
A Delphi consensus on the management of anticoagulation in the COVID-19 pandemic: the MONACO study
Highlight box
Key recommendations
• Oral anticoagulation should be maintained during admission for COVID-19 in haemodynamically stable patients with no disease severity criteria, with prior anticoagulation, and no significant interactions with the specific treatment prescribed for COVID-19.
• The intensity of anticoagulation doses with low molecular weight heparin should be adjusted based on the severity of the disease and the thromboembolic risk and considering the bleeding risk.
• Use of thromboembolic prophylaxis after hospital discharge in patients with COVID-19 should be individualised based on their thromboembolic and bleeding risk.
• During the COVID-19 pandemic, it is recommended that patients newly diagnosed with atrial fibrillation (AF) start anticoagulant therapy with direct oral anticoagulants if there is no contraindication.
• Virtual nursing consultations with predefined protocols are recommended for following-up patients with AF.
What was recommended and what is new?
• Previous guidelines and formal positions on anticoagulation management have been developed, but most of them did not follow a rigorous method to reach consensus. Here, we achieved expert consensus using the Delphi method.
What is the implication, and what should change now?
• These recommendations should be combined with the results of clinical trials addressing anticoagulation management in patients with COVID-19 and AF, when available.
Introduction
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease (COVID-19) outbreaks have spread rapidly worldwide since the first case was reported at the end of 2019 (1). The pandemic has officially left behind around 7 million deaths, but the real number is estimated to be at least double (2,3). The pathophysiology of COVID-19 is complex, and although it mainly compromises the respiratory system, the disease has also major implications for the cardiovascular system (4). Common cardiovascular complications among COVID-19 hospitalised patients include arrhythmias, myocardial injury, cardiac arrest, heart failure, and prothrombotic coagulopathy (4,5). The high risk of venous thromboembolic events (VTEs) in hospitalised patients may be caused by the cytokine storm induced by the virus (6) and the reduced venous flow due to prolonged bed rest (7). The risk is especially high in the intensive care unit (ICU), where VTEs occur in up to 28% of patients (8,9). Among the arrhythmias reported in hospitalised COVID-19 patients, atrial fibrillation (AF) is the most prevalent (10), particularly among patients who are older (≥60 years old) and have severe COVID-19 (11). AF in COVID-19 patients is associated with poor prognosis and higher mortality (11).
It is well established that oral anticoagulation with vitamin K antagonists (VKAs) or direct oral anticoagulants (DOACs) markedly reduces stroke and mortality in patients with AF (12). According to the current guidelines from the European Society of Cardiology (ESC), therapeutic anticoagulation is indicated in patients with CHA2DS2-VASc score ≥2 (men) and ≥3 (women), and it should be considered in patients with CHA2DS2-VASc score ≥1 (men) and ≥2 (women), regardless of COVID-19 infection (13,14). Since hospitalised patients with COVID-19 are usually older than 65 years and have several comorbidities, including cardiovascular diseases (15), a considerable number of patients meet criteria for anticoagulation. In line with this, prophylactic anticoagulation as initial treatment among patients admitted to hospital with COVID-19 has been associated with a decreased risk of mortality and no increased risk of serious bleeding events (16). However, treatments for COVID-19 might interact with anticoagulant agents (17), posing a challenge for physicians when making treatment decisions.
Anticoagulated patients who were not infected with COVID-19 also suffered the effects of the pandemic. Healthcare systems were overwhelmed and had to be adapted to treat the largest number of COVID-19 patients as possible. This situation, together with the restrictions of mobility imposed during the lockdowns, jeopardised the management of patients with various diseases (18), including those receiving anticoagulation treatment. Consequently, medical consultations had to be reorganised to adapt to these changes (19). In this context, telemedicine has played an important role, where procedures and tools for remote follow-up were immediately adopted (20).
Rationale and knowledge gap
Given the urgency of the situation, guideline documents and formal positions on anticoagulation management from scientific societies in Spain (21-24) and other countries (14,25-32) were rapidly published during the pandemic. However, these documents were prepared without following a structured methodology, and there were significant differences between guidelines (33).
Objective
To fill this gap, and to identify recommendations on anticoagulation management during the COVID-19 pandemic, a national panel of experts from different hospitals in Spain participated in the present Delphi consensus. This consensus broadly covers clinical management of patients with AF and management of thromboprophylaxis (both in patients with AF and in patients without this condition) during and after COVID-19 infection. In this article, we provide the results of the consensus recommendations reached. We present this article in accordance with the CREDES reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-76/rc).
Methods
Overview of the method used
A 2-round modified Delphi method was used to reach consensus among experts. The Delphi method allows structured communication in a group of experts by inquiring about their experiences and opinions, when scientific information is lacking or controversial. In iterative rounds, panellists reply to the questionnaire. Each expert provides opinions individually and anonymously, avoiding the biasing effect of dominant individuals and group pressure. The responses from each round are compiled and provided to the participants. This process allows each expert to re-evaluate their initial answer, after considering the response of the other panellists.
The approval of the Institutional Review Board or by equivalent ethics committee(s) was not required as this Delphi study does not involve research on human subjects. No patient data were collected for this study, which was completely based on the feedback provided by the panellists. All panellists accepted to participate in the study.
Selection and composition of the participants
A total of 150 cardiologists with a balanced territorial representation throughout Spain were contacted to participate in the MONACO study as the expert panel. This number represents approximately 4% of the cardiologists in Spain. All the cardiologists had experience in the management of patients with anticoagulation and AF. An expert scientific committee of seven cardiologists was established, based on their extensive experience and their recognised expertise in the field.
Preparation of the questionnaire
The scientific committee undertook a narrative review of the current medical literature of available studies and clinical practice guidelines on the management of anticoagulation during the COVID-19 infection in patients with and without AF that was available up to September 2020. After a careful and critical review of the selected literature, the first set of dimensions and items for the Delphi questionnaire was drafted.
Item rating and consensus levels
The panellists received an information leaflet with the study aims, procedure, and a link to the online questionnaire on the web platform. The two rounds of the Delphi process were conducted anonymously (first round: April to June 2021; second round: September to November 2021).
First round
Panellists were asked to rate each item on a 9-point Likert scale from 1 (completely disagree) to 9 (completely agree). Each item was categorised according to the scores provided by the panellist as rejected (scores 1–3), undetermined (scores 4–6), or accepted (scores 7–9). Panellists were also encouraged to provide comments on the items using open-text fields. Once the questionnaire from the first round was completed, members of the scientific committee participated in an online meeting to interpret and discuss the results. Items agreed by ≥66% of the panellists and the scientific committee were selected. Items that did not achieved 66% of agreement were removed or modified according to the feedback provided by the panellists. The 66% cut-off was chosen as it represents two-thirds of the panellists, and similar cut-offs have been also used in other Delphi studies (34-37). Redundant items were merged or eliminated, and some items were edited, based on the experts’ comments. New items were generated and included when necessary. The updated questionnaire was redistributed to the panellists for the second round.
Second round
The same panellists were asked to rate the items that did not reach consensus from the first round using the same method. For this evaluation, the panellists were provided with a summary of the opinions issued anonymously by the participants in the previous round, together with information considered appropriate by the scientific committee to clarify certain aspects. Panellists were allowed to reflect upon the group’s responses and re-evaluate items that did not achieve agreement in the first round.
Final consensus statements
After the second round was completed, the scientific committee met online on 17 January 2022 and discussed the items that did not reach consensus in the second round. In this meeting, the final document with the guidance statements that reached consensus was approved.
Statistical analysis
Descriptive statistical analyses were conducted on the results obtained from the two rounds. The frequency distribution of responses on the 9-point scale was calculated to establish the consensus level for each item. Consensus in favour was established when items were accepted by ≥66% of experts, and consensus against was established when items were rejected by ≥66% of experts.
A descriptive statistical analysis of the characteristics of the expert panel was also performed, including calculation of measures of central tendency and dispersion [mean ± standard deviation (SD), median and interquartile range (IQR)] for quantitative variables, and frequencies and valid percentages for qualitative variables. IBM-SPSS version 22 (Armonk, NY, USA) for Windows was used for data analysis.
Results
A total of 147 (98%) out of 150 cardiologists participated in the first round. Of these participants, 144 (98%) completed the second round. The characteristics of the panellists are summarised in Table 1. Most participant were associate physicians (91.0%) from public hospitals (85.5%), with a median of 15 years of professional experience. Approximately half (47.6%) were involved in research.
Table 1
| Characteristics | Value |
|---|---|
| Age (years), median (IQR) | 43.0 (37.0–50.5) |
| Male, n (%) | 101 (69.7) |
| Professional experience (years), median (IQR) | 15.0 (10.0–23.0) |
| Hospital position, n (%) | |
| Department head | 8 (5.5) |
| Staff physician | 132 (91.0) |
| Other | 5 (3.4) |
| Research/teaching experience, n (%) | |
| Professor | 12 (8.3) |
| Researcher | 69 (47.6) |
| No research activity | 55 (37.9) |
| Other | 9 (6.2) |
| Type of hospital, n (%) | |
| Public | 124 (85.5) |
| Private | 19 (13.1) |
| Mixed public-private | 2 (1.4) |
Other research or teaching activity: practical classes to medical students occasionally (n=1), co-author in publications (n=1), colaborator in clinical trials and teaching (n=1), training of resident doctors (n=1), attending physician (n=1), associate professor (n=2), residency mentor (n=2). IQR, interquartile range.
Five dimensions were covered: (I) management of anticoagulation in patients with AF without mechanical valves or moderate/severe mitral stenosis (AF, hereafter) during COVID-19 infection; (II) thromboprophylaxis in patients hospitalised for COVID-19; (III) management of anticoagulation at hospital discharge/after COVID-19; (IV) anticoagulation monitoring in the COVID-19 pandemic setting; and (V) role of telemedicine in the management and follow-up of patients with AF in the COVID-19 pandemic setting.
Figure 1 shows a schematic view of the questionnaire during the Delphi process. Briefly, the questionnaire included 160 items in the first round, and 9 of them (5.6%) did not reach consensus. Among these items, seven were considered by the scientific committee to be re-evaluated in the second round. The four items that did not reach consensus in the second round were discussed by the scientific committee. The final set included 161 consensus items.
Tables S1-S5 display the 161 items (157 with consensus in favour and 4 with consensus against), the percentage of agreement among panellists for each item, and the round where consensus was reached for each item.
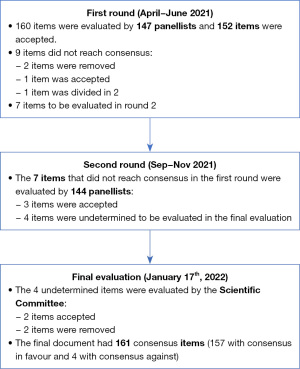
Regarding management of anticoagulation in patients with AF during COVID-19 infection, most panellists agreed that outpatients should continue their oral anticoagulant therapy, except if requiring hospitalisation or an invasive procedure. Consensus was also reached for switching from oral anticoagulants to low molecular weight heparin (LMWH) at therapeutic doses, especially in patients with a treatment for COVID-19 that interacts with the oral anticoagulation, or in hemodynamically unstable patients, with COVID-19 severity criteria, or with an invasive procedure planned. LMWH at intermediate-high doses would be advisable if the patient has a low thromboembolic risk, depending on COVID-19 severity and bleeding risk. The start of treatment with LMWH should follow the same guidelines as in patients hospitalised for other reasons. If oral anticoagulation was maintained, DOACs were considered more advisable than VKAs. An algorithm for management of anticoagulation in patients with AF hospitalised for COVID-19 based on consensus items is shown in Figure 2.
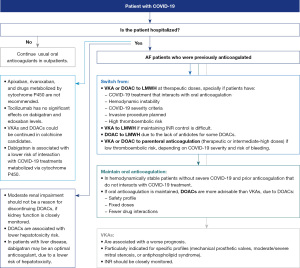
Panellists agreed on providing prophylactic doses of LMWH in all patients hospitalised for COVID-19 with no prior indication for anticoagulation, unless contraindicated. The intensity of anticoagulation doses with LMWH should be adjusted based on the severity of the disease and the thromboembolic and bleeding risk (Figure 3). Regarding parenteral anticoagulation during admission for COVID-19, LMWH (once daily) may be more beneficial than unfractionated heparin (UFH) (twice daily) to minimise exposure of patients and healthcare professionals. Use of UFH was recommended only in certain cases (such as creatinine clearance <15 mL/min or an immediate invasive procedure). Several laboratory parameters and history of high thrombotic risk should be considered to stratify the thrombotic risk of patients at hospital admission (Figure 3).
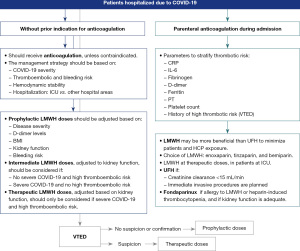
Consensus was reached on the use of thromboembolic prophylaxis after COVID-19 hospitalisation discharge, which should be individualised based on the patient’s thromboembolic and bleeding risk. Maintenance of LMWH for 7–15 days after discharge was also considered prudent. The patient profile for extending thromboembolic prophylaxis, if bleeding risk is low, includes thromboembolic risk factors, elevated D-dimer levels, and reduced mobility, among others (Figure 4). For patients who were receiving anticoagulation with DOACs before hospital admission, DOACs should be restarted when the next LMWH was scheduled, except if patients are receiving a COVID-19 treatment that interacts with the DOAC. If patients were previously treated with VKAs, a switch to DOACs (unless contraindicated) was agreed.

Key aspects of anticoagulation in AF patients without COVID-19 during the pandemic were also addressed. Panellists agreed that anticoagulation with VKAs and the required close monitoring results in an increased risk of contagion both for patients and healthcare professionals. Switching from VKAs to DOACs should be considered, in the absence of contraindications, since DOACs could increase treatment adherence and reduce the risk of patient exposure associated with anticoagulation monitoring. If DOACs are contraindicated, VKAs should be used. Measures to decrease the risk of contagion in patients anticoagulated with VKAs are shown in Table 2.
Table 2
| • Spacing of INR monitoring visits in patients with good therapeutic control |
| • Differentiated INR monitoring circuits for respiratory/nonrespiratory patients |
| • Triage upon entry to the primary care centre and phone triage |
| • Rapid INR monitoring circuits in particularly vulnerable patients (e.g., with mechanical valve prostheses or moderate/severe mitral stenosis) |
| • Appointments with groups of patients based on risk |
| • Schedule appointments for INR monitoring in differentiated areas and at less busy hours |
| • Self-monitoring of INR at home (self-monitoring with portable coagulometer) with phone consultation for dose adjustment |
| • Reinforcement of patient education in oral anticoagulation with VKAs |
| • Switch from VKAs to DOACs |
VKAs, vitamin K antagonists; INR, international normalised ratio; DOACs, direct oral anticoagulants.
Consensus was reached on using, if possible, telemedicine in patients with AF in the pandemic setting, although the most appropriate care management should be individualised considering the patient’s demographic and clinical characteristics and the purpose of the visit. Consultation was agreed to be performed via phone, video call or specific platforms (e.g., TELEA), and using email as a complementary tool. Aspects to be reviewed in online monitoring visits of patients with AF are included in Table 3.
Table 3
| • Signs and symptoms |
| • Thrombotic and bleeding risk |
| • Achievement of anticoagulation monitoring targets |
| • Treatment adherence |
| • Modifications of doses or drugs |
| • Concomitant medication |
| • Self-assessed biometric data (e.g., blood pressure, heart rate, etc.) |
| • Potential side effects |
| • Lifestyle (diet, exercise, smoking, etc.) |
AF, atrial fibrillation (patients without mechanical valves or moderate/severe mitral stenosis).
The nursing staff was recognised as playing a key role in telemedicine, especially in making the first contact with patients, by providing them with information about the visit and identifying patients’ situation (symptoms, signs and potential impairments for conducting remote visits). Nurses were also considered relevant for conducting patients’ follow-up, where predefined protocols should be used. Online referrals between specialties involved in the follow-up of AF patients and the use of the electronic prescription system should be also encouraged.
Discussion
We have presented here consensus-driven recommendations on anticoagulation management in the COVID-19 era reached among 144 national experts in cardiology (MONACO study). Recommendations were obtained using the Delphi process, a rigorous and well-known methodology to reach consensus when scientific knowledge is scarce or uncertain (38), and which has been previously used to reach consensus on oral anticoagulation management (34). The use of this methodology is a major strength of the study, considering the limited evidence-based guidelines on anticoagulation management related to COVID-19 available to date (39). Moreover, the MONACO consensus covers anticoagulation management broadly, including the management of both ‘usual’ anticoagulated patients (who were treated before the pandemic started) and ‘new’ anticoagulated patients (who initiated treatment after COVID-19 infection), along with recommendations on the role of telemedicine. Our results show a high level of agreement between national experts on the broad spectrum of anticoagulation management in the context of the pandemic.
The evidence that anticoagulation improves prognosis in hospitalised COVID-19 patients is compelling (40-42). However, data on the type of anticoagulant treatment and dose that are more beneficial varies among studies (43-49), and findings in patients with AF are not fully available yet (50). In the present Delphi consensus, panellists agreed that the usual oral anticoagulant therapy should be switched to LMWH at therapeutic doses in inpatients with AF, especially in those with hemodynamical instability, severe COVID-19, high thromboembolic risk, an invasive procedure planned, or when treated with antivirals. Switching to therapeutic doses of LMWH in AF patients hospitalised with COVID-19 has been previously recommended by other authors (23,24,31,51). The use of LMWH at therapeutic doses in high-risk inpatients is also supported by results from the HEP-COVID randomised clinical trial, as therapeutic doses of LMWH reduced the risk of thromboembolism and mortality compared with prophylactic or intermediate doses of LMWH or UFH, without increasing major bleeding (48). Results from two other clinical trials (ATTACC and RAPID) also showed a reduction in mortality with therapeutic-dose heparin in noncritically (46) and moderately (52) ill patients. Future studies should provide evidence of the optimal dose for COVID-19 patients who also have AF.
One of the main reasons for recommending switching oral anticoagulants to LMWH when receiving antiviral drugs or immunomodulating agents for COVID-19 (23,30,53) is drug-drug interactions (54). If there are no significant drug-drug interactions between oral anticoagulation and COVID-19 treatment, the consensus among panellists favoured maintaining oral anticoagulation in stable AF, nonsevere COVID-19 inpatients. If oral anticoagulation was maintained, panellist agreed that treatment with DOACs instead of VKAs was preferred, due to fewer drug-drug interactions, better safety profile, and lower monitoring. A switch from a VKA to a DOAC during hospitalisation has been previously suggested (53). Indeed, guidance for the safe switching from VKAs to DOACs in AF patients and VTE were published at the beginning of the pandemic (26).
Panellists supported the use of dabigatran as anticoagulation treatment during hospitalisation in AF. Dabigatran is associated with a lower risk of both hepatotoxicity and interaction with COVID-19 treatments that are metabolised by cytochrome P450 (55). In fact, dabigatran was initially suggested to be the first choice for oral anticoagulation in hospitalised patients with AF and COVID-19 (56,57) due to the low risk of interactions with antiviral therapies, the low risk of hepatotoxicity, the lack of metabolism by cytochrome P450, and the availability of specific reversal agent. With the widespread use of dexamethasone for COVID-19, dabigatran or edoxaban at discharge have been recommended because of the low risk of drug-drug interactions (57,58). The use of dexamethasone concomitantly with apixaban or rivaroxaban in hospitalised COVID-19 patients with coagulopathy should be avoided because of the interactions (59). In line with this, panellists were against switching from DOACs to LMWH in AF patients who are candidates for dexamethasone, although no particular DOAC agent was mentioned.
Thrombotic events occur mainly in the first ten days after admission due to COVID-19 (60). International guidelines recommend that hospitalised, nonpregnant patients with COVID-19 should receive, at a minimum, a prophylactic dose of anticoagulation to prevent VTE (39,61-66). In COVID-19 inpatients with no prior indication for anticoagulation, panellist concurred that the strategy for prevention of thromboembolic events should be based on the patient’s clinical profile. In the absence of any contraindications, prophylactic doses of LMWH were recommended, adjusted according to disease severity, D-dimer levels, body mass index (BMI), kidney function, and bleeding risk, in line with other authors (67). LMWH, in addition to the anticoagulation properties and the lower administration frequency (compared to UFH), has anti-inflammatory properties too (68), an added benefit considering that levels of proinflammatory cytokines are elevated in COVID-19 (6).
Regarding thromboembolic prophylaxis after hospital discharge, panellists suggested to individualise treatment based on thromboembolic and bleeding risk. The ESC guidance, which also made this recommendation, suggests the use of the CHA2DS2-VASc score to assess thromboembolic risk in AF patients (14), as validated thromboembolic risk assessment scores for COVID-19 patients after hospital discharge are not yet available. According to panellists, continuous use of LMWH for 1–2 weeks after discharge is advisable in all patients, but extension of thromboembolic prophylaxis must consider the thromboembolic and bleeding risk of the patient. In the absence of contraindication, panellists recommended to initiate DOACs or switch to DOACs in patients diagnosed with AF during hospitalisation or in patients previously anticoagulated with VKAs, respectively. In a study with 1,936 hospitalised patients, VTE prophylaxis was associated with enhanced survival rates at 30 and 90 days and reduced likelihood readmission 30 days after discharge (69).
In patients with normal liver function and creatinine clearance >30 mL/min, dabigatran was considered by panellists an optimal anticoagulation option, while edoxaban was preferred when creatinine clearance ranges from 15 and 30 mL/min. The choice of DOACs over VKAs for long-term anticoagulant therapy is supported by the CHEST guideline (70). Also, the MICHELLE trial, which evaluated the efficacy and safety of extended thromboprophylaxis after hospitalisation for COVID-19 in patients at high VTE risk and low bleeding risk (71), showed that post-discharge thromboprophylaxis with rivaroxaban 10 mg/day was effective at reducing thrombotic events and thrombotic-related death with a low risk of major bleeding. Results from ongoing trials (HEAL-COVID NCT04801940 and XACT NCT04640181) evaluating post-discharge thromboprophylaxis will provide further evidence to support recommendations in this regard.
The consensus achieved among experts on anticoagulation monitoring in chronically anticoagulated patients not infected with COVID-19 and on the use of telemedicine during the pandemic was particularly high. Panellists presented several measures to decrease the risk of contagion in patients anticoagulated with VKAs, including measures related to changes in international normalised ratio (INR) monitoring and switching from VKAs to DOACs, when possible. The recommendation to prescribe DOACs instead of VKAs was made in England by the National Health Service (NHS) soon after the onset of the pandemic (29). A considerable number of patients switched from a VKA to a DOAC practically in line with NHS guidance (72), and the experience was overall safe and well-received by patients (73). In Spain, the Spanish Medicines Agency restricts the use of DOACs to a second-line treatment in most cases, in contrast with recommendations from Spanish Scientific Societies and the European Cardiology Society. However, during the pandemic, the healthcare departments of most autonomous communities approved anticoagulation with DOACs for patients with recently diagnosed AF. Due to the advantages of DOACs versus VKAs, particularly in situations such as a pandemic, administrative barriers should be minimised, and equal access should be guaranteed for all patients.
A consensus was reached regarding the use of telemedicine to follow up on AF patients during the pandemic, although the most appropriate type of visit should be determined individually. Several remote communication tools (phone calls, video calls, platforms) and the role of the nursing staff in telemedicine were highlighted. Digital health tools provide both healthcare professionals and patients with protection against potential contagious diseases. They are also convenient for situations where access to healthcare is limited due to a future crisis, such as natural disasters or armed conflicts, allowing geographic, individual, and social barriers to be crossed. Panellists also agreed to combine virtual visits with remote heart rate and rhythm monitoring using portable devices for the management of patients with AF. This is in line with the recent recommendation by the National Institute for Health and Care Excellence (NICE) on the KardiaMobile as an option for detecting AF for people with suspected paroxysmal AF (74). The COVID-19 pandemic clearly catalysed the transformation of telemedicine into a tool indispensable for the present and future management of patients outside the hospital setting (75).
These consensus recommendations have several limitations that should be considered. Results from clinical trials on anticoagulation in COVID-19 patients were not available during the Delphi process. This limitation is inherent to a Delphi process where evidence simultaneously emerges, especially in the COVID-19 context. Our recommendations, however, were discussed considering the currently available evidence. Another limitation might be that while the expert panel represented a diversity of expertise from different autonomous communities in Spain, it was conducted at a national level, and therefore the application of these recommendations in other countries might be subject to the characteristics of the healthcare system, the epidemiological situation, and the patient profile in each country. In line with this, another limitation of our study is the lack of racial diversity. We focused on the management of anticoagulation in the COVID-19 pandemic within Spain, a country with a largely homogeneous population in terms of race. Hence, the insights provided might not be entirely representative or applicable to diverse racial and ethnic groups. Given the potential differences in the susceptibility to COVID-19 and its complications, as well as the response to anticoagulation therapy among different races, our findings may not be universally applicable. Our guidelines should be complemented with similar guidelines from other countries.
Conclusions
These recommendations reached with the Delphi process are intended to aid clinicians in making decisions regarding anticoagulation in the context of the COVID-19 pandemic, where results from clinical trials are still limited and, in some cases, conflicting. Our recommendations will be subject to change with increasing knowledge and should be periodically updated. The document could be also used to promote debate about possible future studies that support or question the consensus statements.
Acknowledgments
The authors would like to acknowledge the Delphi panel of the MONACO study for their participation (Delphi panellists available at https://cdn.amegroups.cn/static/public/cdt-23-76-Delphipanel.pdf). We also thank Cristina Vidal and Laura Prieto del Val from Evidenze Clinical Research for their scientific advice and medical writing support, which was funded by Boehringer Ingelheim Spain.
Funding: This work was supported by Boehringer Ingelheim.
Footnote
Reporting Checklist: The authors have completed the CREDES reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-76/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-76/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-76/coif). CE reports that he has received honoraria for lectures and educational events from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer. RBF reports that he has received honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boehringer, Novartis, and Astra Zeneca. AVM reports that he has received consulting fees from AMCA, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events Ferrer, Amgen, Sanofi, Servier, Astra-Zeneca, Boehringer Ingelheim-Lilly, NovoNordisk, Bayer, Daiichi Sankyo, Pfizer-BMS, Novartis, Rovi, Vifor, and Servier. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. The authors did not receive payment related to the development of the manuscript. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The approval of the Institutional Review Board or by equivalent ethics committee(s) was not required as this Delphi study does not involve research on human subjects. No patient data were collected for this study, which was completely based on the feedback provided by the panellists.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen L, Liu W, Zhang Q, et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect 2020;9:313-9. [Crossref] [PubMed]
- Institute for Health Metrics and Evaluation. COVID-19 Projections. Accessed 03/03/2022. Available online: https://covid19.healthdata.org/global?view=social-distancing&tab=trend
- COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet 2022;399:1513-36. Erratum in: Lancet 2022;399:1468. [Crossref] [PubMed]
- Kwenandar F, Japar KV, Damay V, et al. Coronavirus disease 2019 and cardiovascular system: A narrative review. Int J Cardiol Heart Vasc 2020;29:100557. [Crossref] [PubMed]
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020;324:782-93. [Crossref] [PubMed]
- Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med 2020;383:2255-73. [Crossref] [PubMed]
- Tan BK, Mainbourg S, Friggeri A, et al. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax 2021;76:970-9. [Crossref] [PubMed]
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145-7. [Crossref] [PubMed]
- Jiménez D, García-Sanchez A, Rali P, et al. Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest 2021;159:1182-96. [Crossref] [PubMed]
- Peltzer B, Manocha KK, Ying X, et al. Arrhythmic Complications of Patients Hospitalized With COVID-19: Incidence, Risk Factors, and Outcomes. Circ Arrhythm Electrophysiol 2020;13:e009121. [Crossref] [PubMed]
- Li Z, Shao W, Zhang J, et al. Prevalence of Atrial Fibrillation and Associated Mortality Among Hospitalized Patients With COVID-19: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2021;8:720129. [Crossref] [PubMed]
- Denas G, Gennaro N, Ferroni E, et al. Reduction in all-cause mortality in COVID-19 patients on chronic oral anticoagulation: A population-based propensity score matched study. Int J Cardiol 2021;329:266-9. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. [Crossref] [PubMed]
- Task Force for the management of COVID-19 of the European Society of Cardiology. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 1-epidemiology, pathophysiology, and diagnosis. Eur Heart J 2022;43:1033-58. Erratum in: Eur Heart J 2022;43:1776. [Crossref] [PubMed]
- Becerra-Muñoz VM, Núñez-Gil IJ, Eid CM, et al. Clinical profile and predictors of in-hospital mortality among older patients hospitalised for COVID-19. Age Ageing 2021;50:326-34. [Crossref] [PubMed]
- Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ 2021;372: [Crossref] [PubMed]
- Landayan RP, Saint-Felix S, Williams A. Probable Interaction Between Warfarin and the Combination of Remdesivir With Dexamethasone for Coronavirus Disease 2019 (COVID-19) Treatment: A 2 Case Report. J Pharm Pract 2022;35:1039-43. [Crossref] [PubMed]
- Moynihan R, Sanders S, Michaleff ZA, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open 2021;11:e045343. [Crossref] [PubMed]
- Mazón Ramos P, Virgós Lamela A, González-Juanatey JR. Reorganizing outpatient care in the era of COVID-19. Time for e-consultations. Revista Española de Cardiología Suplementos 2020;20:21-6.
- Barrios V, Cosín-Sales J, Bravo M, et al. Telemedicine consultation for the clinical cardiologists in the era of COVID-19: present and future. Consensus document of the Spanish Society of Cardiology. Revista Española de Cardiología 2020;73:910-8. [Crossref]
- Grupo de Trabajo de Cardiovascular y Diabetes de la Sociedad Española de Médicos Generales y de Familia (SEMG). Anticoagulación oral en tiempos de COVID-19.
- Piera Carbonell A, Frías Vargas M, García Vallejo O, et al. COVID-19 and thromboprophylaxis: Recommendations for our clinical practice in Primary Care. Semergen 2020;46:479-86. [Crossref] [PubMed]
- Vivas D, Roldán V, Esteve-Pastor MA, et al. Recommendations on antithrombotic treatment during the COVID-19 pandemic. Position statement of the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology. Rev Esp Cardiol (Engl Ed) 2020;73:749-57. [Crossref] [PubMed]
- Llau JV, Ferrandis R, Sierra P, et al. SEDAR-SEMICYUC consensus recommendations on the management of haemostasis disorders in severely ill patients with COVID-19 infection. Rev Esp Anestesiol Reanim (Engl Ed) 2020;67:391-9. [Crossref] [PubMed]
- Belgian Society on Thrombosis and Haemostasis (BSTH). Anticoagulation management in COVID-19 positive patients BSTH consensus guideline. Available online: https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_Anticoagulation_Management_1.pdf
- College of General Practitioners BHS. Guidance for the safe switching of warfarin to direct oral anticoagulants (DOACs) for patients with non-valvular AF and venous thromboembolism (DVT / PE) during the coronavirus pandemic. 2020. Available online: https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Coronavirus/FINAL%20Guidance%20on%20safe%20switching%20of%20warfarin%20to%20DOAC%20COVID-19%20Mar%202020.pdf?ver=2020-03-26-180945-627
- University health system. Guidelines for Anticoagulation in Hospitalized COVID-19 Patients ≥ 18 Years of Age. Available online: https://www.strac.org/files/Incident%20Specific/2019nCoV/Guidelines_for_Anticoagulation_in_Hospitalized_COVID19_Patients.pdf
- Song JC, Wang G, Zhang W, et al. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil Med Res 2020;7:19. [Crossref] [PubMed]
- National Health Service (NHS) England. Clinical guide for the management of anticoagulant services during the coronavirus pandemic. 2020. Available online: https://www.nice.org.uk/media/default/about/covid-19/specialty-guides/specialty-guide-anticoagulant-services-and-coronavirus.pdf
- Rattanawong P, Shen W, El Masry H, et al. Guidance on Short-Term Management of Atrial Fibrillation in Coronavirus Disease 2019. J Am Heart Assoc 2020;9:e017529. [Crossref] [PubMed]
- Tomaszuk-Kazberuk A, Koziński M, Domienik-Karłowicz J, et al. Pharmacotherapy of atrial fibrillation in COVID-19 patients. Cardiol J 2021;28:758-66. [Crossref] [PubMed]
- Poli D, Tosetto A, Palareti G, et al. Managing anticoagulation in the COVID-19 era between lockdown and reopening phases. Intern Emerg Med 2020;15:783-6. [Crossref] [PubMed]
- Flaczyk A, Rosovsky RP, Reed CT, et al. Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations. Crit Care 2020;24:559. [Crossref] [PubMed]
- Escobar C, Borrás X, Bover Freire R, et al. A Delphi consensus on the management of oral anticoagulation in patients with non-valvular atrial fibrillation in Spain: ACOPREFERENCE study. PLoS One 2020;15:e0231565. [Crossref] [PubMed]
- Puglisi F, Bisagni G, Ciccarese M, et al. A Delphi consensus and open debate on the role of first-line bevacizumab for HER2-negative metastatic breast cancer. Future Oncol 2016;12:2589-602. [Crossref] [PubMed]
- Burton JO, Coats AJS, Kovesdy CP, et al. An international Delphi consensus regarding best practice recommendations for hyperkalaemia across the cardiorenal spectrum. Eur J Heart Fail 2022;24:1467-77. [Crossref] [PubMed]
- de Pablos-Velasco P, Venegas EM, Álvarez Escolá C, et al. Diagnosis, treatment and follow-up of patients with acromegaly in a clinical practice setting in Spain: the ACROPRAXIS program Delphi survey. Pituitary 2020;23:129-39. [Crossref] [PubMed]
- Niederberger M, Spranger J. Delphi Technique in Health Sciences: A Map. Front Public Health 2020;8:457. [Crossref] [PubMed]
- Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: January 2022 update on the use of therapeutic-intensity anticoagulation in acutely ill patients. Blood Adv 2022;6:4915-23. [Crossref] [PubMed]
- Hozayen SM, Zychowski D, Benson S, et al. Outpatient and inpatient anticoagulation therapy and the risk for hospital admission and death among COVID-19 patients. EClinicalMedicine 2021;41:101139. [Crossref] [PubMed]
- Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094-9. [Crossref] [PubMed]
- Baumann Kreuziger L, Sholzberg M, Cushman M. Anticoagulation in hospitalized patients with COVID-19. Blood 2022;140:809-14. [Crossref] [PubMed]
- Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19. J Am Coll Cardiol 2020;76:1815-26. [Crossref] [PubMed]
- INSPIRATION Investigators. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021;325:1620-30. [Crossref] [PubMed]
- Lopes RD, de Barros E, Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet 2021;397:2253-63. [Crossref] [PubMed]
- ATTACC Investigators. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med 2021;385:790-802. [Crossref] [PubMed]
- REMAP-CAP Investigators. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med 2021;385:777-89. [Crossref] [PubMed]
- Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern Med 2021;181:1612-20. [Crossref] [PubMed]
- Rauniyar R, Kuikel S, Mishra A, et al. Safety and efficacy of prophylactic anticoagulation versus therapeutic anticoagulation in hospital-admitted COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials. Clin Respir J 2023;17:73-9. [Crossref] [PubMed]
- Olivera P, Velásquez-Escandón C, Campoy D, et al. Edoxaban Versus Low-Molecular-Weight Heparin in Hospitalized COVID-19 Patients With Atrial Fibrillation. Clin Appl Thromb Hemost 2023;29:10760296231180865. [Crossref] [PubMed]
- López-Reyes R, Oscullo G, Jiménez D, et al. Thrombotic Risk and Covid-19: Review of Current Evidence for a Better Diagnostic and Therapeutic Approach. Archivos de Bronconeumología 2021;57:55-64. [PubMed]
- Sholzberg M, Tang GH, Rahhal H, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021;375: [Crossref] [PubMed]
- Papakonstantinou PE, Borovac JA, Gąsecka A, et al. Anticoagulation therapy in non-valvular atrial fibrillation in the COVID-19 era: is it time to reconsider our therapeutic strategy? Eur J Prev Cardiol 2022;29:2069-71. [Crossref] [PubMed]
- The Liverpool Drug Interaction Group. Interactions with Experimental COVID-19 therapies. Available online: https://www.covid19-druginteractions.org/
- European Medicines Agency (EMA). Pradaxa®, Summary of product characteristics. Accessed March 9, 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/pradaxa-epar-product-information_en.pdf
- Iturbe-Hernandez T, García de Guadiana Romualdo L, Gil Ortega I, et al. Dabigatran, the oral anticoagulant of choice at discharge in patients with non-valvular atrial fibrillation and COVID-19 infection: the ANIBAL protocol. Drugs Context 2020;2020: [Crossref] [PubMed]
- Cerezo-Manchado JJ, Meca Birlanga O, García de Guadiana Romualdo L, et al. Dabigatran in patients with atrial fibrillation after COVID-19 hospitalization: an update of the ANIBAL protocol. Drugs Context 2021;2022: [PubMed]
- Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021;23:1612-76. [Crossref] [PubMed]
- Smythe MA, Burns C, Liu Q, et al. Potential Dexamethasone-Direct Oral Anticoagulant Drug Interaction: Is This a Concern in COVID? Ann Pharmacother 2022;56:319-29. [Crossref] [PubMed]
- Tacquard C, Mansour A, Godon A, et al. Anticoagulation in COVID-19: not strong for too long? Anaesth Crit Care Pain Med 2021;40:100857. [Crossref] [PubMed]
- Moores LK, Tritschler T, Brosnahan S, et al. Thromboprophylaxis in Patients With COVID-19: A Brief Update to the CHEST Guideline and Expert Panel Report. Chest 2022;162:213-25. [Crossref] [PubMed]
- Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis 2020;50:72-81. [Crossref] [PubMed]
- Marietta M, Ageno W, Artoni A, et al. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus 2020;18:167-9. [PubMed]
- National Institute for Health Care Excellence. COVID-19 rapid guideline: managing COVID-19. 2022. Accessed March 3, 2022. Available online: https://www.nice.org.uk/guidance/ng191
- National Health Service (NHS). Antithrombotic Therapy in Patients With COVID-19. Accessed March 10, 2022. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/
- Kyriakoulis KG, Kollias A, Kyriakoulis IG, et al. Thromboprophylaxis in Patients with COVID-19: Systematic Review of National and International Clinical Guidance Reports. Curr Vasc Pharmacol 2022;20:96-110. [Crossref] [PubMed]
- McBane RD 2nd, Torres Roldan VD, Niven AS, et al. Anticoagulation in COVID-19: A Systematic Review, Meta-analysis, and Rapid Guidance From Mayo Clinic. Mayo Clin Proc 2020;95:2467-86. [Crossref] [PubMed]
- Poterucha TJ, Libby P, Goldhaber SZ. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb Haemost 2017;117:437-44. [Crossref] [PubMed]
- Patel L, Stenzel A, Van Hove C, et al. Outcomes in patients discharged with extended venous thromboembolism prophylaxis after hospitalization with COVID-19. Vasc Med 2023;28:331-9. [Crossref] [PubMed]
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Ramacciotti E, Barile Agati L, Calderaro D, et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet 2022;399:50-9. [Crossref] [PubMed]
- OpenSAFELY Collaborative. OpenSAFELY: impact of national guidance on switching anticoagulant therapy during COVID-19 pandemic. Open Heart 2021;8:e001784. [Crossref] [PubMed]
- Patel R, Czuprynska J, Roberts LN, et al. Switching warfarin patients to a direct oral anticoagulant during the Coronavirus Disease-19 pandemic. Thromb Res 2021;197:192-4. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. Evidence-based recommendations on KardiaMobile for detecting atrial fibrillation. 2022. Available online: https://www.nice.org.uk/guidance/MTG64/chapter/1-Recommendations
- Varma N, Marrouche NF, Aguinaga L, et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA Worldwide Practice Update for Telehealth and Arrhythmia Monitoring During and After a Pandemic. J Am Coll Cardiol 2020;76:1363-74. [Crossref] [PubMed]


