
Pacemaker leads as a potential source of problems in patients who might need a central venous access port
Highlight box
Key findings
• Insertion of the venous port in patients with cardiac implantable electronic devices (CIEDs) can be difficult due to venous occlusion on both the side of the implanted CIED and the contralateral side, therefore the initial assessment of venous flow is very important.
What is known and what is new?
• Lead-dependent venous occlusion is a frequent finding in patients with CIEDs and is usually asymptomatic.
• The main thoracic veins on the opposite side to the intracardial leads may also be severely narrowed, which can be challenging in patients requiring vascular ports and dialysis fistulas.
What is the implication, and what should change now?
• Attempting to introduce a venous port in patients with endocardial leads requires prior venography. It also seems recommended to perform it in the case of implantation of the port on the side of the chest opposite to the leads.
Introduction
Over 3 million cardiovascular implantable electronic devices (CIEDs) are implanted annually in the United States, and nearly as many central vascular access devices (CVADs) are used each year (1,2). Lead-dependent venous obstruction (LDVO) is common in CIED patients (3-10). LDVO is usually asymptomatic, but it may prevent the placement of a venous port or interfere with its proper functioning. CVADs is a significant medical advance providing important benefits in the management of cancer and kidney patients (10-15). Insertion of a CVAD may interfere with the existing CIED and cause complications. So far, no detailed analysis of the location and degree of venous obstruction in patients with CIED has been performed, especially in terms of the chance of implantation and long-term normal functioning of CVAD.
This study aimed to describe obstruction of the major veins of the thorax, location and range of LDVO in relation to the device side and to examine the chances of success using different venous port approaches (cephalic, subclavian, brachiocephalic and jugular) in relation to lead location. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-104/rc).
Methods
Design of the study
This is a cohort study in which a retrospective analysis of the population of patients undergoing transvenous lead extraction (TLE) between June 2008 and July 2021 was performed. Subsequent patients undergoing TLE in three centers in Poland were included in the study: in Lublin, Zamość and Radom. In all centers, the TLE procedure was performed by one key operator. The only criterion for exclusion from the study was contraindication to venography. Patients with renal failure, contrast allergy, lack of an available peripheral vein, or presence of an arteriovenous fistula were excluded from the study. The flow diagram of the patients undergoing the study is presented in Figure 1.
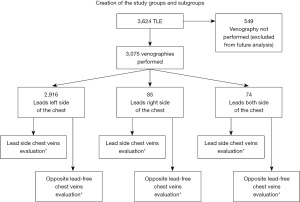
Study population
We reviewed and analyzed retrospectively data from 3,075 patients who had a venogram routinely performed before TLE. All information about patients and procedures was entered into a computer database on an ongoing basis.
Venographic data collection
Venography was performed usually on the implant side because there was no medical indication for additional contrast dose. However, due to collateral flow, an evaluation of veins on the opposite side was possible in some patients. The volume of 20–40 mL of high-quality contrast medium (350 g iodine/mL) was administered using a forearm placed venous catheter, and venous blood flow through the arm, neck and thoracic veins was recorded in an anteroposterior view as described previously (8,9).
All the venograms were reviewed retrospectively by our TLE team consisting of an experienced cardiothoracic surgeon, cardiologist and anesthesiologist, all with more than 30 years of experience in central vein catheterization for different goals.
Venographic data analysis
Venous patency, in the plane projection, was assessed on a 5-point scale from normal flow to complete venous occlusion. The degree of narrowing of all visible/dyed veins was determined as patent (no stenosis in an in-plane view), mild obstruction (<30% stenosis), moderate obstruction (30–60% narrowing), severe obstruction (>60% stenosis), and total obstruction (100%) of subclavian, brachiocephalic veins and superior vena cava (SVC). Despite ipsilateral contrast injection, the new regional collateral veins and significant collateral flow via the neck veins enabled an evaluation of the brachiocephalic vein on the opposite side of the chest in some patients. In patients without collateral circulation and without contralateral leads there was no reason to suspect venous obstruction, if they had not previously undergone removal of leads, ports, and dialysis catheters on that side of the chest, as the risk of underestimating obstruction incidence was low.
Evaluation of the chance of CVAD insertion and proper function
There are several equivalent techniques for introducing venous ports, depending on the operator’s preferences and routines in the facility. It is possible to introduce the port through the cephalic vein and puncture of the axillary, subclavian, brachiocephalic and jugular veins. When evaluating the venograms, we considered all possible access routes. An occlusion of the axillary vein alone did not preclude the possibility of introducing the port via other routes. Therefore, the results of the assessment of the axillary and cephalic veins were omitted in the tables. An obstruction of the subclavian vein did not preclude the possibility of introducing the port by puncture of the jugular vein or, ultimately, the brachiocephalic vein. An obstruction of the brachiocephalic vein basically prevented port insertion, whereas in cases of moderate or severe obstruction, the chances of port insertion were assessed as difficult or doubtful. Similarly, in the case of SVC obstruction, due to its larger diameter, the assessment was more tolerant. On the other hand, severe stenosis or occlusion of the SVC was classified as inability to introduce the port despite sufficient patency of the remaining veins.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee board of Regional Chamber of Physicians in Lublin (No. 288/2018/KB/VII) and informed consent was taken from all the patients.
Statistical analysis
Due to non parametric distribution continuous variables were expressed as median and interquartile range (IQR) categorical variables were reported as numbers and percentages and were compare using the Pearson’s Chi-square test. P value <0.05 was considered statistically significant.
Statistical analysis was performed with Statistica 13.3 (TIBCO Software Inc.).
Results
The study group consisted of 3,075 patients, average age 66.85 years (60.94 women) having a wide variety of CIEDs: pacemakers (PM), implantable cardioverter-defibrillator (ICD) and implantable cardiac defibrillators with ventricular resynchronization [cardiac resynchronization therapy with high voltage lead (CRT-D)]. The average implant duration was 6.17 years. Infectious complications were observed in 616 (20.03%) of patients. The leads were usually placed on the left side of the chest in 2,916 (94.83%) of patients; leads on the right or both sides were rare (2.76% and 2.41%, respectively). Severe or total venous obstruction was observed in 1,278 (41.56%) of individuals (Table 1).
Table 1
| All patients’ characteristics (n=3,075) | Values |
|---|---|
| Patient’s age during TLE (years) | 69.00 (17.00) |
| Sex (male patients) | 1,874 (60.94) |
| Underlying disease (IHD, MI) | 1,770 (57.56) |
| LVEF (%) | 54.00 (25.00) |
| Renal failure (any) | 607 (19.74) |
| Charlson comorbidity index (points) | 4.00 (4.00) |
| System’s infection | 616 (20.03) |
| Non-infective indications (lead failure/replacement, upgrading, overmuch of leads) | 2,459 (79.97) |
| Kind of CIED: pacemaker (any) | 2,133 (69.37) |
| Kind of CIED: ICD (VVI, DDD) | 704 (22.89) |
| Kind of CIED: CRT-D | 238 (7.74) |
| Dwell time of the oldest one lead in the patient before TLE (months) | 85.91 (87.00) |
| Cumulative dwell time of leads before TLE (years) | 12.17 (14.67) |
| Completed venography—chest side | |
| Left chest side | 2,916 (94.83) |
| Right chest side | 85 (2.76) |
| Both chest side | 74 (2.41) |
| Chest side of lead’s location | |
| Left chest side | 2,916 (94.83) |
| Right chest side | 85 (2.76) |
| Both chest sides | 74 (2.41) |
| Venous obstruction (the maximal degree in the patient, any location) | |
| Lack of obstruction | 547 (17.79) |
| Small obstruction | 613 (19.93) |
| Moderate obstruction | 637 (20.72) |
| Severe obstruction | 608 (19.77) |
| Total obstruction (occlusion) | 670 (21.79) |
Data are presented as count (%) or median (IQR). TLE, transvenous lead extraction; IHD, ischemic heart disease; MI, myocardial infarction; LVEF, left ventricle ejection fraction; CIED, cardiac implantable electronic device; ICD, implantable cardioverter-defibrillator; VVI, ventricular pacemaker; DDD, dual chamber pacemaker; CRT-D, cardiac resynchronization therapy with high voltage lead; IQR; interquartile range.
Table 2 summarizes the difficulty and possible complications of central venous port insertion in patients with CIEDs assessed by two experienced specialists. Future function of the port was evaluated based on possible port tip location at the junction of the SVC and the right atrium (±2 cm) and the dynamics of collateral venous flow.
Table 2
| Chances of port insertion and its future proper function | Rating of big chest veins—CIED side (pts with complete information) | Rating of big chest veins—opposite to CIED side (pts with complete information) | Pearson’s χ2 P | |||
|---|---|---|---|---|---|---|
| Patients | % | Patients | % | |||
| Possible/easy | 2,318 | 75.38 | 2,291 | 97.91 | <0.001 | |
| Difficult insertion, performance questionable | 246 | 8.00 | 22 | 0.94 | ||
| Doubtful or impossible insertion, performance questionable | 511 | 16.62 | 27 | 1.15 | ||
| All patients | 3,075 | 100.0 | 2,340 | 100.0 | ||
CIED, cardiac implantable electronic device; pts, patients.
It is important to remember that the contralateral side does not always mean the “lead-free side” because 74 of the 3,075 patients (2.41%) had abandoned leads on the opposite side of the chest. The table shows that the chances of port insertion were estimated as easy on CIED side/opposite side in 2,318 (75.38%)/2,291 (97.91%), difficult insertion/questionable performance in 246 (8.00%)/22 (0.94%) and doubtful or impossible insertion/questionable performance in 511 (16.62%)/27 (1.15%) of patients with CIEDs.
Forty-nine (2.09%) of cases with complete venographic information, obstruction of the great veins precluded implantation of a central port on the contralateral side of the chest due to previous patient history (Table 2).
Table 3 shows patency of the major veins of the thorax (right and left subclavian veins, brachiocephalic veins and SVC) according to lead location. Data shows that if leads were located on the left side (most typical location), a significant narrowing was more common in the subclavian than in brachiocephalic vein [1,595 (55.29%) vs. 830 (28.63%)] or SVC [21 (0.73%)]. The narrowing of left subclavian and brachiocephalic veins on the opposite lead-free side was also observed [35 (2.35%) and 27 (1.24%)].
Table 3
| Variables | CIED/leads left side of the chest, pts (%) | CIED/leads right side of the chest, pts (%) | Leads both side of the chest, pts (%) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veins of left side of the chest (containing leads) | Vena cava superior | Veins of right (opposite) side of the chest (free of leads) | Pearson’s χ2 P | Veins of right side of the chest (containing leads) | Vena cava superior | Veins of left (opposite) side of the chest (free of leads) | Pearson’s χ2 P | Veins of right side of the chest (containing leads) | Vena cava superior | Veins of left side of the chest (containing leads too) | Pearson’s χ2 P | |||||||||||||||
| Left SC vein | Left BC vein | Right SC vein | Right BC vein | Left vs. right SC vein | Left vs. right BC vein | Right SC vein | Right BC vein | Left SC vein | Left BC vein | Right vs. left SC vein | Right vs. left BC vein | Right SC vein | Right BC vein | Left SC vein | Left BC vein | Right vs. left SC vein | Right vs. left BC vein | |||||||||
| All patients | 2,916 (100.00) | 2,916 (100.00) | 2,916 (100.00) | 2,916 (100.00) | 2,916 (100.00) | – | – | 85 (100.00) | 85 (100.00) | 85 (100.00) | 85 (100.00) | 85 (100.00) | – | – | 74 (100.00) | 74 (100.00) | 74 (100.00) | 74 (100.00) | 74 (100.00) | – | – | |||||
| Complete information | 2,885 (98.94) | 2,899 (99.42) | 2,882 (98.83) | 1,486 (50.96) | 2,183 (74.86) | <0.001 | <0.001 | 69 (81.18) | 74 (87.06) | 84 (98.82) | 82 (96.47) | 84 (98.82) | <0.001 | <0.001 | 49 (66.22) | 61 (82.43) | 73 (98.65) | 70 (94.59) | 73 (98.65) | <0.001 | <0.001 | |||||
| Lack of obstruction | 617 (21.39) | 1,663 (57.36) | 2,837 (98.44) | 1,434 (96.50) | 2,139 (97.98) | 44 (63.77) | 53 (71.62) | 83 (98.81) | 42 (51.22) | 57 (67.86) | 20 (40.82) | 33 (54.10) | 69 (94.52) | 9 (12.86) | 33 (45.21) | |||||||||||
| Small obstruction | 673 (23.33) | 406 (14.00) | 24 (0.83) | 17 (1.14) | 17 (0.78) | 7 (10.14) | 11 (14.86) | 0 (0.00) | 14 (17.07) | 11 (13.10) | 9 (18.37) | 7 (11.48) | 1 (1.37) | 16 (22.86) | 12 (16.44) | |||||||||||
| Moderate obstruction | 646 (22.39) | 246 (8.49) | 9 (0.31) | 7 (0.47) | 8 (0.37) | 7 (10.14) | 3 (4.05) | 0 (0.00) | 9 (10.98) | 7 (8.33) | 9 (18.37) | 6 (9.84) | 1 (1.37) | 7 (10.00) | 7 (9.59) | |||||||||||
| Severe obstruction | 516 (17.89) | 205 (7.07) | 5 (0.17) | 14 (0.94) | 9 (0.41) | 4 (5.80) | 1 (1.35) | 0 (0.00) | 11 (13.41) | 8 (9.52) | 4 (8.16) | 5 (8.20) | 1 (1.37) | 14 (20.00) | 3 (4.11) | |||||||||||
| Total obstruction (occlusion) | 433 (15.01) | 379 (13.07) | 7 (0.24) | 14 (0.94) | 10 (0.46) | 7 (10.14) | 6 (8.11) | 1 (1.19) | 6 (7.32) | 1 (1.19) | 7 (14.29) | 10 (16.39) | 1 (1.37) | 24 (34.29) | 18 (24.66) | |||||||||||
| Lack of information | 31 (1.06) | 17 (0.58) | 34 (1.17) | 1,430 (49.04) | 733 (25.14) | – | – | 16 (18.82) | 11 (12.94) | 1 (1.18) | 3 (3.53) | 1 (1.18) | – | – | 25 (33.78) | 13 (17.57) | 1 (1.35) | 4 (5.41) | 1 (1.35) | – | – | |||||
CIED, cardiac implantable electronic device; SC, subclavian; BC, brachiocephalic; pts, patients.
If leads were located on the right side (much less typical location), a significant narrowing of the subclavian and brachiocephalic vein was less frequent [18 (26.09%), and 10 (13.51%) of cases, respectively]. Also, the right subclavian vein was narrowed more frequently than the right brachiocephalic vein. In patients with right-sided lead system, stenosis of the subclavian and brachiocephalic veins on the opposite lead-free side was unexpectedly common [26 (31.71%) and 16 (19.04%) of cases, respectively]. This seemingly strange phenomenon can be explained by the patient’s history (previous presence of leads or catheters).
Leads on both sides created more complex conditions for venous port placement. Right-sided veins (subclavian and brachiocephalic) were affected slightly less frequently [20 (40.84%) and 21 (34.43%) of cases, respectively] than those on the left side of the chest [45 (64.29%) and 38 (38.36%) of cases, respectively] in this group. Furthermore, SVC was narrowed in 3 (4.11%) of patients with bilateral chest lead location (Table 3).
Discussion
Occlusion of the main thoracic veins is a well-known complication in patients with CIED. The incidence of venous obstruction is as high as 30–45%, with an average incidence of mild stenosis between 10% and 40%, moderate stenosis between 6% and 50%, and severe stenosis/total occlusion between 3% and 22% (2-9). This study supports evidence from previous observations. Maximum obstruction was related to lead location and vein involvement. One of the important factors contributing to the development of venous obstruction in patients with CIEDs is inflammation. CIED-related infections increase the risk of venous obstruction. It could be an example of a defence mechanism by which the flow of pus into the circulatory system is blocked. Our observations indicate that this is a permanent and even progressive phenomenon, which is not reversed by lead removal (9).
Lead-dependent venous occlusion makes it difficult to implant a new lead and insert the lead for temporary pacing. Electrocardiologists are very aware of this problem (2-9), however, this aspect of venous obstruction is less known among vascular surgeons and anesthesiologists. Ipsilateral central venous catheters should also be avoided as the presence of two systems (CVAD or dialysis catheter and CIED) in the same vein may increase the risk of co-infection between devices (15-20).
Our subjective grading of venous stenosis in the context of central line insertion has practical implications. In our opinion, mild and moderate narrowing should not interfere with future implantation of the venous port and its proper function.
In the case of moderate or severe stenosis of the distal section of the left brachiocephalic vein or at the confluence with the SVC, it is often possible to insert a hydrophilic guidewire and a long introducer (usually of the peel-away type). Such procedures may need fluoroscopic control for proper catheter navigation, avoidance of perforation or prevention of catheter placement in low-flow collateral veins.
Temporary or permanent PM leads will function properly despite venous obstruction, whereas venous ports need free blood flow around the distal port end for proper function. Long-term catheter function may depend on external compression from electrodes or fibrous tissue, or insufficient caliber of the vein with the positioned tip.
Drop in the blood flow caused by venous outflow obstruction leads results in drug-induced venous wall irritation, producing additional lumen narrowing and, finally, port dysfunction or vein thrombosis.
Previous reports on risk factors for LDVO demonstrated that patient age and gender had no effect on venous obstruction, but there is still controversy as to whether the number of leads (lead burden) and implant duration may contribute to LDVO (2-9).
Recently, multiple studies on venous port implantation (have concentrated on comparing different approaches to venous port insertion and techniques (cephalic vs. subclavian vs. jugular vein) (21-33), methods of evaluating correct tip location [external measurements only, fluoroscopy (21) or intracardiac electrocardiogram (ECG)] (21), surface catheter measurements (30) or transesophageal echocardiography (TEE) (34). Guidance for puncture of the subclavian, brachiocephalic and jugular vein is most frequently ultrasonic (25,31,34-45), rarely fluoroscopic (37,45) or venipuncture is performed without imaging (43).
There is a wide variety of studies that compare the incidence of intraoperative complications (hemothorax, hemopneumothorax or pinch-off syndrome, pneumothorax, nerve damage, arterial puncture and hematoma, wrong tip location in the internal thoracic vein, or in the collateral circulation with subsequent severe consequences) when different safety approaches are used (21,26-31,33,35,37,43,44,46,47).
Several investigators have described periprocedural or late complications of venous port implantation (21,23,24,26,27,30,32-36,38,41,42,46,47). These include: venous thrombosis, infection, catheter-related infection, skin complications at the port site, port infection, post-operative venous access obstruction, central venous stenosis, catheter damage, port and catheter disconnection, flexion at the catheter-port interface, infusion disorders, thrombotic obstruction of the catheter or spontaneous dislocation of the port tip into the azygos or other small veins, and unexpected difficulties during removal of the port (22).
Ultrasonic control of the subclavian, brachiocephalic, or jugular vein puncture site is an easy and widely used option for patients with normal, previously untouched venous system without stenosis (48-50). Standardized protocols such as RaCeVa have been established and are widely used in hospital practice (48-50). Problems may appear when a narrowed vein segment cannot be visualized with ultrasound. In our opinion, venography is still a valuable option in patients with CIEDs. Ultrasound monitoring perfectly shows possible sites for cannulation but may not show the dynamics of collateral flow or obstacles. In such cases the guidewire, instead of entering the SVC and right atrium can be displaced to a small thoracic vein or the left SVC (if present). The likelihood of an inappropriate guidewire path is minimal in healthy patients, but the risk grows dramatically in patients with preexisting venous stenosis or obstruction. Attempts at device insertion can result in its placement in the collateral circulation or in dissection of the obstructed vein wall. Finally, port dysfunction and severe complications can occur if the procedure is performed without fluoroscopic control.
Figures 2,3 show that insertion of a venous port may be difficult or even dangerous in spite of the unchanged lumen of the jugular, subclavian or auxiliary veins and blood flow visible in ultrasound because of stenosis or occlusion in the lower segments, inaccessible by ultrasound.


To the best of our knowledge, no one has investigated the causes of difficult or impossible port insertion, and the results of venographic imaging obtained for different indications prompted us to present the data. Especially that the risk of developing cancer should be considered in aging patients with CIED leads who may need venous port implantation.
The problem still exists and one of the reports provided an important conclusion: “in the light of the findings, there is a need for standardized guidelines for evaluation before central venous port catheter insertion and follow-up after insertion to detect and to avoid possible complications” (25).
The current study describes occlusion of the main thoracic veins with permanently implanted leads and, in some patients, occlusion of the main veins on the opposite side of the chest. An explanation of this phenomenon is that right-sided thoracic veins were previously used for other medical purposes, i.e., temporary pacing leads or another central venous catheter [perioperative, at intensive care unit (ICU), rarely venous port or temporary or permanent catheter for hemodialysis]. And any temporary lead or temporary catheter can cause or initiate the narrowing of the vein in which it has been placed.
It seems that an important message is the recognition of patients with CIED as candidates for the placement of a central venous port. It could also suggest that every attempt at venous port insertion in patients with CIEDs, especially on the implant side, may be safer when preceded by venography. Venography is also recommended if the contralateral side of the chest is to be used.
Study limitations
Routine pre-TLE venography was performed in all patients without contrast contraindications. Thus, analysis does not include all the patients referred for TLE. Although the analysis was carried out on data prospectively entered into the database, the study is retrospective. In most patients, venography was performed only on the side of the implant to reduce the dose of contrast. In some patients, we were able to evaluate patency of the contralateral brachiocephalic vein thanks to collateral circulation through the neck veins and opposite jugular vein, but not subclavian or auxiliary veins.
Conclusions
- Varying degrees of lead-dependent venous occlusion are a common finding in patients with pacing or defibrillation leads;
- Large thoracic veins on the opposite side of the chest may also be occluded in a small percentage of patients;
- Patients with CIED may benefit from fluoroscopic control during venous port insertion;
- Venography should be considered before attempted CVAD insertion in patients with endocardial leads;
- Venography seems to be valuable also in cases of planned contralateral port placement.
Acknowledgments
The authors would like to thank all those participants in the lead extraction procedures.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-104/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-104/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-104/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-104/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee board of Regional Chamber of Physicians in Lublin (No. 288/2018/KB/VII) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wadhwani L, Occhipinti K, Selim A, et al. Time to diagnosis of acute complications after cardiovascular implantable electronic device insertion and optimal timing of discharge within the first 24 hours. Heart Rhythm 2021;18:2110-4. [Crossref] [PubMed]
- Conley SB, Buckley P, Magarace L, et al. Standardizing Best Nursing Practice for Implanted Ports: Applying Evidence-based Professional Guidelines to Prevent Central Line-Associated Bloodstream Infections. J Infus Nurs 2017;40:165-74. [Crossref] [PubMed]
- Li X, Ze F, Wang L, et al. Prevalence of venous occlusion in patients referred for lead extraction: implications for tool selection. Europace 2014;16:1795-9. [Crossref] [PubMed]
- Korkeila P, Nyman K, Ylitalo A, et al. Venous obstruction after pacemaker implantation. Pacing Clin Electrophysiol 2007;30:199-206. [Crossref] [PubMed]
- van Rooden CJ, Molhoek SG, Rosendaal FR, et al. Incidence and risk factors of early venous thrombosis associated with permanent pacemaker leads. J Cardiovasc Electrophysiol 2004;15:1258-62. [Crossref] [PubMed]
- Albertini CMM, Silva KRD, Leal Filho JMDM, et al. Usefulness of preoperative venography in patients with cardiac implantable electronic devices submitted to lead replacement or device upgrade procedures. Arq Bras Cardiol 2018;111:686-96. [Crossref] [PubMed]
- Lickfett L, Bitzen A, Arepally A, et al. Incidence of venous obstruction following insertion of an implantable cardioverter defibrillator. A study of systematic contrast venography on patients presenting for their first elective ICD generator replacement. Europace 2004;6:25-31. [Crossref] [PubMed]
- Sticherling C, Chough SP, Baker RL, et al. Prevalence of central venous occlusion in patients with chronic defibrillator leads. Am Heart J 2001;141:813-6. [Crossref] [PubMed]
- Czajkowski M, Jacheć W, Polewczyk A, et al. Risk Factors for Lead-Related Venous Obstruction: A Study of 2909 Candidates for Lead Extraction. J Clin Med 2021;10:5158. [Crossref] [PubMed]
- Czajkowski M, Jacheć W, Polewczyk A, et al. The Influence of Lead-Related Venous Obstruction on the Complexity and Outcomes of Transvenous Lead Extraction. Int J Environ Res Public Health 2021;18:9634. [Crossref] [PubMed]
- Czajkowski M, Polewczyk A, Jacheć W, et al. How does a CIED presence influence chances and safety of haemodialysis access? Conclusions from over 3000 thoracic venografies. Clin Physiol Funct Imaging 2023;43:47-57. [Crossref] [PubMed]
- Expert Panel on Interventional Radiology. ACR Appropriateness Criteria® Radiologic Management of Central Venous Access. J Am Coll Radiol 2017;14:S506-29. [Crossref] [PubMed]
- Zhang KC, Chen LChinese Research Hospital Association Digestive Tumor Committee, et al. Chinese expert consensus and practice guideline of totally implantable access port for digestive tract carcinomas. World J Gastroenterol 2020;26:3517-27. [Crossref] [PubMed]
- Ding X, Ding F, Wang Y, et al. Shanghai expert consensus on totally implantable access ports 2019. J Interv Med 2019;2:141-5. [Crossref] [PubMed]
- Maňásek V, Charvát J, Chovanec V, et al. Indications for venous access in oncology - recommendations of national professional societies and current state in the Czech Republic. Klin Onkol 2021;34:192-201. [PubMed]
- Salmeri M, Sorbello MG, Mastrojeni S, et al. Infections of cardiovascular implantable electronic devices: 14 years of experience in an Italian hospital. Infez Med 2016;24:131-6. [PubMed]
- Guha A, Maddox WR, Colombo R, et al. Cardiac implantable electronic device infection in patients with end-stage renal disease. Heart Rhythm 2015;12:2395-401. [Crossref] [PubMed]
- Hickson LJ, Gooden JY, Le KY, et al. Clinical presentation and outcomes of cardiovascular implantable electronic device infections in hemodialysis patients. Am J Kidney Dis 2014;64:104-10. [Crossref] [PubMed]
- Othman H, Fishbain JT, Khatib R. The role of intravenous catheters in cardiovascular implantable electronic device infections: identifying potential targets for prevention. Am J Infect Control 2013;41:376-7. [Crossref] [PubMed]
- Asif A, Salman L, Lopera G, et al. Transvenous cardiac implantable electronic devices and hemodialysis catheters: recommendations to curtail a potentially lethal combination. Semin Dial 2012;25:582-6. [Crossref] [PubMed]
- Liu Z, Zheng X, Zhen Y, et al. Efficacy, Safety, and Cost-Effectiveness of Intracavitary Electrocardiography-Guided Catheter Tip Placement for Totally Implantable Venous Access Port. Ann Vasc Surg 2022;83:168-75. [Crossref] [PubMed]
- Kinoshita M, Takao S, Hiraoka J, et al. Risk factors for unsuccessful removal of central venous access ports implanted in the forearm of adult oncologic patients. Jpn J Radiol 2022;40:412-8. [Crossref] [PubMed]
- Iguchi T, Hiraki T, Matsui Y, et al. Contrast examination of central venous access port implanted through internal jugular vein for evaluation of suspected complications. Jpn J Radiol 2021;39:1103-10. [Crossref] [PubMed]
- Nadjiri J, Geith T, Waggershauser T, et al. Risk factors assessment for radiographically guided port implantations with forearm access. PLoS One 2021;16:e0259127. [Crossref] [PubMed]
- Bawazir OA, Bawazir A. Ultrasound guidance for Port-A-Cath insertion in children; a comparative study. Int J Pediatr Adolesc Med 2021;8:181-5. [Crossref] [PubMed]
- Moralar DG, Turkmen UA, Bilen A, et al. Our central venous port catheter system practice - a retrospective study. J Pak Med Assoc 2021;71:1442-5. [PubMed]
- Becker F, Wurche LA, Darscht M, et al. Totally implantable venous access port insertion via open Seldinger approach of the internal jugular vein-a retrospective risk stratification of 500 consecutive patients. Langenbecks Arch Surg 2021;406:903-10. [Crossref] [PubMed]
- Frias PF, Cross CG, Kaufman CS, et al. Port malposition in the azygos vein resulting in a veno-broncho and broncho-esophageal fistula: A case report. J Vasc Access 2022;23:632-5.
- Otsubo R, Yano H, Matsumoto M, et al. Comparison of Central Venous Port Procedures Between Puncture vs. Cut-down and Residents vs. Senior Surgeons. In Vivo 2021;35:1197-204. [Crossref] [PubMed]
- Zhou C, Lu L, Yang L, et al. Modified surface measurement method to determine catheter tip position of totally implantable venous access port through right subclavian vein. J Vasc Surg Venous Lymphat Disord 2021;9:409-15. [Crossref] [PubMed]
- Yu Z, Sun X, Bai X, et al. Perioperative and Postoperative Complications of Supraclavicular, Ultrasound-Guided, Totally Implantable Venous Access Port via the Brachiocephalic Vein in Adult Patients: A Retrospective Multicentre Study. Ther Clin Risk Manag 2021;17:137-44. [Crossref] [PubMed]
- Han L, Zhang J, Deng X, et al. Totally implantable venous access ports: A prospective randomized study comparing subclavian and internal jugular vein punctures in children. J Pediatr Surg 2021;56:317-23. [Crossref] [PubMed]
- Salavracos M, Deprez FC. Endovascular Repositioning of a Central Venous Port Malposition in the Internal Thoracic Vein. J Belg Soc Radiol 2021;105:3. [Crossref] [PubMed]
- Yang S, Kong X, Liu L, et al. Application of transesophageal echocardiography for localization in totally implantable venous access port implantation through subclavian approach in children. Clin Cardiol 2021;44:129-35. [Crossref] [PubMed]
- Hüttner FJ, Bruckner T, Hackbusch M, et al. Primary Open Versus Closed Implantation Strategy for Totally Implantable Venous Access Ports: The Multicentre Randomized Controlled PORTAS-3 Trial (DRKS 00004900). Ann Surg 2020;272:950-60. [Crossref] [PubMed]
- Chen YB, Bao HS, Deng HR, et al. Comparison of comfort and complications in breast cancer patients of implantable venous access port (IVAP) with ultrasound guided internal jugular vein (IJV) and axillary vein/subclavian vein (AxV/SCV) puncture: a randomized controlled study protocol. Ann Palliat Med 2020;9:4323-31. [Crossref] [PubMed]
- Ince ME, Ozkan G, Ors N, et al. Complications and pitfalls of central venous port catheters: experience with 782 patients with cancer. Ir J Med Sci 2020;189:1371-7. [Crossref] [PubMed]
- Liu Y, Li LL, Xu L, et al. Comparison between Arm Port and Chest Port for Optimal Vascular Access Port in Patients with Breast Cancer: A Systematic Review and Meta-Analysis. Biomed Res Int 2020;2020:9082924. [Crossref] [PubMed]
- Xu L, Qin W, Zheng W, et al. Ultrasound-guided totally implantable venous access ports via the right innominate vein: a new approach for patients with breast cancer. World J Surg Oncol 2019;17:196. [Crossref] [PubMed]
- Rhu J, Jun KW, Song BJ, et al. Cephalic vein approach for the implantable central venous access: A retrospective review of the single institution's experiences; Cohort Study. Medicine (Baltimore) 2019;98:e18007. [Crossref] [PubMed]
- Matsunari K, Watanabe K, Hishizume N, et al. Influence of venipuncture point and port chamber site on the risk of catheter fracture in right internal jugular port placements. J Vasc Access 2019;20:666-71.
- Yao M, Xiong W, Xu L, et al. A modified approach for ultrasound-guided axillary venipuncture in the infraclavicular area: A retrospective observational study. J Vasc Access 2019;20:630-5.
- Paprottka KJ, Voelklein J, Waggershauser T, et al. Retrospective outcome analysis of rates and types of complications after 8654 minimally invasive radiological port implantations via the subclavian vein without ultrasound guidance. Radiol Med 2019;124:926-33. [Crossref] [PubMed]
- Sun D, Kobayashi K, Samuel M, et al. Right- versus Left-Sided Chest Ports in Oncologic Patients with a History of Right-Sided Port Removal: Are There Any Differences in the Complication Rates? J Vasc Interv Radiol 2019;30:726-33. [Crossref] [PubMed]
- Dore M, Barrena S, Triana Junco P, et al. Is Intraoperative Fluoroscopy Necessary for Central Venous Port System Placement in Children? Eur J Pediatr Surg 2019;29:108-12. [Crossref] [PubMed]
- Song MG, Seo TS, Kang EY, et al. Innominate vein stenosis in breast cancer patients after totally implantable venous access port placement. J Vasc Access 2015;16:315-20.
- Manimaran PA, Agarwal P, Ramasundaram M, et al. Rare cause of extravasation from implantable venous access device. BMJ Case Rep 2021;14:e239103. [Crossref] [PubMed]
- Saugel B, Scheeren TWL, Teboul JL. Ultrasound-guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care 2017;21:225. [Crossref] [PubMed]
- Forneris G, Marciello A, Savio D, et al. Ultrasound in central venous access for hemodialysis. J Vasc Access 2021;22:97-105.
- Spencer TR, Pittiruti M. Rapid Central Vein Assessment (RaCeVA): A systematic, standardized approach for ultrasound assessment before central venous catheterization. J Vasc Access 2019;20:239-49.


