
Diagnostic accuracy of superb microvascular imaging for detecting intraplaque neovascularization: a systematic review and meta-analysis
Highlight box
Key findings
• This meta-analysis shows that superb microvascular imaging (SMI) has a sensitivity of 93% [95% confidence interval (CI): 87–96%] and a specificity of 80% (95% CI: 71–87%) for detecting intraplaque neovascularization (IPN), making it a good tool for diagnosis.
What is known and what is new?
• IPN is a known risk factor for atherosclerotic plaque vulnerability.
• Contrast-enhanced ultrasonography (CEUS) and SMI are both tools for detecting IPN, but SMI is less invasive.
• This meta-analysis synthesized data to assess the accuracy of SMI for detecting IPN.
What is the implication, and what should change now?
• SMI is a less invasive alternative to CEUS for the purposes of diagnosing IPN. Additional large-scale studies should be performed to confirm our findings using pathological results as the reference test.
Introduction
Background
Atherosclerotic disease (myocardial infarction and stroke) is one of the leading causes of death worldwide (1-3). Atherosclerotic plaques can cause carotid artery stenosis, and “vulnerable plaques” can even lead to ischemic stroke due to plaque rupture and thrombi formation (4). Therefore, to optimize the management of atherosclerotic disease, evaluation of atherosclerotic plaque vulnerability is vital.
Rationale and knowledge gap
Intraplaque neovascularization (IPN) is a known risk factor for atherosclerotic plaque vulnerability (5). Ultrasound imaging techniques, including contrast-enhanced carotid ultrasonography (CEUS) and non-invasive superb microvascular imaging (SMI), can effectively indicate IPN features in patients with carotid stenosis. SMI does not use contrast agents while CEUS uses adaptive principles to display low-velocity blood flow signals (6). Two recently published meta-analysis demonstrated that SMI and CEUS display excellent diagnostic value for detecting carotid IPN (7,8). However, the analysis could not draw conclusions regarding the accuracy of SMI since the sample sizes in the relevant studies were small (7,8).
Objective
To the best of our knowledge, there are no reports of synthesized data on the accuracy of SMI for detecting IPN. The objective of this study was to assess the accuracy of SMI in the detection of carotid IPN in patients with atherosclerotic plaques via conducting a meta-analysis of the relevant literature. We present this article in accordance with the PRISMA-DTA reporting checklist (9) (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-202/rc).
Methods
This systematic review and meta-analysis did not require the approval of a Research Ethics Board because we used published evidence in our data analysis. The study was registered at PROSPERO (No. CRD42023399416). A protocol was not prepared.
Eligibility criteria
The inclusion criteria for this meta-analysis were as follows: (I) original studies [randomized controlled trials (RCTs), cohort studies, cross-sectional studies, and case-control studies]; (II) patients with carotid plaques; (III) having information on the diagnostic accuracy of SMI for the evaluation of carotid IPN; and (IV) having information on pathologic evaluations or CEUS as the reference test.
The exclusion criteria were as follows: (I) duplicate publications; (II) study design was a systematic review, meta-analysis, editorial, protocol, letters, or case reports; (III) full text was not available; and (IV) studies without sufficient data to perform SMI accuracy assessment.
Literature search
We searched the Cochrane Library, Embase, Medline, and Wanfang databases until January 17, 2023. The full search strategy is presented in Appendix 1. English was applied as the language restriction when searching the Cochrane Library, Embase and Medline. The key search terms included “carotid”, “plaque”, “fatty streak”, “fibroatheroma”, “neovascularization”, and “superb microvascular imaging”. The bibliographies of the related papers (reviews, meta-analysis, and potential eligible studies) were checked to find other potential articles. We also checked published conference proceedings for eligible data or references.
Study selection
Two independent reviewers (Zhao L and Han Y) performed title and abstract screening to generate a potentially relevant study list according to the eligibility criteria. The full texts of these papers were then reviewed to further confirm the eligibility of the studies. Any inconsistency between the two reviewers was resolved by discussion or referred to a third reviewer (Li J). The study selection workflow is shown in Figure 1.
Data collection
Two study team members (Zhao L and Han Y) independently performed the data extraction using a pre-test data collection form. Discrepancies were resolved by discussion or by referral to a third reviewer. The data collection included information for authors, publication year, study country, study design, patient demographics, SMI-related data, and index test information.
Outcomes
The primary outcome of the study was the accuracy of SMI for the detection of carotid IPN in patients with atherosclerotic plaques, which was measured using sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), and diagnostic odds ratio (DOR) analyses. The definitions and equations of each parameter are described in Appendix 2.
In this study, we applied both histology results and the CEUS results as reference tests to define the target condition of carotid IPN. For the different categories of carotid IPN for SMI, CEUS, and pathologic evaluation results, we used light (spot IPN), moderate (linear IPN), and severe (multiple linear IPNs) diagnoses as the positive results, and no IPN as a negative result.
Study risk of bias assessment
Two study team members (Zhao L and Han Y) independently performed the risk of bias analysis using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) criteria (10). The QUADAS-2 tool includes four primary parameters: patient selection, index test, reference standards, and methodological quality. Quality was graded as “no” for low quality, “yes” for high quality, or “unclear” if the information was not available.
Statistical analysis and synthesis methods
We performed the statistical analysis of SMI accuracy according to the Cochrane guidelines for diagnostic test accuracy reviews (11). All data analyses were performed using the STATA software, version 15.0 (Stata Corp., College Station, USA) (12). The STATA commands METANDI and MIDAS were used to perform the meta-analysis. We applied forest plots and receiver operating characteristic (ROC) plots to visualize the variation between sensitivity and specificity of SMI and their 95% confidence intervals (CIs) for detecting IPN. The bivariate random effects model was used to summarize sensitivity and specificity. The sensitivity, specificity, LR+, LR−, and DOR with their 95% CIs were achieved. We applied Cochran’s Q-statistic and I2 tests to evaluate potential heterogeneity between studies. The random effects model was applied if significant heterogeneity (P<0.1 for Q test or I2 test exceeded 50%) was detected; otherwise, we used the fixed effects model.
Subgroup analyses were performed to explore variation in test performance according to different reference indexes (CEUS and pathologic evaluation). These estimates were used to indirectly compare each parameter of accuracy by checking the overlap of the 95% CIs.
Fagan’s nomogram was used to estimate the clinical value of SMI for detecting IPN. We used this analysis to evaluate how much the result of SMI changed the probability that a plaque has IPN. Deeks’ funnel plot was used to assess publication bias (9). Meta-regression analysis was applied to investigate potential heterogeneity by including variables of age, sex, thickness of the plaques, percentage of having hypertension (HTN), percentage of having diabetes mellitus, dyslipidemia, and probe velocity scale.
Results
Study selection
A total of 103 articles were identified after searching the databases, and five more articles were found after checking the bibliographies of the potential related papers. After omitting duplicated studies, 74 articles were further screened for title and abstract. Of these, 28 articles were selected for full-text review. Finally, 20 articles were included in the data quality assessment and data analysis (6,13-31). The study selection process is shown in Figure 1 (32).
Study characteristics
A total of 1,225 patients with 1,589 carotid plaques were studied in all included papers. The mean age of the included patients ranged from 59.3 to 71.0 years. The study patients had >50% carotid stenosis and maximum carotid plaque thickness >1.5 mm. Most studies used the Toshiba Aplio500 and linear probes with slightly different MHz (range, 3–12 MHz) to detect plaques. The study characteristics of the included studies are summarized in Table 1.
Table 1
| First author [year], country | Study design | Patient type | Sample size, n | Age (years), mean or specified | Male, n (%) | DM/HTN/smoking/dyslipidemia, n | Plaques, n | Reference test | Machine/probe of SMI |
|---|---|---|---|---|---|---|---|---|---|
| Zamani et al. [2019], Norway (6) | One-arm CS | Carotid stenosis ≥50% | 31 | 70.0 | 20 (64.5) | 3/21/17/13 | 31 | Plaque histology | Toshiba Aplio500/L 7.5 MHz |
| Chen et al. [2016], China (13) | One-arm CS | Carotid plaque thickness >1.5 mm | 56 | NA | NA | NA | 80 | CEUS | Toshiba Aplio500/L 4–11 MHz |
| Chen et al. [2020], China (14) | One-arm CS | Carotid stenosis ≥50% | 28 | 63.4 | 22 (78.6) | 12/23/18/18 | 28 | Plaque histology | Toshiba Aplio500/L 4–9 MHz |
| Cheng et al. [2015], China (15) | One-arm CS | Carotid plaque thickness 2.6–5.7 mm | 57 | 61.8 | 44 (77.2) | 33/42/NA/31 | 33 | Enhanced CEUS | GE Logid E9/L 7–10 MHz |
| 13 | Plaque histology | ||||||||
| Ding et al. [2020], China (16) | One-arm CS | Carotid plaque thickness >1.5 mm | 89 | 68.5 | 45 (50.6) | NA | 89 | CEUS | Toshiba Aplio500/L 7.5 MHz |
| Forsberg et al. [2019], USA (17) | One-arm CS | Carotid plaque thickness >2 mm | 30 | 71.0 | 12 (40.0) | NA | 28 | Plaque histology | Toshiba Aplio500/L 7.5 MHz |
| Guo et al. [2023], China (18) | One-arm CS | Carotid plaque thickness >2 mm | 45 | 64.5 | 24 (53.3) | 21/38/26/32 | 76 | CEUS | Toshiba Aplio500/L 4–11 MHz |
| He et al. [2018], China (19) | One-arm CS | Carotid plaque thickness ≥1.5 mm | 40 | 64.2 | 25 (62.5) | NA | 72 | CEUS | Toshiba Aplio500/L 7–12 MHz |
| Huang et al. [2015], China (20) | One-arm CS | Carotid plaque thickness >2 mm | 62 | 61.6 | 50 (80.6) | NA | 103 | CEUS | Toshiba Aplio500/L 5–10 MHz |
| Jin et al. [2017], China (21) | One-arm CS | Carotid plaque thickness >2 mm | 146 | 67.6 | 76 (52.1) | NA | 146 | CEUS | Toshiba Aplio500/L 4–9 MHz |
| Liu et al. [2016], China (22) | One-arm CS | Carotid plaque thickness >3 mm | 52 | 65.4 | 40 (76.9) | NA | 67 | CEUS | Toshiba Aplio500/NA |
| Ma et al. [2018], China (23) | One-arm CS | Carotid plaque thickness ≥1.5 mm | 46 | 61.7 | 34 (73.9) | NA | 55 | CEUS | Toshiba Aplio500/L 4–9 MHz |
| Meng et al. [2021], China (24) | One-arm CS | Carotid plaque thickness >2.5 mm | 78 | 67.3 | 63 (80.8) | 28/59/39/46 | 104 | CEUS | Toshiba Aplio500/L 3–9 MHz |
| Oura et al. [2018], Japan (25) | One-arm CS | Carotid plaque thickness ≥2 mm | 27 | 71.0 (median) | 25 (92.6) | 14/18/NA/17 | 27 | CEUS | Toshiba Aplio500/L 7.5 MHz |
| Tang et al. [2019], China (26) | One-arm CS | Carotid plaque thickness >2 mm | 92 | 66.4 | 52 (56.5) | NA | 92 | CEUS | Toshiba Aplio500/L 7.5 MHz |
| Wang et al. [2021], China (27) | One-arm CS | Carotid plaque thickness ≥1.5 mm | 100 | 62.3 | 63 (63.0) | 32/88/43/NA | 134 | CEUS | Toshiba Aplio500/NA |
| Xie et al. [2018], China (28) | One-arm CS | Carotid plaque thickness ≥2.5 mm | 69 | 68.1 | 53 (76.8) | 21/51/31/47 | 108 | CEUS | Toshiba Aplio500/L 4–9 MHz |
| Yi et al. [2018], China (29) | One-arm CS | Carotid plaque thickness ≥1.5 mm | 95 | 59.3 | 56 (58.9) | NA | 157 | CEUS | Toshiba Aplio500/L 4–9 MHz |
| Zhang et al. [2017], China (30) | One-arm CS | Carotid plaque thickness ≥2 mm | 39 | 66.8 | 31 (79.5) | NA | 64 | CEUS | Toshiba Aplio500/L 4–11 MHz |
| Zhu et al. [2019], China (31) | One-arm CS | Carotid plaque thickness >1.5 mm | 43 | 66.0 | 26 (60.5) | 23/23/20/28 | 82 | CEUS | Toshiba Aplio500/L 7.5 MHz |
DM, diabetes mellitus; HTN, hypertension; SMI, superb microvascular imaging; One-arm CS, one-arm comparative study; NA, not available; CEUS, contrast-enhanced carotid ultrasonography.
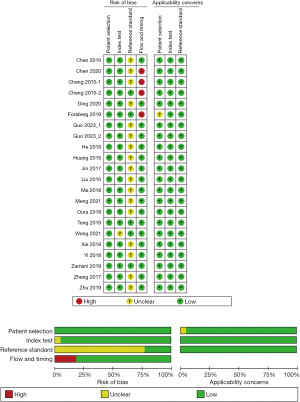
Risk of bias and applicability
The methodological quality for each included study and the overall summary of the analysis are shown in Figure 2. The risk of bias of the flow and timing domain in the final analysis was reported in three studies (14,15,17). Another risk of bias came from the reference test. Although the CEUS can diagnose IPN with high accuracy, it was not used for final pathological results. Therefore, we defined it as an unclear risk of bias in 17 included studies (Figure 2).
Accuracy of SMI for detecting IPN
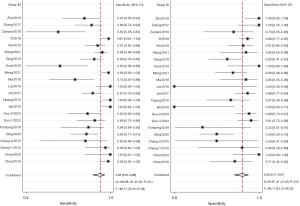
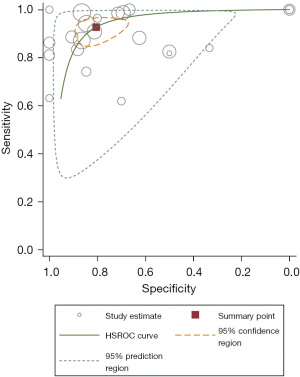
Overall, the sensitivity and specificity of SMI for detecting IPN were 93% (95% CI: 87–96%) and 80% (95% CI: 71–87%), respectively. We applied the random effects model due to the high heterogeneity in the results of the included studies, which is shown in a forest plot (Figure 3). In addition, the LR+, LR−, and DOR were 4.75 (95% CI: 3.16–7.15), 0.09 (95% CI: 0.05–0.16), and 52.4 (95% CI: 26.6–103.0), respectively. The area under the summary ROC curve (AUC) was 0.93 (95% CI: 0.91–0.95), and the graphs of the hierarchical summary ROC (HSROC) curves of the individual studies for the diagnostic accuracy of the test analyzed are shown in Figure 4.
Subgroup analysis
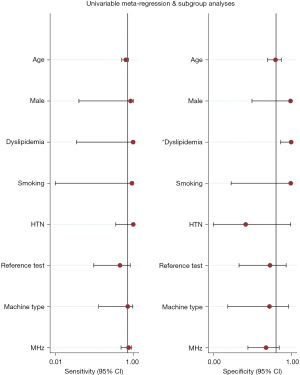
We performed subgroup analyses according to the different reference tests (pathological results versus CEUS). Details of the accuracy data are listed in Table 2. According to the current value of each diagnostic accuracy parameter, the accuracy of the CEUS group was better than the pathological group. However, according to the indirect comparison, there was no statistical difference between the two groups. Meta-regression analysis showed that age, male (proportion), smoking (proportion), HTN (proportion), different reference tests, different machine types, and probe velocity scale did not contribute to heterogeneity among studies; only dyslipidemia (proportion) contributed to study heterogeneity (Figure 5).
Table 2
| Accuracy | Reference tests | |
|---|---|---|
| CEUS | Pathological results | |
| Sensitivity, % (95% CI) | 93.6 (88.1–96.7) | 86.5 (58.9–96.6) |
| Specificity, % (95% CI) | 80.9 (69.8–88.6) | 78.8 (45.4–94.3) |
| LR+, ratio (95% CI) | 4.90 (3.06–7.82) | 4.07 (1.11–4.89) |
| LR−, ratio (95% CI) | 0.08 (0.04–0.14) | 0.17 (0.04–0.76) |
| DOR, ratio (95% CI) | 62.3 (32.0–121.2) | 23.8 (1.73–327.80) |
SMI, superb microvascular imaging; IPN, intraplaque neovascularization; CEUS, contrast-enhanced carotid ultrasonography; CI, confidence interval; LR+, positive likelihood ratio; LR−, negative likelihood ratio; DOR, diagnostic odds ratio.
Post-test probability
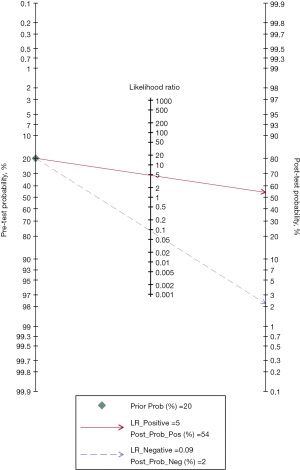
A pretest probability of 20% of SMI for detecting IPN was fixed, which was defined from the number of IPN cases in the included studies. SMI for detecting IPN had a post-test probability of 54%. Thus, with a prevalence of 20% for IPN, the post-test probability that a patient truly has IPN would be 54% if this patient tests positive. In contrast, the post-test probability that a patient truly has IPN would be 2% if the patient tests negative (Figure 6).
Publication bias
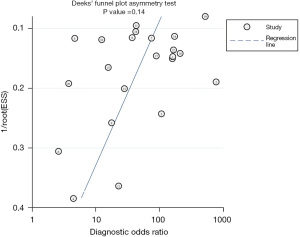
Although significant heterogeneity was detected for the test (80.2% for sensitivity and 68.2% for specificity), the funnel plots showed that there was no potential publication bias (P=0.14) (Figure 7).
Discussion
Key findings
This systematic review showed that SMI can be a highly accurate technique for detecting IPN according to the meta-analysis results for each key accuracy parameter. This meta-analysis of 20 studies with 1,589 carotid plaques in 1,225 patients reported a summary sensitivity and specificity of SMI for detecting IPN of 93% (95% CI: 87–96%) and 80% (95% CI: 71–87%), respectively. Summary estimates of each diagnostic accuracy parameter of the CEUS group were better than the pathological group. However, according to the indirect comparison, there was no statistical difference between the two groups. The risk of bias across QUADAS domains was low; only the proportion of dyslipidemia influenced the estimates of sensitivity and specificity.
Explanation of findings
The present study demonstrated that the sensitivity of SMI for detecting IPN was 93% (87–96%), which means the test is suitable as a screening tool for IPN in patients with carotid plaques (33). The high specificity of the test [80% (95% CI: 71–87%)] indicates a good capacity for diagnosing IPN in the clinic (33). Both the DOR [52.4 (95% CI: 26.6–103.0)] and the AUC [0.93 (95% CI: 0.91–0.95)] confirmed that SMI can effectively detect IPN (34,35).
Comparison with similar research
Previous studies have confirmed the validity of CEUS for detecting IPN (36,37). However, CEUS has the limitations of high cost and invasive characteristics of the contrast agent. Furthermore, compared to SMI, CEUS does not define the direction or velocity of blood flow (38,39). According to a recent meta-analysis, there is high consistency between SMI and CEUS for diagnosing IPN (7,8). However, no studies have reported the accuracy of SMI for detecting IPN. In this study, we applied CEUS as the reference test, in addition to using pathological evaluation standards, because CEUS has a high diagnosis validity for detecting IPN. However, considering the potential discrepancy between the real diagnosis and the results of the CEUS detection, we also performed subgroup analysis. According to our analysis, the accuracy estimates of SMI based on the CEUS results are better than those based on the pathological results. However, there was no statistical difference between the two groups, as there was overlap in the 95% CIs for each parameter. According to the meta-regression analysis (Figure 5), the difference in the reference tests did not contribute to the heterogeneity of the sensitivity and the specificity through the included studies. However, this may be due to the small sample sizes of the included studies for the pathological results group. Future studies with large sample sizes for pathological results as the reference test are needed to confirm our findings.
An interesting finding of this study was that among the variables that could influence SMI’s ability to detect IPN, only the proportion of dyslipidemia contributed significantly to the heterogeneity for the sensitivity and specificity analyses (Figure 4). It has been reported that patients with dyslipidemia have a higher prevalence of carotid plaques (40-43). Thus, the apparent increased IPN prevalence due to the increased prevalence of carotid plaques can influence the sensitivity and specificity (44).
Implications of results
The results of the present study provide new insight for the clinical management of IPN. If patients have a positive SMI result for detecting IPN, the post-test likelihood that the patient has IPN will be 54% (given that the population prevalence in this study was 20%). These patients may need further tests to confirm the diagnosis. On the other hand, if a patient has a negative SMI result, the probability of having the condition of IPN will be 2%. This analysis indicates that SMI is an excellent tool for detecting IPN. Further research and high-quality studies on this subject are needed to confirm our findings.
In clinical practice, plaques are categorized as homogeneous and heterogeneous using ultrasonography. It is generally believed that homogeneous plaques are mostly stable with uniform internal echoes and intensity. Stable plaques are characterized by a smooth surface, regular shape, uniform and strong echoes, as well as a stable internal tissue structure. Heterogeneous plaques are mostly unstable. Unstable plaques are characterized by irregular morphology, non-smooth surface, low or predominantly low echo, and some plaques have a detectable juxtaluminal black area, which is mostly due to hemorrhage and ulceration. The surface of the plaque will have at least two sections with detectable depressions; the depressions will be bordered, the surface of the plaque will be markedly defective, and color Doppler can be used to visualize the formation of filling defects.
To distinguish homogeneous plaques from heterogeneous plaques, clinicians can only rely on the appearance of the plaque and the homogeneity of the echoes to determine whether the plaque is stable or not. Furthermore, this determination is highly subjective and uncertain and lacks a prospective approach for evaluating clinical events, which can result in the occurrence of acute events, such as strokes, that cannot be predicted. In clinical practice, most hypoechoic or isoechoic plaques with smooth borders and intact fibrous caps are treated conservatively as stable plaques. However, not all of these plaques are actually stable, and some may have undergone neovascularization, which cannot be accurately detected by conventional ultrasound and Doppler ultrasound techniques. Moreover, neither of these techniques can accurately obtain clinically meaningful blood flow information, making it difficult to judge plaque stability.
Strengths and limitations
The present review is, to the best of our knowledge, the first meta-analysis to summarize the accuracy of SMI for detecting IPN. We applied rigorous methodology in this systematic review. However, there are still some limitations to our analysis. First, only a few of the included studies provided pathological results to confirm the test results, which may lead to an overestimation of the accuracy of SMI. In addition, the prevalence of IPN varied across the included studies, which may have significantly influenced the overall estimates of sensitivity and specificity. The protocol which was uploaded to PROSPERO contains some inaccuracies, which we were regrettably not able to properly update. A version of the protocol in line with the review’s methods can be found in the supplementary file (available at https://cdn.amegroups.cn/static/public/cdt-23-202-1.pdf). Finally, most of the studies were from China, which may have limited the external validity of the findings.
Conclusions
In summary, the present review suggests that SMI is a non-invasive ultrasound method that has a good diagnostic performance for detecting IPN. The high sensitivity and excellent post-test probability indicated that SMI can be recommended to screen for IPN among patients with carotid plaques. Additional large-scale studies should be performed to confirm our findings using pathological results as the reference test.
Acknowledgments
The authors would also like to thank Medjaden Bioscience Limited for their assistance in polishing the language of the manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (Youth Science Foundation Program) (Batch No. 82205060). The funding bodies had no role in the design of the study, the collection, analysis, or interpretation of the data, or writing of the manuscript.
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-202/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-202/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-202/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fan J, Watanabe T. Atherosclerosis: Known and unknown. Pathol Int 2022;72:151-60. [Crossref] [PubMed]
- Agarwal S, Sud K, Thakkar B, et al. Changing Trends of Atherosclerotic Risk Factors Among Patients With Acute Myocardial Infarction and Acute Ischemic Stroke. Am J Cardiol 2017;119:1532-41. [Crossref] [PubMed]
- Mattingly Q. Cardiovascular Diseases: WHO (2023). Available from: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1
- Baradaran H, Gupta A. Brain imaging biomarkers of carotid artery disease. Ann Transl Med 2020;8:1277. [Crossref] [PubMed]
- Boswell-Patterson CA, Hétu MF, Kearney A, et al. Vascularized Carotid Atherosclerotic Plaque Models for the Validation of Novel Methods of Quantifying Intraplaque Neovascularization. J Am Soc Echocardiogr 2021;34:1184-94. [Crossref] [PubMed]
- Zamani M, Skagen K, Scott H, et al. Carotid Plaque Neovascularization Detected With Superb Microvascular Imaging Ultrasound Without Using Contrast Media. Stroke 2019;50:3121-7. [Crossref] [PubMed]
- Song Y, Xing H, Zhang Z, et al. Detection of Carotid Atherosclerotic Intraplaque Neovascularization Using Superb Microvascular Imaging: A Meta-Analysis. J Ultrasound Med 2021;40:2629-38. [Crossref] [PubMed]
- Yang F, Wang C. Consistency of superb microvascular imaging and contrast-enhanced ultrasonography in detection of intraplaque neovascularization: A meta-analysis. PLoS One 2020;15:e0230937. [Crossref] [PubMed]
- McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018;319:388-96. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Macaskill P, Gatsonis C, Deeks J, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. The Cochrane Collaboration; 2010.
- Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. [Crossref] [PubMed]
- Chen J. Using SMI and CEUS to Evaluate the Neovascular in Different Thickness Carotid Atherosclerotic Plaque. Master's thesis. Shijiazhuang: Hebei Medical University; 2016.
- Chen X, Wang H, Jiang Y, et al. Neovascularization in carotid atherosclerotic plaques can be effectively evaluated by superb microvascular imaging (SMI): Initial experience. Vasc Med 2020;25:328-33. [Crossref] [PubMed]
- Cheng LG, He W, Zhang HX, et al. Superb Microvascular Imaging in Evaluation of Neovascularization in Carotid Atherosclerotic Plaque. Chinese Journal of Medical Imaging Technology 2015;31:647-50.
- Ding L. The Clinical Application Effect of Ultra-microvascular Imaging and Contrast-enhanced Ultrasound in Carotid Plaque Neovascularization with Different Thickness. World Journal of Complex Medicine 2020;6:110-2.
- Forsberg F, Machado P, Stanczak M, et al. Assessing carotid plaque neovascularity and calcifications in patients prior to endarterectomy. J Vasc Surg 2019;70:1137-44. [Crossref] [PubMed]
- Guo Y, Wang X, Wang L, et al. The Value of Superb Microvascular Imaging and Contrast-enhanced Ultrasound for the Evaluation of Neovascularization in Carotid Artery Plaques. Acad Radiol 2023;30:403-11. [Crossref] [PubMed]
- He CY, Fang B, Li KL, et al. The Comparison of the Diagnostic Value of Superb Microvascular Imaging and Contrast-Enhanced Ultrasonography in Carotid Plaque Neovascularization. Jilin Medical Journal 2018;39:2142-43.
- Huang JL, Ding Z. Clinical Value of SMI in Detecting Neovascularization in Carotid Artery Plaque: Compared to Enhanced-Contrast Ultrasound. Master Thesis. Bengbu: Bengbu Medical College; 2015.
- Jin L, Xu R, Feng L, et al. Application of Superb Microvascular Imaging (SMI) in Evaluating the Relationship between Carotid Atherosclerotic Plaque Neovascularization and Clinical Symptoms. Journal of Xinxiang Medical University 2017;34:1033-36.
- Liu TT, Wei L. Comparative Study of Ultra-Microvascular Imaging and Contrast-Enhanced Ultrasonography in Evaluating Neovascularization in Carotid Plaque. Women's Health Research 2016;221.
- Ma L, Ma XH, Zhang YT, et al. Evaluation of Neovascularization in the Different Risk Levels of Carotid Atherosclerotic Plaque: Compared SMI with CEUS. Ningxia Medical Journal 2018;40:307-10.
- Meng Q, Xie X, Li L, et al. Assessment of neovascularization of carotid artery atherosclerotic plaques using superb microvascular imaging: a comparison with contrast-enhanced ultrasound imaging and histology. Quant Imaging Med Surg 2021;11:1958-69. [Crossref] [PubMed]
- Oura K, Kato T, Ohba H, et al. Evaluation of Intraplaque Neovascularization Using Superb Microvascular Imaging and Contrast-Enhanced Ultrasonography. J Stroke Cerebrovasc Dis 2018;27:2348-53. [Crossref] [PubMed]
- Tang J. Chinese Journal of CT and MRI 2019;113. [Application Value of Superb Microvascular Imaging and Contrast-Enhanced Ultrasound in N Eovascularization of Carotid Plaques with Different Thiclrness].
- Wang XZ, Wang L, Lu RG, et al. Clinical Application of SMI Technology Neovascularization in Carotid Plaques. Imaging Science and Photochemistry 2021;39:569-73.
- Xie X, Bai ZY, Liu Y, et al. Value of Superb Micro-vascular Imaging in Diagnosing Carotid Artery Vulnerable Plaque. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2018;40:444-9. [PubMed]
- Yi XL, Ni CF. The value of routine ultrasound combined with superb microvascular imaging in evaluating the stability of carotid artery plaque. PhD Thesis. Soochow: Soochow University; 2018.
- Zhang H, Du J, Wang H, et al. Comparison of diagnostic values of ultrasound micro-flow imaging and contrast-enhanced ultrasound for neovascularization in carotid plaques. Exp Ther Med 2017;14:680-8. [Crossref] [PubMed]
- Zhu YC, Jiang XZ, Bai QK, et al. Evaluating the Efficacy of Atorvastatin on Patients with Carotid Plaque by an Innovative Ultrasonography. J Stroke Cerebrovasc Dis 2019;28:830-7. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Parikh R, Mathai A, Parikh S, et al. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol 2008;56:45-50. [Crossref] [PubMed]
- Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56:1129-35. [Crossref] [PubMed]
- Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 2010;5:1315-6. [Crossref] [PubMed]
- Dong S, Hou J, Zhang C, et al. Diagnostic Performance of Atherosclerotic Carotid Plaque Neovascularization with Contrast-Enhanced Ultrasound: A Meta-Analysis. Comput Math Methods Med 2022;2022:7531624. [Crossref] [PubMed]
- Huang R, DeMarco JK, Ota H, et al. Prognostic Value of Intraplaque Neovascularization Detected by Carotid Contrast-Enhanced Ultrasound in Patients Undergoing Stress Echocardiography. J Am Soc Echocardiogr 2021;34:614-24. [Crossref] [PubMed]
- Sato W, Suto Y, Yamanaka T, et al. An advanced ultrasound application used to assess peripheral vascular diseases: superb microvascular imaging. J Echocardiogr 2021;19:150-7. [Crossref] [PubMed]
- Mantella LE, Liblik K, Johri AM. Vascular imaging of atherosclerosis: Strengths and weaknesses. Atherosclerosis 2021;319:42-50. [Crossref] [PubMed]
- Mi T, Sun S, Zhang G, et al. Relationship between dyslipidemia and carotid plaques in a high-stroke-risk population in Shandong Province, China. Brain Behav 2016;6:e00473. [Crossref] [PubMed]
- Geng Y, Liu Y, Chen Y, et al. Association of LDLc to HDLc ratio with carotid plaques in a community-based population with a high stroke risk: A cross-sectional study in China. Clin Biochem 2021;88:43-8. [Crossref] [PubMed]
- Gorgui J, Gasbarrino K, Georgakis MK, et al. Circulating adiponectin levels in relation to carotid atherosclerotic plaque presence, ischemic stroke risk, and mortality: A systematic review and meta-analyses. Metabolism 2017;69:51-66. [Crossref] [PubMed]
- Lin PC, Chen CY, Wu C, et al. Synergistic Effects of Inflammation and Atherogenic Dyslipidemia on Subclinical Carotid Atherosclerosis Assessed by Ultrasound in Patients with Familial Hypercholesterolemia and Their Family Members. Biomedicines 2022;10:367. [Crossref] [PubMed]
- Leeflang MM, Rutjes AW, Reitsma JB, et al. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ 2013;185:E537-44. [Crossref] [PubMed]



