
Persistent right venous valve as a cause of fetal and neonatal pathology from prenatal to postnatal periods: a case report and review
Highlight box
Key findings
• Persistent right venous valve (PRVV) can cause increased nuchal translucency thickness, pericardial effusion, and Doppler flow reversal in ductus venosus in early pregnancy; right atrium (RA) outlet anomalies in middle and late pregnancy; and neonatal cyanosis.
What is known and what is new?
• PRVV can divert blood from vena cava directly into left atrium through foramen ovale during fetal period and after birth.
• PRVV can confine tricuspid flow, resulting in decreased blood flow for oxygen resaturation and increased RA pressure, which can boost right-to-left shunting, potentially causing impaired venous drainage, fluid accumulation, and reduced perfusion in RA outlet.
What is the implication, and what should change now?
• The management options for PRVV depend on its variant forms and associated flow patterns during fetal period and after birth.
• For early PRVV diagnosis, watchful waiting is advised; if instability arises, surgery or pregnancy termination may be considered.
Introduction
Background
The venous sinus valve is a structure that appears in the right atrium (RA) in early embryonic development. In the fetal circulation, the right and left venous valves facilitate oxygenated blood flow from the umbilical vein into the left atrium, allowing oxygenated blood to be delivered preferentially to the head and neck vessels. These valves usually degenerate gradually by 9–15 weeks of pregnancy; however, if they fail to degenerate completely, their remnants, known as persistent right venous valve (PRVV), may affect caval flow and contribute to pathophysiological conditions during the fetal and neonatal periods (1-3).
Rationale and knowledge gap
Sonography can identify PRVV variants and associated flow patterns. However, information on intrauterine ultrasound results and subsequent follow-up in infancy is lacking. We analyzed antenatal and postnatal sonographic images of PRVV variants from our case and past studies (Tables S1,S2). Antenatal and postnatal management options vary depending on the course of the abnormality. We report a case of a fetus with suspected PRVV and right ventricle (RV) hypoplasia, who was monitored closely during mid-to-late gestation. After birth, we inferred that PRVV caused right-to-left shunting, affecting right heart development. Unlike typical cases, our patient showed no immediate symptoms but developed hypoxemia after pulmonary infection. The swift hypoxemia resolution after PRVV surgery and subsequent right heart normalization offer insights into the clinical course and management of PRVV.
Objective
We report a case of a newborn diagnosed with PRVV at 31 weeks of gestation (w.g.) that resulted in potential tricuspid valve (TV) obstruction and RV hypoplasia. We reviewed the current case, as well as previous cases, to summarize the sonographic appearance of PRVV variants before and after birth to facilitate early diagnosis and allow more informed prenatal counseling regarding prognosis and perinatal planning. We present this case in accordance with the CARE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-288/rc).
Case presentation
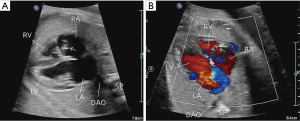
A 28-year-old primigravida woman had abnormal cardiac features during a routine obstetric scan at 24 w.g. An abnormal TV and mild RV hypoplasia [long-axis diameter, 12 mm (Z-score, −3.6)] were diagnosed at 29 w.g., and she was referred to our center for fetal echocardiography at 31 w.g. She received normal prenatal care and had no family history of congenital heart disease. All routine maternal prenatal screening results were negative. Fetal ultrasound at 12 w.g. revealed normal nuchal translucency. Fetal echocardiography showed moderate RV hypoplasia with a long-axis diameter of 12 mm (Z-score, −4.1). The TV appeared to be inserted normally but had a narrowed annulus (7 mm; Z-score, −2.2) resulting in restricted forward blood flow. The pulmonary artery was slightly narrowed with a diameter at the lower limit for the gestational age [main pulmonary artery diameter, 6 mm (Z score, −1.8)]. Sonography revealed a mobile echogenic membrane in the RA prolapsing into the TV orifice. Diagnoses of RV hypoplasia and a prominent Eustachian valve (EV) or Chiari network (CN) were considered (Figure 1, Videos 1,2). The RV and TV dimensions remained unchanged at 37 w.g. (Z-scores, −4.0 and −2.0, respectively). No other fetal developmental abnormalities were observed (Table S1).
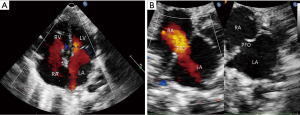
The woman delivered a male baby weighing 2,680 g at 38 w.g. at another center. The baby had difficulty feeding after birth and was followed up at outpatient clinics. Transthoracic echocardiography (TTE) on postnatal day ten demonstrated bidirectional flow at the foramen ovale (FO) (6.5 mm) and left-to-right shunting at the ductus arteriosus (1.0 mm). The RV was tripartite but moderately hypoplastic, with a basal diameter of 8.2 mm (Z-score, −3.4), long-axis diameter of 13.8 mm (Z-score, −4.6), and outflow tract diameter of 6.8 mm (Z-score, −4.4). The TV was hypoplastic but otherwise morphologically normal, with an annulus diameter of 7.2 mm (Z-score, −2.5). However, a mobile, echogenic membrane was observed in the RA near the superior vena cava (SVC), which prolapsed through the TV into the RV, resulting in a narrowed tricuspid inflow of 3 mm and a monophasic Doppler velocity of 1.4 m/s (Figure 2, Videos 3,4). The pulmonary valve (PV) annulus diameter was 6.9 mm (Z-score, −2.3), with no evidence of abnormal valve thickening, flow acceleration, or regurgitation, and the pulmonary artery diameter was 6.7 mm (Z-score, −2.7), with no flow acceleration. A lengthy CN leading to supravalvular obstruction of the TV and secondary RV hypoplasia was considered (Table 1).

Table 1
| Date | Initial and follow-up visits | Diagnostic testing | Interventions |
|---|---|---|---|
| Jun. 2022 | Patient’s condition: difficulty feeding | 2022/6/15; TEE: a lengthy CN leading to TV supravalvular obstruction and secondary RA outlet hypoplasia; bidirectional shunt at PFO; left-to-right shunt at PDA | Outpatient clinics follow-up |
| Patient’s condition: intermittent cyanosis (SaO2 70–84%) | 2022/6/27; chest X-rays: diffuse bilateral ground-glass opacities; bedside TTE: bidirectional flow at PFO (5.0 mm) and left-to-right shunt at PDA (1.0 mm) | Empirical antibiotic treatment: cefotaxime IV q8h for 1 day; 100% O2 via 2-L high-flow nasal cannula; continuous prostaglandin infusion | |
| Patient’s condition: cyanosis (SaO2 with a nadir of approximately 40%) | 2022/6/30; bedside TTE: absolute right-to-left flow at PFO; left-to-right shunt at PDA | Invasive mechanical ventilation; inhaled nitric oxide | |
| Jul. 2022 | Patient’s condition: continuous cyanosis | 2022/7/1; TEE: fibrous, cord-like structure originating from the anterior margin of the SVC in RA | Minimally invasive surgical correction |
| Oct. 2022 | Patients condition: improved oxygen saturation (>90%); TTE: normalized RA outlet portion; left-to-right shunt at PFO | – | – |
TEE, transesophageal echocardiography; CN, Chiari network; TV, tricuspid valve; RA, right atrium; PFO, patent foramen ovale; PDA, patent ductus arteriosus; SaO2, oxygen saturation; TTE, transthoracic echocardiography; IV, intravenous; SVC, superior vena cava.
The infant exhibited intermittent cyanosis on postnatal day 23, which had recently worsened, along with decreased arterial oxygen saturation (SaO2) that fluctuated between 70% and 84%. The newborn was admitted to our neonatal intensive care unit. On admission, the neonate’s lips and mouth turned purplish during feeding and crying, and this was relieved by rest. A small amount of ecchymosis on the chest was observed. Physical examination showed a heart rate of 150 beats/min and a respiratory rate of 42 breaths/min with no signs of respiratory distress. A grade 2/6 systolic murmur was audible at the left sternal border, and normal femoral pulses were noted. Arterial blood gas analysis showed pH 7.42, partial pressure of carbon dioxide of 41.3 mmHg, partial pressure of oxygen of 41.3 mmHg, SaO2 of 72.2%, and oxygen saturation of 80% on pulse oximetry, with no difference between the upper and lower extremities. Electrocardiography was unremarkable. Chest X-ray revealed diffuse bilateral ground-glass opacities. Suspecting an underlying infection exacerbating the decreased SaO2, we immediately started empirical antibiotic treatment with cefotaxime administered intravenously every 8 hours. Bedside TTE showed bidirectional flow at the patent FO (5.0 mm) and left-to-right shunting at the ductus arteriosus (1.0 mm). There were no other significant echocardiographic changes compared with the prior evaluations. He was placed on 100% oxygen delivered through a 2-L high-flow nasal cannula, and a continuous prostaglandin infusion was started to maintain ductus arteriosus patency and to provide an additional source of pulmonary blood flow. Continued desaturations were observed, with a nadir of approximately 40%, accompanied by cyanosis. The neonate was placed on invasive mechanical ventilation and inhalational nitric oxide, resulting in transient clinical improvement, but with no stable effective response. TTE was reviewed and remained unchanged on postnatal day 25, but the atrial level shunt had transitioned to an absolute right-to-left shunt.
The newborn was referred for urgent surgical repair at 26 days of age because of his unstable condition and because the FO was at risk of becoming restrictive. Transesophageal echocardiography (TEE) revealed a fibrous, cord-like structure originating from the anterior margin of the SVC in the RA, which was attached to the TV and restricted its opening. A small midline skin incision was made to access the chest cavity through the right fourth intercostal space. After opening the pericardium, a delivery sheath and biopsy forceps were inserted into the right atrium. Under TEE guidance, the fibrous fenestrated network was captured and removed successfully (Figure 3). The infant’s oxygen saturation improved to >90%, and his cyanosis was immediately reversed following surgery. He was doing well at the 3-month follow-up. Repeated echocardiography revealed normalized dimensions of the TV [annulus, 11.0 mm (Z-score, −0.3)] and a tripartite RV [RV outflow tract diameter, 12.7 mm (Z-score, −0.4); RV basal diameter, 14.3 mm (Z score, −0.2); RV long-axis diameter, 25.6 mm (Z-score, 0.3)]. The shunt direction at the FO had changed from left to right (Figure 4). Throughout the course of the investigation and treatment, there were no adverse or unanticipated events.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
PRVV can divert blood from the vena cava to the left atrium via the FO, causing right-to-left shunting. It can also restrict tricuspid flow, reducing blood flow to the pulmonary circulation for oxygenation while increasing RA pressure, which can further boost right-to-left shunting and lead to impaired venous drainage, fluid accumulation, and decreased perfusion in the RA outlet (4-12). The current report and review suggest that these changes can lead to increased nuchal translucency thickness (4,5), pericardial effusion (7), and Doppler reversal of flow with atrial contraction in the ductus venosus (5,7,8) in early pregnancy, as well as RV anomalies, such as hypoplasia, tricuspid atresia, and pulmonary stenosis (7) in middle and late pregnancy. In addition, persistent right-to-left shunting at the atrial level and decreased systemic SaO2 may lead to cyanosis after birth, usually during the neonatal period. Obstruction between the RA and RV caused by PRVV is the major clinical sign after birth.
Strengths and limitations
We identified similar cases reported in the literature. The prenatal echocardiographic features are described in Table S1, and the echocardiographic and clinical features, as well as surgical repair, are described in Table S2. However, our observations may be subject to bias, and it was not possible to control for confounding factors in this case series review, which may have affected the interpretation of the results. Additional research is required before the results can be applied to a larger population. Nevertheless, the current study may serve as a foundation for subsequent large-scale studies.
Comparison with similar research
Right and left venous valves are embryonic structures in the RA that direct oxygen-enriched blood from the inferior vena cava (IVC) to the FO in the fetal circulation. The left venous valve is generally absorbed into the right side of the interatrial septum, while the right venous valve regresses into the crista terminalis, EV, and Thebesian valve (1-3). The CN is an extended, fenestrated, echogenic structure that originates from either the Thebesian valve or the EV and attaches to the RA wall or interatrial septum (13). The EV is located at the entry of the IVC without attachment to the upper wall of the RA or interatrial septum. Additionally, cor triatriatum dexter (CTD) originates from the crista terminalis. Differentiating CTD from giant EV or CN can be challenging; EV and CN are more likely to be fenestrated and tend to display mobility, while CTD usually has fewer or no fenestrations and is characterized by RA partitioning, leading to a triatrial heart.
The prenatal diagnosis of PRVV primarily relies on echocardiography, which may reveal an undulating echogenic mass or membranous strands in the RA, typically visible after 19 w.g. (4,5,7,8,10,11). During diastole, PRVV can obstruct RV inflow by protruding into the TV or RV, causing various non-specific indirect signs. Non-specific extracardiac indirect signs, such as fetal hydrops, may be observed as early as 12 w.g. (7,8), while non-specific cardiac indirect signs in the second and third trimesters may include TV hypoplasia, RV hypoplasia, and pulmonary stenosis. These prenatal ultrasound features are summarized in Table S1. Postnatal cases of PRVV involving 25 infants under 6 weeks of age (Table S2) (14-33) all displayed membranous structures in the RA, with right-to-left shunting in 22 of 25 cases. RA-RV flow was affected in seventeen of 25 patients; nine patients had clear TV obstruction, while PRVV was closely related to the TV in eight others, either overlaying the valve or extending into the RV through the TV. Differential diagnoses should include Ebstein’s anomaly due to membrane movement mimicking TV displacement and RV hypoplasia (17,20). Cyanosis associated with true Ebstein’s anomaly and/or atrial septal defect is noteworthy, and thorough scanning of the TV and membrane features is crucial. Some cases of PRVV may partially regress, potentially mimicking cardiac masses or thrombi (34). el-Khouri et al. reported PRVV fibers trapped in the FO with no size change over 6 months (33). PRVV can cause thrombus formation, as shown in a neonate with CTD who suffered embolic stroke (30).
Explanations of findings
In the current case, antenatal to postnatal sonography revealed a mobile echogenic membrane consistent with CN. This membrane was identified in the RA, proximal to the SVC entrance, and prolapsed through the TV into the RV, resulting in constricted tricuspid inflow. This constriction may have contributed to cyanosis by facilitating right-to-left shunting via the patent FO and by reducing the pulmonary circulation. We also observed moderate RV hypoplasia along with narrowed but morphologically normal TV and PV, suggesting that the PRVV was directly impeding normal RV blood flow during development.
Implications and actions needed
PRVV may continue to regress throughout pregnancy and may therefore have no clinical relevance. Even if valve remnants remain, the prognosis can be similar to that of regressed remnants and may be favorable with follow-up ultrasonography. Valve remnants were observed in several cases in the review, except for one case with EV or CN in which surgery was performed after birth (10). However, further developments remain unknown in fetuses diagnosed at an early stage of pregnancy with an impaired fetal condition from other causes. In the previous cases, a joint decision was made between the pregnant women and the clinicians to terminate pregnancy. The treatment options for PRVV after birth vary depending on the course of the abnormality. PRVV remnants in infants have been managed conservatively with spontaneous symptom resolution or by surgery in cases with persistent hypoxia (15-24). In cases of PRVV that deteriorate progressively to cardiogenic shock and respiratory failure, the patent ductus arteriosus should be kept open with prostaglandins to improve systemic oxygenation (24,25). Surgical PRVV resection is the treatment of choice, and it usually has excellent short- and long-term outcomes (15-17,19-24). Surgical or percutaneous intervention is advised in patients with a high degree of obstruction, indicated by symptoms or clinical evidence of restricted anterograde flow, and balloon dilation is the usual approach for alleviating obstruction (35). Open thoracotomy was performed in most pediatric cases reviewed in this study. Alghamdi et al. reported a case of a newborn with CTD in whom cardiac catheterization was attempted but failed to disrupt the membrane, and open-heart surgery was subsequently performed (22). In the current case, the obstructive CN was effectively removed using a minimally invasive approach. Redundant surgical intervention should be avoided in patients with a self-limited PRVV course. In our review, ten cases were managed conservatively, and the infants’ oxygen saturation was normal after an average follow-up of 4 months (5,15,26-30). This finding could have been due to somatic growth reducing membrane obstruction over time, gradually decreasing pulmonary artery pressure and improving RV compliance. Regarding RV hypoplasia, the prolapsing membrane across the TV might have obstructed RV inflow, potentially interfering with RV development. This possibility is supported by normalization of the RV dimensions after obstruction removal. In the present case, RV hypoplasia could have occurred secondary to supravalvular obstruction of the lengthy CN, which was ameliorated after obstruction relief. In our review, follow-up ultrasonography also showed that PRVV might persist, partially regress, or be completely absorbed, with improved RV filling and normalized dimensions (5,26,28,30).
Conclusions
Although PRVV is often a benign structural variant, it can have major clinical implications, especially in fetuses and neonates. Echocardiographers need to accurately identify and report PRVV variants and their associated flow patterns, while clinicians should be aware that a redundant PRVV could regress spontaneously over time, and catch-up growth of the RV and pulmonary artery can be expected. Correct recognition and understanding are important to inform decision-making regarding conservative treatment or surgical intervention for PRVV.
Acknowledgments
The authors are very grateful to the patient’s parents. We thank Emily Woodhouse, PhD; Jane Charbonneau, DVM; and Susan Furness, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the English text of drafts of this manuscript.
Funding: This work was supported by the National Natural Science Foundation of China Joint Fund for Regional Innovation and Development (No. U21A20333); Fundamental Research Funds for the Central Universities (No. SCU2022D022); the National Key R&D Program of China (No. 2017YFC0113905); Fundamental Research Funds for the Central Universities, the Key R&D Program of the Science and Technology Department of Sichuan Province (No. 2019YFS0403); and the Popularization and Application Project of the Sichuan Health and Family Planning Commission (No. 17PJ415).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-288/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-288/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-288/coif). H.L. received funding from the National Natural Science Foundation of China Joint Fund for Regional Innovation and Development (No. U21A20333) and Fundamental Research Funds for the Central Universities (No. SCU2022D022); J.C. received funding from the National Key R&D Program of China (No. 2017YFC0113905), Fundamental Research Funds for the Central Universities, the Key R&D Program of the Science and Technology Department of Sichuan Province (No. 2019YFS0403), and the Popularization and Application Project of the Sichuan Health and Family Planning Commission (No. 17PJ415). The funding agency had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moral S, Ballesteros E, Huguet M, et al. Differential Diagnosis and Clinical Implications of Remnants of the Right Valve of the Sinus Venosus. J Am Soc Echocardiogr 2016;29:183-94. [Crossref] [PubMed]
- Kadiyala M, Hui K, Banga S, et al. Persistent Right Venous Valve: Insights From Multimodality Imaging. Circ Cardiovasc Imaging 2021;14:e010977. [Crossref] [PubMed]
- Chakkarapani AA, Roehr CC, Hooper SB, et al. Transitional circulation and hemodynamic monitoring in newborn infants. Pediatr Res 2023; Epub ahead of print. [Crossref] [PubMed]
- Maroun LL, Graem N, Skibsted L. Fetal cor triatriatum dexter: a report of two cases associated with nuchal edema in early second trimester. Pediatr Dev Pathol 2008;11:59-62. [Crossref] [PubMed]
- Bendadi F, van Tijn DA, Pistorius L, et al. Chiari's network as a cause of fetal and neonatal pathology. Pediatr Cardiol 2012;33:188-91. [Crossref] [PubMed]
- Ghi T, Perolo A, Prandstraller D, et al. Antenatal sonography of eustachian valve aneurysm. Ultrasound Obstet Gynecol 2002;20:206-8. [Crossref] [PubMed]
- Lasa JJ, Westover T, Khandelwal M, et al. Cor triatriatum dexter and right ventricular hypoplasia in a fetus. J Ultrasound Med 2011;30:1744-7. [Crossref] [PubMed]
- Arenas Ramírez J, Fernandez Castro C, Otero Chouza M, et al. Persistent and redundant eustachian valve simulating atrial tumor: prenatal diagnosis. Ultrasound Obstet Gynecol 2007;29:704-7. [Crossref] [PubMed]
- Bhatia S, Qasim A, Jiwani AK, et al. Benign Structures Mimicking Right Atrial Masses on Prenatal Ultrasound. Case Rep Pediatr 2021;2021:8889941. [Crossref] [PubMed]
- Fesslova V, Saracino A, Nuri H, et al. Cor triatriatum dexter: unusual features in utero and after birth. Interact Cardiovasc Thorac Surg 2012;14:330-2. [Crossref] [PubMed]
- Vigna R, De Paola N, Cignini P, et al. An isolated fetal cor triatriatum dexter during a targeted anatomic survey at 22 weeks' gestation. J Prenat Med 2008;2:47-8. [PubMed]
- McLean G, Menahem S, Teoh M. Prenatal diagnosis of cor triatriatum dexter. Ultrasound Obstet Gynecol 2010;36:777-8. [Crossref] [PubMed]
- Kim MJ, Jung HO. Anatomic variants mimicking pathology on echocardiography: differential diagnosis. J Cardiovasc Ultrasound 2013;21:103-12. [Crossref] [PubMed]
- Sommer RJ, Hijazi ZM, Rhodes JF Jr. Pathophysiology of congenital heart disease in the adult: part I: Shunt lesions. Circulation 2008;117:1090-9. [Crossref] [PubMed]
- Sunthankar S, Do NL, Parra D, et al. Mysterious Infantile Cyanosis: An Imaging Case Series. CASE (Phila) 2021;5:267-72. [Crossref] [PubMed]
- Galli MA, Galletti L, Schena F, et al. A rare case of neonatal cyanosis due 'cor triatriatum dexter' and a review of the literature. J Cardiovasc Med (Hagerstown) 2009;10:535-8. [Crossref] [PubMed]
- Salam S, Gallacher D, Uzun O. Cor triatriatum dexter masquerading as Ebstein's anomaly. Cardiol Young 2011;21:354-6. [Crossref] [PubMed]
- van Ledden-Klok M, de Mol A, Backx A. Images in Congenital Heart Disease. Symptomatic divided right atrium in a newborn. Cardiol Young 2007;17:110. [Crossref] [PubMed]
- Tueche S. Cor triatriatum dextrum. Surgical treatment in a neonate. Acta Cardiol 2003;58:39-40. [Crossref] [PubMed]
- Cartón AJ, González Rocafort Á, Rubio D, et al. Persistent embryonic right venous valve giving a cor triatriatum dexter appearance in a cyanotic neonate. J Thorac Cardiovasc Surg 2011;142:e147-8. [Crossref] [PubMed]
- Qureshi AU, Latiff HA, Sivalingam S. Persistent valve of systemic venous sinus: a cause of neonatal cyanosis. Cardiol Young 2014;24:756-9. [Crossref] [PubMed]
- Alghamdi MH. Cor triatriatum dexter: A rare cause of cyanosis during neonatal period. Ann Pediatr Cardiol 2016;9:46-8. [Crossref] [PubMed]
- Picciolli I, Francescato G, Colli AM, et al. Cor Triatriatum Dexter: Contrast Echocardiography Is Key to the Diagnosis of a Rare but Treatable Cause of Neonatal Persistent Cyanosis. Children (Basel) 2022;9:676. [Crossref] [PubMed]
- Verde BL, Kaushal S, Slack MC, et al. Venous-Arterial ECMO as a Vital Bridge for Survival in a Neonate with Cor-triatriatum Dexter. Ann Clin Case Rep 2017;2:1409.
- Khalil M, Jux C, Rueblinger L, et al. Acute therapy of newborns with critical congenital heart disease. Transl Pediatr 2019;8:114-26. [Crossref] [PubMed]
- Gad A, Mannan J, Chhabra M, et al. Prominent Eustachian Valve in Newborns: A Report of Four Cases. AJP Rep 2016;6:e33-7. [PubMed]
- Doğan V, Ertuğrul İ, Kayalı Ş, et al. Severe Persistant Cyanosis in a Newborn Due to Prominent Eustachian Valve. Iranian Journal of Neonatology 2017;8:37-9.
- Aljemmali S, Bokowski J, Morales R, et al. Chiari Network Associated with Hypoxemia in a Neonate: Case Report and Review of the Literature. Pediatr Cardiol 2020;41:1529-31. [Crossref] [PubMed]
- Salameh N, Nair P, Salamehova L. G414(P) Unusual cause of cyanosis in a newborn. Archives of Disease in Childhood 2017;102:A163.
- León RL, Zaban NB, Schamberger MS, et al. Cyanosis and Stroke due to Functional Cor Triatriatum Dexter in a Neonate. Neonatology 2018;113:231-4. [Crossref] [PubMed]
- Ko HS, Chen MR, Lin YC. A huge Chiari network presenting with persistent cyanosis in a neonate. Pediatr Cardiol 2011;32:239-40. [Crossref] [PubMed]
- Hurtado-Sierra D, Fernández-Gómez O, Manrique-Rincón F, et al. Cor Triatriatum Dexter: an unusual cause of neonatal cyanosis. Arch Cardiol Mex 2020;91:361-3. [Crossref] [PubMed]
- el-Khouri H, Putman D, Rutkowski M. Unusual case of prominent Chiari network trapped in the left atrium. J Am Soc Echocardiogr 1998;11:71-3. [Crossref] [PubMed]
- Zaher W, Balland A, De Cubber M, et al. Thrombosis of Chiari's network in the setting of non-bacterial thrombotic endocarditis occurring under non-vitamin K antagonist oral anticoagulation: a case report. Eur Heart J Case Rep 2023;7:ytad227. [Crossref] [PubMed]
- Derimay F, Gamondes D, Rioufol G. First Case of Complete Percutaneous Correction of Isolated Divided Atrium (or Cor Triatriatum) Dexter. Can J Cardiol 2021;37:1867-9. [Crossref] [PubMed]


