
A case report of giant cell myocarditis complicated by severe heart failure: the value of early endomyocardial biopsy and mechanical circulatory support
Highlight box
Key findings
• Sustained treatment with a combination of immunosuppressive agents together with optimal heart failure medication can lead to full or partial myocardial recovery, which remains stable over several years.
What is known and what is new?
• Historical data showed that death or transplantation occurred in the vast majority (89%) of giant cell myocarditis (GCM) patients, with a median survival from symptom onset to death or transplantation of 5.5 months, which improved to 11 months with the combination immunosuppressive therapy and guideline-directed medical therapy.
• Rapid initiation of methylprednisolone had an immediate effect on reducing myocardial inflammation.
• We show that urgent treatment with access to mechanical circulatory support (MCS) is crucial for better long-term outcomes.
What is the implication, and what should change now?
• The most important factors associated with favourable outcome in GCM are focus on rapid diagnostic measures including early endomyocardial biopsy, access to MCS, early initiation and sustained treatment with combined immunosuppression.
Introduction
Giant cell myocarditis (GCM) is a rare but often life-threatening condition. The exact pathogenesis is not fully understood, but GCM is attributed to a progressive T-lymphocyte-mediated inflammation of the myocardium that can lead to severe heart failure, malignant arrhythmias and heart block (1,2). GCM can also be mistaken with acute myocardial infarction, other inflammatory myocardial diseases like cardiac sarcoidosis or lymphocytic myocarditis, or it can present as a sudden cardiac death. In the latter situation the diagnosis may be verified by autopsy. Although cardiac biomarkers and multimodality imaging are used as initial diagnostic tests in most patients, endomyocardial biopsy (EMB) is often required for a definitive diagnosis. Early and accurate diagnosis verified by EMB, and initiation of immunosuppressive treatment are essential to reduce myocardial inflammation and damage, and the risk of death (1-4). Furthermore, access to mechanical circulatory support (MCS) is crucial for better outcomes (1), reducing the risk of complications such as severe heart failure, need for heart transplantation (HTx), and death. This case is unique in terms of long-term clinical outcome and survival.
Rationale and knowledge gap
Highlighting the urgency of an early EMB, access to MCS and the efficacy of immunosuppressive treatment along with guideline-directed medical therapy in the management of GCM patients deserve more attention in the literature. There are still gaps in our knowledge in terms of the etiology, incidence, diagnosis, prognosis and management of GCM. The incidence is mainly known from autopsy studies (5-7). Furthermore, randomized controlled trials (RCTs) are limited. Rapid initiation of immunosuppressive treatment has an immediate effect on reducing myocardial inflammation, and sustained treatment with a combination of immunosuppressive agents along with optimal heart failure medications have led to greater chance of complete or partial myocardial recovery, clinical remission over several years and improvement in overall and transplantation-free survival (1,3). Despite this, our understanding regarding the predictors of response in those patients treated with immunosuppressive therapy is limited. Furthermore, scientific evidence on which agent or combination regiment and their optimal duration is not fully investigated and good RCTs are missing. In the future, carefully designed prospective studies should investigate these important knowledge gaps to increase our understanding of GCM.
Objective
This report describes the case history of a middle-aged male patient admitted to Haukeland University Hospital with fulminant course of GCM. Based upon a review of the literature and experiences from Scandinavia and other Nordic countries, we also present an updated guidance on the contemporary management of GCM. We present this case in accordance with the CARE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-265/rc).
Case presentation
A man in his 50s was admitted to a local hospital with a two-week history of progressive shortness of breath and 10 kg weight gain. On admission, he had dyspnoea at rest and bilateral pitting oedema below the knees. There were no clinical signs of infection. His past medical history included lichen planus and hypertension. He was obese with a body mass index of 38.8 kg/m2. Shortly after admission, he developed rapid ventricular tachycardia (Figure 1A) leading to acute circulatory collapse. An immediate cardioversion was performed with successful restoration of sinus rhythm. He was subsequently airlifted to our institution which is the regional cardiothoracic centre. Upon arrival at the intensive care unit, he had cold extremities and clammy skin, but was mentally alert and orientated. His blood pressure was 87/50 mmHg and heart rate was 97 bpm. The electrocardiogram (ECG) (Figure 1B) showed sinus rhythm with low voltage, poor R-wave progression in leads V1–V3 and a premature ventricular contraction.

Investigations and treatment
The blood tests at admission showed elevated troponin T (TnT) at 5,500 ng/L, pro-B-type natriuretic peptide (pro-BNP) 2,900 pmol/L, creatinine 165 µmol/L and C-reactive protein (CRP) at 35 mg/L. The echocardiogram revealed a dilated left ventricle (LV) with end-diastolic dimension of 6.2 cm, septal thickness 1.6 cm and posterior wall thickness 1.3 cm (LV mass index 161 g/m2 and relative wall thickness 0.42 cm). LV ejection fraction (LVEF) was 15% (Figure 2A, Video 1).

The tricuspid annular plane systolic excursion (TAPSE) was 1.1 cm (Figure 2B). All heart valves appeared normal. A selective coronary angiography showed normal coronary arteries. Despite continuous infusion of vasoactive agents, he suffered sustained hypotension and developed profound oliguria and anuria. Acute heart failure due to a fulminant myocarditis was suspected. The rapid progression with severely reduced LVEF, ventricular arrhythmias and hemodynamic instability, as well as elevated cardiomyocyte-specific biomarkers in a relatively young patient, were all suggestive of a possible GCM, and he therefore received 1,000 mg methylprednisolone. An intra-aortic balloon pump (IABP) was inserted followed by implantation of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) (Figure 3). On day 3, EMB was performed and a temporary atrial pacing lead was inserted due to sinus bradycardia. The EMB confirmed the diagnosis of GCM with intense leucocyte infiltration and multiple giant cells, causing myocardial damage (Figure 4). Immunosuppressive therapy with 150 mg anti-thymocyte globulin (ATG) infusion was initiated and continued for 3 days. The patient continued to receive intravenous methylprednisolone (1,000 mg once daily) for a total of 7 days. We then began up-titration of combined immunotherapy with cyclosporine (aimed at 100–150 ng/mL), mycophenolate mofetil (1,000 mg twice daily) and oral prednisolone (0.2 mg/kg body weight).
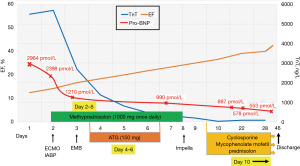

The patient remained on VA-ECMO for 6 days during which he was conscious and alert. Echocardiography demonstrated some improvement of cardiac function with LVEF of 25%. An Impella CP® was inserted using subclavian access and the VA-ECMO circuit together with the IABP was removed. Four days after removal of VA-ECMO, he deteriorated rapidly and developed hypotension. The echocardiogram showed cardiac tamponade which required an urgent pericardiocentesis and drainage of 350 mL bloody pericardial effusion. His condition immediately improved but the following day he again became hemodynamically unstable and developed hypotension, shortness of breath and hypoxia. A repeated echocardiogram revealed pericardial effusion and signs of tamponade. Chest X-ray showed increased cardiac shadow and a white left lung (Figure 5). A CT angiography showed extra-pleural bleeding leading to both massive left-sided hemothorax and hemopericardium, for which he underwent an immediate pericardiocentesis followed by endovascular coiling of a bleeding intercostal artery. A chest tube was then inserted under general anaesthesia and a total of 1,500 mL of blood was drained from the pleural cavity. This was managed without the need of reintroducing VA-ECMO. The patient was stabilized on immunosuppressive treatment and optimal medical management for heart failure, and remained in hospital for 46 days. A cardiac magnetic resonance scan demonstrated multiple patchy formed pathological areas of oedema as reflected by late gadolinium enhancement (Figure 6). On the last echocardiography before discharge, LVEF was just below 40%. Given the fact that at presentation he was critically ill and experienced ventricular tachycardia leading to circulatory collapse, we deemed a secondary prophylactic implantable cardioverter-defibrillator (ICD) necessary for this patient, and a dual-chamber ICD was implanted prior to discharge. On discharge, he was on immunosuppressive therapy with a combination of cyclosporine that has been gradually reduced, mycophenolate mofetil (1,000 mg twice a day) and oral prednisolone (10 mg). In addition, his heart failure was treated with ramipril 10 mg, metoprolol 200 mg. He was also put on apixaban 10 mg due to severely reduced LVEF and short runs of atrial fibrillation.


Outcome and follow-up
At 6-month follow-up, LVEF increased to 60% (Video 2) and remained stable (55%) at 4-year follow-up (Videos 3,4), with a LV end-diastolic dimension of 5.7 cm and septal thickness of 1.2 cm, suggesting reverse LV remodelling compared with baseline study. Creatinine was 133 µmol/L, N-terminal pro b-type natriuretic peptide (NT pro-BNP) 891 ng/L and CRP <1 mg/dL. The right ventricle (RV) systolic function was normal (TAPSE 1.9 cm) and there were no signs of significant tricuspid regurgitation or pulmonary hypertension. The patient reported to have resumed work 6 months after his initial admission and is currently working full time. There has been no clinical evidence of GCM recurrence during 5-year follow-up. His maintenance cyclosporine levels are 60–80 ng/mL. No adverse and unanticipated events were reported during the follow-up.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
GCM is a rare, probably underdiagnosed, and often life-threatening condition. The exact pathogenesis is not fully understood, but it is commonly attributed to T cell-mediated autoimmunity. The incidence is mainly known from autopsy studies and varies from 0.007% to 0.051% (5-7). The condition has often a rapid progression and is associated with poor prognosis if immunosuppressive treatment is not timely initiated, or there is no access to MCS, particularly VA-ECMO or Impella®. Until the 1980s the diagnosis was mainly made at autopsy or from the explanted heart (8). A multicentre study from 1997 showed a median transplant-free survival of 5.5 months from the onset of symptoms. When the diagnosis was confirmed by biopsy, and early immunosuppressive treatment was initiated, the transplant-free survival improved from 3 months to nearly one year (3). Increased awareness of the condition, early diagnosis confirmed by EMB and rapid initiation of immunosuppressive treatment, and access to MCS are crucial for better outcomes (1), and reduce the risk of complications such as severe heart failure, need for HTx, and death.
The importance of early and correct histologic diagnosis
GCM is one of the many causes of myocardial inflammation. A myocardial biopsy is essential as the heart is the only organ to collect representative specimens. Biopsy must be performed as soon as there is a diagnostic suspicion of fulminant myocarditis, and analysed by an experienced pathologist. The difference between a lymphocitic myocarditis and GCM is the presence of giant cells that requires a representative specimen and can be hard to differentiate from sarcoid cells. If in doubt, repeated biopsies should be performed. EMB is recommended by European Society of Cardiology (ESC) guidelines in cases of progressive and persistent cardiac dysfunction and/or life-threatening ventricular arrhythmias or high degree AV blocks on optimal medical management during the first 2 weeks (9). A positive EMB can reveal the direct cause of heart failure and greatly influences both treatment and prognosis. This was also clearly illustrated by the present case report. The sensitivity of transvenous EMB for GCM is reported to be 68% in a series from Finland and may further enhance the sensitivity to over 90% with a second biopsy performed (2). An EMB may be hesitated as clinical deterioration progresses and the risk of complications increases, a rationale that should actually prompt the biopsy to be performed at first suspicion. Myocardial biopsies on VA-ECMO are more complicated but can and must be performed in trained hands if indicated. The right atrial ECMO cannula and pacemaker leads may cause instrumental challenges and anticoagulation increase the risk of bleeding. On the other hand, a spontaneous remission of a GCM in cardiogenic shock without immunosuppression is not reported and a rapid diagnostic work up is essential.
Bridging to MCS and the choice of circulatory support
Bridging patients with fulminant myocarditis to MCS with vasoactive medications is difficult and sometimes futile as hypotension and poor cardiac function often will not improve sufficiently to stabilize the patient (10). Our patient was treated initially with norepinephrine and Levosimendan infusion but with little or no effect as the condition was continuously deteriorating. However, the use of these agents may not be appropriate or insufficient in the setting of severe acute heart failure secondary to fulminant myocarditis. The balance between a preferred afterload reduction to reduce LV end-diastolic pressure and increase cardiac output is often in conflict with the need of adequate blood pressure and adequate tissue perfusion. The risk of cardiogenic shock and need of MCS in GCM is high. If GCM is suspected an urgent transfer to a hospital with MCS facility is required to have a backup solution. MCS should be installed rapidly if deterioration occurs before cardiogenic shock and multiorgan failure is manifest. In a series from France, 13 patients were identified with GCM between 2002 and 2016, all of which required MCS. Of these, 4 died on treatment with MCS and 9 went on to receive successful HTx (11). The choice of MCS is not established and no MCS has demonstrated better survival compared to the other. In our patient, a balloon pump was initially inserted prior to the establishment of VA-ECMO. Due to the frequently fulminant course of GCM with biventricular failure, VA-ECMO may be required. Peripheral VA-ECMO is usually insertable within short time, and provides adequate perfusion of cerebral and abdominal organs, but a retrograde perfusion of oxygenated blood to the descending aorta increases afterload to the heart. LV unloading and adequate oxygenation of the pulmonary blood flow is required to secure coronary and cerebral perfusion with oxygenated blood. Otherwise, the Harlequin syndrome (poor oxygenation of the upper part of the body) may appear with deoxygenated blood in part of the aortic arch. Hence, a LV unloading that reduces LV end-diastolic pressure may be needed. An Impella® combined with VA-ECMO, often called “ECPELLA” are often the two preferred MCSs to maintain pulmonary flow and secure LV unloading. However, by applying more invasive procedures, increased complication rates will challenge the balance between volume and flow, pressures and the need of anticoagulation and risk of bleeding. In our case, IABP was sufficient as unloading of the LV during the time he was on VA-ECMO. If patient is not weaned of short term MCS, options are termination of treatment, urgent HTx or implant of a long-term MCS [LV assist device (LVAD)]. The two latter options require preservation of other organs or potential for recovery. An urgent HTx may be the safest and best option for the patient, but with increasing risk of recurrence of GCM in the transplanted graft, and shorter post-transplant survival is reported in urgent immune active GCM. Limited donor pool challenges this ethical dilemma, how to utilize the potential organs to the best for the potential heart failure recipients, and how to optimize post-transplant results. LVAD may be implanted with the risk of right heart failure due to a progressive destructive inflammatory process and ventricular arrhythmia post-LVAD. The evaluation of right ventricular function of patients on VA-ECMO can be challenging. Generally, because of the complex anatomy of the RV, precise function assessment is difficult. Moreover, recent case reports have shown that in fulminant heart failure due to GCM, the pattern of reverse remodelling may be different between LV and RV (12). Prednisolone and cylosporin treatment resulted in the RV function recovery and improvement of heart failure symptoms, while the LVEF did not recover. Of note, RV function, a low pressure system, was assessed by fractional area change, an echocardiographic measure which has been shown to correlate better with cardiac magnetic resonance imaging-derived RV ejection fraction (RVEF) than with TAPSE (13). TAPSE is a standard measure of RV systolic function, but is load dependent and may be normal until late stage and in case of tricuspid regurgitation (14). By contrast, RV systolic velocity (S') and RV fractional area change are considered as the measurements of choice in defining RV function in critically ill patients, and RV free wall strain is a better predictor of mortality than TAPSE or RV S'. Furthermore, the novel RV-pulmonary artery coupling markers derived from either TAPSE/systolic pulmonary artery pressure ratio or fractional area change/SPAP ratio seems to provide additional information about the causes and consequences of RV impairment in patients with significant tricuspid regurgitation or critically ill COVID-19 patients (14,15). However, there are very limited data available on the assessment of RV function in GCM patients, particularly RV-pulmonary artery coupling markers. The clinical significance and prognostic impact of these important non-invasive markers of RV function warrant further investigation in GCM patients. RV evaluation while on ECMO needs a multifaceted approach with echo and invasive parameters. While turning down the ECMO rpm, less unloading and higher preload of the RV occurs. In case of recovery, RV ECMO functional parameters improve, central venous pressure maintains within acceptable range, mixed venous oxygen saturation (SvO2) is maintained or improved and LV responds positively to increased preload with output increase and wedge pressure within acceptable values. In the opposite case, RV and LV recovery is limited and weaning may be difficult.
Finally, a bi-ventricular VAD can be performed with success, but balancing the two devices can be challenging and the risk of LVAD complications are greatly increased (16).
Choice of immunosuppressive strategy
When GCM is suspected, early initiation of immunosuppressive treatment is vital to reduce the existing and prevent further progression of myocardial inflammation and limit myocardial damage that results in severe heart failure, cardiogenic shock and life-threatening ventricular arrhythmias (4,10). It has to be emphasized that no RCT has been performed and all protocols are developed based on local traditions and scientific rationale. In a systematic review by Patel et al. (17), which included 43 patients from 2009 to 2019, one-year survival was 72.7% (16/22) for patients receiving both immunosuppressive therapy and MCS versus 31.3% (5/16) in MCS alone. Data from the early Lewis rat model, and the dominance of lymphocytes and T cells in the biopsy, suggest GCM to be T lymphocytes-mediated with secretion of interleukin gamma by CD4 positive T cells and macrophage activation (18). Immunosuppressive treatment is aimed at attenuating T cell function. Therefore, the initial use of corticosteroids is a cornerstone in the management of GCM (19). The choice of cyclosporine/tacrolimus and either azathioprine or mycophenolate is based on the experience from successful T cell inhibition in HTx. Initiation of high blood concentration calcineurin inhibitors can be relatively rapidly reduced to early maintenance transplant blood cyclosporine levels usually aimed around cyclosporine 100–150 ng/nL or tacrolimus blood levels of 5–15 ng/mL to be further reduced when the patient is stabilized or nephrotoxic side effect occurs. Two RCTs initiated in 1999 and 2001 assessing the effect of immunosuppression with muromonab-CD3, cyclosporine, methylprednisolone, and prednisone versus standard care in terms of death, HTx, or LV assist device placement in patients with GCM was terminated prematurely due to recruitment problems. A preliminary report from 20 cases demonstrated effect of cyclosporine but no additive effect of muromonab-CD3 (8). Thymoglobulin is used due to the wide recognition of T cells in the immune activation (CD2, CD3, CD4, CD8, CD 11, CD25, HLA-DR and class I) that are eliminated from circulation by complement dependent lysis. Usual dose is 1–2 mg/kg in 3 days depending on the effect on the CD4 cell count. The documentation is empiric and there are no randomized studies. ATG, well known from HTx induction or rejection treatment is a potent T-cell inhibitor, which was used in our case, is off-label and normally preserved for patients with severe illness and poor prognosis. No report exists on successful termination of immunosuppression.
Already after the initiation of the first high-dose systemic steroid (methylprednisolone) on day 2, TnT, a sensitive marker of cardiac damage, began to decline in our patient. However, MCS might also have contributed to acute decompression of the heart and subsequent reduction of myocardial damage.
Experience from other Nordic countries
There is no evidence that epidemiology of GCM differs by region. In a retrospective study from Finland, Kandolin et al. presented data from 32 consecutive patients admitted with GCM between 1991 and 2011 (2). Transplant-free survival was (impressive) 69% at 1 year, 58% at 2 years, and 52% at 5 years. All the patients received conventional treatment for heart failure and anti-arrhythmic drugs. A total of 26 patients were treated with combined immunosuppression, and cyclosporine was used in 20 patients. HTx was performed in 10 patients and in 2 patients the diagnosis was first made in the explanted heart. A previous study from Norway including 11 patients (median age 46 years) with GCM verified by biopsy also have provided important results (20). Similar to our patient, in their study a rapid progressive heart failure was the most common presentation. Two-thirds of patients had severe ventricular arrhythmias, and 4 had pre-existing autoimmune disease. Other case reviews have previously demonstrated that approximately 20% of patients with GCM had prior auto-immune disease (21). In addition, some patients with GCM may present with ocular symptoms (18). In the literature, a total of 10 cases of GCM with orbital myositis or other ocular symptoms have been reported (22-24). Orbital myositis is a nonspecific inflammatory disorder of the extraocular muscles, and is generally considered an autoimmune disease. Of note, our patient had a previous history of autoimmune disease (Lichen planus) but did not report any ocular symptoms. Similarly, in another single case study from Denmark involving a 52-year-old male with GCM, autoimmune disorders in the form of type-1 diabetes, Graves’ disease and total alopecia were reported (25). The patient underwent HTx and at 1-year follow-up there was no evidence of GCM recurrence in EMBs. In the study of Aarønaes et al., 8 out of 11 patients received a HTx, of which 2 suffered recurrent GCM in the transplanted heart (20). Average time from diagnosis to death or transplant was 6 months, and only one patient survived without transplantation. Whether the impressive transplant free survival from the Finish report relies on early diagnosis and/or immunosuppressive therapy, or the fact that other centres favour transplant due to lack of early recovery, is unknown. However, the strategy must be individualized based on the clinical course of the patient. Overall, there is little evidence in the literature from prospective studies. To address the existent knowledge gaps in the field, there is need for well-designed prospective research studies. Furthermore, when dealing with the effectiveness of treatments, RCTs provide the most reliable source of evidence for treatment recommendations, reducing the chance of bias. To our knowledge, no proper RCTs have been performed to evaluate the effectiveness of immunosuppressive therapy in GCM, and this should be the aim of future research.
Conclusions
Historically, GCM has been associated with a very poor prognosis. However, over the past two decades, there have been several examples of successful treatments resulting in good clinical outcomes. Experiences from single case reports, including ours, and case series from other centres, suggest that the most important factors associated with favourable outcomes in GCM are: (I) focus on rapid diagnostic measures and early EMB; (II) access to MCS as a bridge to recovery or HTx; and (III) early initiation and sustained treatment with combined immunosuppression.
Acknowledgments
The authors would like to thank Dr. Stig Urheim for his valuable input in the manuscript preparation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-265/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-265/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-265/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kociol RD, Cooper LT, Fang JC, et al. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation 2020;141:e69-92. [Crossref] [PubMed]
- Kandolin R, Lehtonen J, Salmenkivi K, et al. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail 2013;6:15-22. [Crossref] [PubMed]
- Cooper LT Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis--natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med 1997;336:1860-6. [Crossref] [PubMed]
- Cooper LT Jr, ElAmm C. Giant cell myocarditis. Diagnosis and treatment. Herz 2012;37:632-6. [Crossref] [PubMed]
- Vaideeswar P, Cooper LT. Giant cell myocarditis: clinical and pathological features in an Indian population. Cardiovasc Pathol 2013;22:70-4. [Crossref] [PubMed]
- WHITEHEAD R. ISOLATED MYOCARDITIS. Br Heart J 1965;27:220-30. [Crossref] [PubMed]
- Okada R, Wakafuji S. Myocarditis in autopsy. Heart Vessels Suppl 1985;1:23-9. [Crossref] [PubMed]
- Cooper LT Jr, Hare JM, Tazelaar HD, et al. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol 2008;102:1535-9. [Crossref] [PubMed]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. [Crossref] [PubMed]
- Hang W, Chen C, Seubert JM, et al. Fulminant myocarditis: a comprehensive review from etiology to treatments and outcomes. Signal Transduct Target Ther 2020;5:287. [Crossref] [PubMed]
- Montero S, Aissaoui N, Tadié JM, et al. Fulminant giant-cell myocarditis on mechanical circulatory support: Management and outcomes of a French multicentre cohort. Int J Cardiol 2018;253:105-12. [Crossref] [PubMed]
- Yokoyama H, Yamaguchi M, Tobita K, et al. Different reverse remodelling between left ventricle and right ventricle in fulminant heart failure due to giant cell myocarditis: a case report. Eur Heart J Case Rep 2021;5:ytab214. [Crossref] [PubMed]
- Lee JZ, Low SW, Pasha AK, et al. Comparison of tricuspid annular plane systolic excursion with fractional area change for the evaluation of right ventricular systolic function: a meta-analysis. Open Heart 2018;5:e000667. [Crossref] [PubMed]
- Saeed S, Smith J, Grigoryan K, et al. The tricuspid annular plane systolic excursion to systolic pulmonary artery pressure index: Association with all-cause mortality in patients with moderate or severe tricuspid regurgitation. Int J Cardiol 2020;317:176-80. [Crossref] [PubMed]
- Bleakley C, Singh S, Garfield B, et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int J Cardiol 2021;327:251-8. [Crossref] [PubMed]
- Gude E, Hoel TN, Sørensen G, et al. Long-term continuous flow mechanical biventricular support: 9 years and counting. Interact Cardiovasc Thorac Surg 2020;30:81-4. [Crossref] [PubMed]
- Patel PM, Saxena A, Wood CT, et al. Outcomes of Mechanical Circulatory Support for Giant Cell Myocarditis: A Systematic Review. J Clin Med 2020;9:3905. [Crossref] [PubMed]
- Kodama M, Matsumoto Y, Fujiwara M, et al. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol 1990;57:250-62. [Crossref] [PubMed]
- Bang V, Ganatra S, Shah SP, et al. Management of Patients With Giant Cell Myocarditis: JACC Review Topic of the Week. J Am Coll Cardiol 2021;77:1122-34. [Crossref] [PubMed]
- Aarønaes M, Haugaa KH, Andreassen AK, et al. Giant cell myocarditis--a rare, but dangerous disease. Tidsskr Nor Laegeforen 2005;125:2198-201. [PubMed]
- Blauwet LA, Cooper LT. Idiopathic giant cell myocarditis and cardiac sarcoidosis. Heart Fail Rev 2013;18:733-46. [Crossref] [PubMed]
- Kitano S, Awaya T, Moroi M, et al. Two Cases of Giant Cell Myocarditis With Ocular Symptoms. Can J Cardiol 2023;39:985-7. [Crossref] [PubMed]
- Ali MS, Mba BI, Husain AN, et al. Giant cell myocarditis: a life-threatening disorder heralded by orbital myositis. BMJ Case Rep 2016;2016:bcr2015213759. [Crossref] [PubMed]
- Garg V, Tan W, Ardehali R, et al. Giant cell myocarditis masquerading as orbital myositis with a rapid, fulminant course necessitating mechanical support and heart transplantation. ESC Heart Fail 2017;4:371-5. [Crossref] [PubMed]
- Rasmussen TB, Dalager S, Andersen NH, et al. Fatal giant cell myocarditis in a patient with multiple autoimmune disorders. BMJ Case Rep 2009;2009:bcr09.2008.0997.


