |
| Non-Q-wave | 3 (2.8) | 2 (1.8) | 1.556 (0.260–9.310) | 0.628 |
| Target vessel-related reinfarction | 1 (0.9) | 1 (0.9) | 1.033 (0.065–16.515) | 0.982 |
| Q-wave | 0 | 0 | ||
| Non-Q-wave | 1 (0.9) | 1 (0.9) | 1.033 (0.065–16.515) | 0.982 |
| Any revascularization | 16 (14.8) | 24 (21.4) | 0.658 (0.350–1.239) | 0.192 |
| TVR | 4 (3.7) | 15 (13.4) | 0.255 (0.085–0.768) | 0.009 |
| No TVR | 12 (11.1) | 11 (9.8) | 1.123 (0.496–2.545) | 0.781 |
| TLR | 3 (2.8) | 9 (8.0) | 0.329 (0.089–1.215) | 0.079 |
| Definite stent thrombosis | 1 (0.9) | 1 (0.9) | 1.029 (0.064–16.451) | 0.984 |
| Patient oriented endpoint | 26 (24.1) | 34 (30.4) | 0.750 (0.450–1.250) | 0.268 |
| Device oriented endpoint | 7 (6.5) | 14 (12.5) | 0.489 (0.197–1.211) | 0.114 |
| At 8 years | ||||
| All cause of death | 13 (12.1) | 14 (12.6) | 0.930 (0.436–1.981) | 0.850 |
| Cardiovascular death | 4 (3.7) | 7 (6.3) | 0.587 (0.172–2.006) | 0.396 |
| Reinfarction | 6 (5.7) | 6 (5.4) | 1.032 (0.333–3.202) | 0.956 |
| Q-wave | 2 (1.9) | 4 (3.6) | 0.517 (0.095–2.824) | 0.446 |
| Non-Q-wave | 4 (3.8) | 2 (1.8) | 2.101 (0.385–11.475) | 0.376 |
| Target vessel-related reinfarction | 2 (1.9) | 2 (1.8) | 1.078 (0.152–7.665) | 0.940 |
| Q-wave | 1 (0.9) | 1 (0.9) | 1.124 (0.070–18.009) | 0.934 |
| Non-Q-wave | 1 (0.9) | 1 (0.9) | 1.033 (0.065–16.515) | 0.982 |
| Any revascularization | 18 (16.7) | 25 (22.3) | 0.709 (0.387–1.299) | 0.263 |
| TVR | 4 (3.7) | 16 (14.3) | 0.243 (0.081–0.727) | 0.006 |
| No TVR | 14 (13.0) | 12 (10.7) | 1.202 (0.556–2.598) | 0.640 |
| TLR | 3 (2.8) | 10 (8.9) | 0.300 (0.083–1.090) | 0.052 |
| Definite stent thrombosis | 1 (0.9) | 2 (1.8) | 0.542 (0.049–5.981) | 0.611 |
| Patient oriented endpoint | 30 (27.8) | 39 (34.8) | 0.743 (0.461–1.196) | 0.219 |
| Device oriented endpoint | 8 (7.4) | 16 (14.3) | 0.493 (0.211–1.152) | 0.095 |
DCB-combined strategy, paclitaxel-drug-coated balloon after a bare-metal stent implantation; BMS, bare-metal stent; HR, hazard ratio; CI, confidence interval; TVR, target vessel revascularization; TLR, target lesion revascularization.
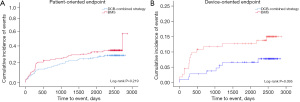
From day 0 onwards, the time-to-event curves demonstrated a divergence for both the device-oriented and patient-oriented endpoints, favoring the DCB-combined strategy (Figure 2). Time-to-event curves for each individual component of the device-oriented endpoints are shown in Figure 3. The results of the stratified analysis showed no statistically significant interaction between the tested variables and the device-oriented composite endpoints (Figure 4), similar results were obtained for the patient oriented composite endpoints (data not shown).

Discussion
Key findings
We sought to assess if our DCB-combined strategy for STEMI patients’ treatment, sustains its favorable effects over time. The two main findings of the 8-year clinical follow-up of the PEBSI-1 clinical trial are: first, the short-term efficacy of a single dose of paclitaxel in STEMI patients treated with BMS is maintained at very long-term follow-up with an 8-year TLR of 2.8%; second, the safety of this single dose of paclitaxel is excellent. In the DCB-combined strategy group, no stent thrombosis occurred from the first to the next 8 years and no cardiovascular deaths, TVR and TLR occurred after 5 years.
Strengths and limitations
DCB-combined strategy shows a very low rate of TLR (2.8%) and TVR (3.7%) with no episode of stent thrombosis after the first year, representing the lowest rates of revascularizations and VLST ever reported in a STEMI clinical trial. However, several limitations of this study should be mentioned. This is a study with a relatively small sample size. The result of the present study is only valid for those STEMI patients who have had a good angiographic result after BMS implantation, therefore safety and efficacy could not be generalized to all STEMI patients. This study was adequately powered to assess a significant reduction of LLL by 9 months QCA but not for successive clinical events comparison. Therefore, our results must be interpreted with caution. All patients received the Prokinetic® platform (Biotronik AG, Bülach, Switzerland) and the DCB-combined strategy group also received the Pantera Lux® balloon (Biotronik AG), thereby our results cannot be extrapolated to patients who receive another stent platforms or another DCBs.
Comparison with similar researches and Explanations of findings
Second-generation DES in STEMI provide excellent clinical results but are associated with a low but persistent requirement of repeat revascularization over time. TVR rates from the first to the fifth year, increased from 4% to 7% in the EXAMINATION trial (2). The same was found in the COMFORTABLE AMI trial where TVR increased from 2% to 6.5% (3). Similar results were seen regarding TLR that increased from 1.6% to 4.4% in EXAMINATION and from 1.6% to 4.4%, in COMFORTABLE AMI. We previously demonstrated in the PEBSI-1 clinical trial (4) that the DCB-combined strategy achieved an excellent clinical efficacy, with 1-year results comparable to the best DES results published in STEMI patients (TVL and TLR: 1.8%). The present extended follow-up study provides further insights regarding long-term efficacy of this unique strategy. It also addresses the burning question of whether a brief and single application of paclitaxel from a balloon can maintain favorable anti-restenotic effects in the very long term. Similar to the studies with DES in STEMI, late revascularizations in the DCB-combined strategy still are needed but with a frequency much lower (cumulative TVR of 3.7% and TLR of 2.8%, which are the lowest rate published in long-term STEMI studies). A possible explanation for these good results, would be due to the fact that DCB-combined strategy produces a different and unique neointimal growth as seen in studies with OCT (7). DCB-combined strategy induces the generation of a “homogeneous type” neointimal thickness, which is known to correlate with both: a high proportion of fibrous connective tissue deposition and low inflammation (9) and a clinical superior long-term clinical outcome (10). Besides, it has a characteristic bright neointimal pattern that has also been correlated with the presence of smooth muscle cells and dense collagen fibers on histology, which can also result in a protective effect. It is well known that time from PCI is an independent predictor of neo-atherosclerosis. Interestingly, in our study, revascularization rates did not increase from the fifth to the eighth year. This might suggest, like that seen with “plain old balloon angioplasty” (POBA), that a paclitaxel balloon may prevent neo-atherosclerosis formation and therefore avoiding very late target vessel myocardial infarctions or the need for revascularization.
Despite the in-stent restenosis’ drastic reduction obtained with DES, there is also a never-ending concern regarding VLST risk which, although rare, still persists with newer generation DES devices (1). This concern has been also seen in STEMI patients. In the only two clinical trials with long-term data at 5 years, both almost doubled their rate of stent thrombosis. In the COMFORTABLE AMI trial, the definite stent thrombosis rate rose from 2.2% in the first year to 3.9% at 5-year, and from 2.5% to 4.1% when the combined definition of “definitive or probable stent thrombosis” was used (3). Similarly, in the EXAMINATION trial, the definite stent thrombosis rate rose from 1 to 5 years from 0.5% to 1.6% and from 0.9% to 2% in the “definitive or probable” stent thrombosis definition was used (2). Our current data demonstrates that the stent thrombosis rate in our DCB-combined strategy is indeed very low even, in this challenging scenario (definitive or probable 0.9%) and, interestingly enough, no stent thrombosis occurred from the first to the next 8 years. This cumulative stent thrombosis rate of 0.9% at 5 and 8 years is the lowest rate ever published in STEMI studies. Notably, the only stent thrombosis event in the DCB-combined strategy occurred in a patient who discontinued all medications including aspirin and clopidogrel due to a depression, 4 months after the STEMI. Our DCB-combined strategy results in STEMI patients are very consistent with DCB data published in other clinical and anatomic coronary scenarios where when compared with current DES generation, a significantly lower rate of target lesion thrombosis has been systematically found favoring DCBs [propensity matched cohort adjusted risk ratio (RR): 0.18; 95% CI: 0.04–0.82; P=0.03] (11). A delayed and incomplete healing after DES implantation appears to be the main underlying pathologic substrate favoring stent thrombosis in STEMI patients (12). Paclitaxel-coated balloons are capable of short-term transfer and short drug retention of paclitaxel in the arterial wall. This could reduce (on vessel healing) the adverse effects of the prolonged drug release that is associated with DES technologies. Paclitaxel applied to the coronary arteries typically produces a characteristic media smooth muscle cells loss and fibrin deposits which exert an influence on the future cicatrization regarding quantity and type neointimal formation (13). As we previously demonstrated, the DCB-combined strategy induces a different neointimal strut coverage that appears to indicate a more favorable vessel healing by a competent neointima compared to new-generation DES (7) which could also explain the good long-term results obtained with our strategy. We also demonstrated by OCT that the DCB-combined strategy in STEMI shows an excellent strut coverage at 9 months (99.5% of struts covered) (4). All these superior vessel healing parameters favoring the DCB-combined strategy, may have contributed to the current excellent long-term safety results. Likewise, the absence of polymer foreign bodies at the coronary wall in the DCB-combined strategy may also have further contributed to this favorable long-term safety profile. VLTS of the BMS group was also low. A probable explanation of this good result in this group could be in the ultrathin stent struts of the Prokinetic® platform used in this study. This singularity may have favored the excellent strut coverage in the BMS group (100.0%±0.0% of struts covered) found at 9 months in the PEBSI-1 OCT substudy (4).
The use of DCBs, mainly those of paclitaxel, as sole therapy in STEMI has also been previously explored. With a high but highly variable percentage of bailout stenting [from 18% in the REVELATION trial (14) to 41% in PAPPA trial (15)], this therapy has shown to be effective in the medium term (14-16), however there are very few data beyond 1 year. Recently the authors of the REVELATION study have published their 2-year results with a TLR of 5.4% in the DCB group and 1.9% in the DES group (HR: 2.86; 95% CI: 0.30–27.53; P=0.34). Between 9 months and 2 years, 1 additional TLR occurred in the DCB group (17). These results are significantly worse than those found in our strategy even at 8 years follow-up. Proper lesion preparation is crucial to achieve favorable long-term outcomes with the DCB-only strategy. It ensures an adequate lumen gain, reducing vessel recoil and maximizing the contact area between the DCB and the vessel wall. However, this approach may result in more coronary dissections, leading to subsequent TLR due to vessel occlusions or stenosis. Our superior results, both in the short and long term, compared to DCB-only treatment, can be attributed to the additional stent implantation. This approach provides fixation of flow-limiting dissections and addresses vessel elastic recoil, thereby preventing future TLRs. Although there is growing interest in limus-coated balloons, data on the biological response in human coronary arteries to these devices are currently limited.
In the past years, there has been great concern with paclitaxel containing devices due to a possible increased late all-cause mortality at 2 and 5 years in peripheral artery disease (18). We have not seen in our 8-year follow-up clinical trial any increase in mortality signal when paclitaxel is used at the coronary level, both in all cause of death (12.1 vs. 12.6; HR: 0.93; 95% CI: 0.436–1.981; P=0.85) and in cardiovascular mortality (3.7 vs. 6.3; HR: 0.587; 95% CI: 0.172–2.006; P=0.396). Our results reinforce in the very long-term, and complement in the STEMI, the results of a recent meta-analysis with a follow-up of up to 3 years that showed that DCB are safe when used in the coronary territory, with no sign of mortality risk or vessel thrombosis risk (19).
Implications and actions needed
New therapeutic devices and strategies, able to improve safety and efficacy after a primary PCI in STEMI patients, are still required to advance the field. The present work should be considered an exploratory and “hypothesis generating study” derived from the follow-up of a clinical trial with a specific endpoint. However, the very good results that we have consistently obtained demonstrating the safety and efficacy of the DCB-combined strategy, indicate that there might be signs that DCB-combined strategy (with an immediate, brief and homogeneous drug delivery in the coated vessel site, without the need of implanted polymers and with a different and unique neointimal growth) may be an alternative to DES in STEMI and especially considering the very long-term follow-up. Further studies are needed to further examine this hypothesis in this complex and challenging clinical scenario. Finally, the DCB-combined strategy implies greater expense and procedure time by having to use a stent first and then a DCB. According to the results of this study, a BMS mounted on the DCB or full drug-coated system including stent and balloon could be a potential development direction of a new stent system.
Conclusions
Our DCB-combined strategy in STEMI primary angioplasty, offers excellent very long-term safety and efficacy results. Our numbers represent the lowest revascularizations and VLST ever reported in STEMI clinical trials. Therefore, paclitaxel-coated balloons might be an alternative to DES in STEMI particularly when considering the very long-term clinical outcome. Development of this novel strategy on a large-scale trial is warranted in order to address the unmet need of the potential long-term risk associated to DES implantation.
Acknowledgments
Funding: This investigation has received the support of the Spanish Clinical Research Network (SCReN), financed by the ISCIII-Subdirección General de Evaluación y Fomento de la Investigación, under the project PT13/0002/0005 (Plan Estatal de I+D+I 2013-2016) and co-funded by the European Regional Development Fund (FEDER). An unrestricted research grant was received from Biotronik.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-623/rc
Trial Protocol: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-623/tp
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-623/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-623/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-623/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was carried out according to the Declaration of Helsinki (as revised in 2013) and approved by the local ethic committee of Puerta de Hierro University Hospital (No. 19/2019). All participating hospitals were informed and agreed on the study. Signed informed consent was obtained from all patients included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet 2016;387:357-66. [Crossref] [PubMed]
- Räber L, Yamaji K, Kelbæk H, et al. Five-year clinical outcomes and intracoronary imaging findings of the COMFORTABLE AMI trial: randomized comparison of biodegradable polymer-based biolimus-eluting stents with bare-metal stents in patients with acute ST-segment elevation myocardial infarction. Eur Heart J 2019;40:1909-19. [Crossref] [PubMed]
- García-Touchard A, Goicolea J, Sabaté M, et al. A randomised trial of paclitaxel-eluting balloon after bare metal stent implantation vs. bare metal stent in ST-elevation myocardial infarction (the PEBSI study). EuroIntervention 2017;12:1587-94. [Crossref] [PubMed]
- Sabate M, Cequier A, Iñiguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet 2012;380:1482-90. [Crossref] [PubMed]
- Räber L, Kelbæk H, Ostojic M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA 2012;308:777-87. Erratum in: JAMA 2012;308:1526. [Crossref] [PubMed]
- García-Touchard A, Gonzalo N, Goicolea J, et al. Early coronary healing in ST segment elevation myocardial infarction: sirolimus-eluting stents vs. drug-coated balloons after bare-metal stents. The PEBSI-2 optical coherence tomography randomized study. Coron Artery Dis 2021;32:673-80. [Crossref] [PubMed]
- Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation 2018;137:2635-50. [Crossref] [PubMed]
- Kim JS, Afari ME, Ha J, et al. Neointimal patterns obtained by optical coherence tomography correlate with specific histological components and neointimal proliferation in a swine model of restenosis. Eur Heart J Cardiovasc Imaging 2014;15:292-8. [Crossref] [PubMed]
- Kim JS, Lee JH, Shin DH, et al. Long-term outcomes of neointimal hyperplasia without neoatherosclerosis after drug-eluting stent implantation. JACC Cardiovasc Imaging 2014;7:788-95. [Crossref] [PubMed]
- Venetsanos D, Lawesson SS, Panayi G, et al. Long-term efficacy of drug coated balloons compared with new generation drug-eluting stents for the treatment of de novo coronary artery lesions. Catheter Cardiovasc Interv 2018;92:E317-26. [Crossref] [PubMed]
- Nakazawa G, Finn AV, Joner M, et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 2008;118:1138-45. [Crossref] [PubMed]
- Granada JF, Stenoien M, Buszman PP, et al. Mechanisms of tissue uptake and retention of paclitaxel-coated balloons: impact on neointimal proliferation and healing. Open Heart 2014;1:e000117. [Crossref] [PubMed]
- Vos NS, Fagel ND, Amoroso G, et al. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction: The REVELATION Randomized Trial. JACC Cardiovasc Interv 2019;12:1691-9. [Crossref] [PubMed]
- Vos NS, Dirksen MT, Vink MA, et al. Safety and feasibility of a PAclitaxel-eluting balloon angioplasty in Primary Percutaneous coronary intervention in Amsterdam (PAPPA): one-year clinical outcome of a pilot study. EuroIntervention 2014;10:584-90. [Crossref] [PubMed]
- Gobić D, Tomulić V, Lulić D, et al. Drug-Coated Balloon Versus Drug-Eluting Stent in Primary Percutaneous Coronary Intervention: A Feasibility Study. Am J Med Sci 2017;354:553-60. [Crossref] [PubMed]
- Niehe SR, Vos NS, Van Der Schaaf RJ, et al. Two-Year Clinical Outcomes of the REVELATION Study: Sustained Safety and Feasibility of Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction. J Invasive Cardiol 2022;34:E39-42. [PubMed]
- Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2018;7:e011245. [Crossref] [PubMed]
- Scheller B, Vukadinovic D, Jeger R, et al. Survival After Coronary Revascularization With Paclitaxel-Coated Balloons. J Am Coll Cardiol 2020;75:1017-28. [Crossref] [PubMed]


