
Bypass failure of internal mammary artery caused by subclavian artery stenosis: its clinical characteristics and cardiovascular outcomes in patients receiving coronary artery bypass graft surgery
Highlight box
Key findings
• Subclavian artery stenosis (SAS)-related internal mammary artery (IMA) failure (SAS-IMAF) was associated with a 5.82-fold greater likelihood of experiencing a composite of cardiac death, non-fatal myocardial infarction and non-fatal ischemic stroke.
What is known and what is new?
• While IMA has become a major conduit of coronary artery bypass graft (CABG) surgery, SAS could cause subsequent coronary events due to ischemia of myocardial territory supplied by IMA.
• In the current study, 5.5% of patients with CABG using IMA exhibited coronary events due to SAS-IMAF, reflecting a high-risk phenotype of polyvascular disease.
• SAS-IMAF was associated with a significantly elevated prospective risk of cardiovascular events.
What is the implication, and what should change now?
• It is required for interventionalists to improve their awareness toward the importance of meticulous evaluation about SAS in patients with a history of CABG using IMA.
Introduction
Internal mammary artery (IMA) has become a major conduit of coronary artery bypass graft (CABG) surgery with its favorable long-term patency. This is based on accumulating evidences which showed greater durability of IMA compared to saphenous vein and radial artery grafts. One observational study conducted follow-up angiography at 10 years after CABG. In this study, the patency of IMA at 10 years was 85%, which was better than saphenous vein graft (61%) (1). However, coronary events still occur after CABG using IMA due to such as progression of native coronary artery and/or failure of bypass graft itself.
In addition to these mechanisms, subclavian artery stenosis (SAS) could be another cause. Given that subclavian artery supplies blood to IMA, its atherosclerotic progression could induce ischemia of myocardial territory supplied by IMA. Published case reports have shown functional ipsilateral IMA graft failure due to SAS or coronary subclavian steal syndrome (2-5). However, there is no systematic analysis which focuses on how SAS affects cardiovascular outcomes in patients who received CABG using IMA. Since IMA is mostly anastomosed to left anterior descending (LAD) artery, IMA supplies a large area of myocardium, and therefore, the presence of SAS may profoundly worsen cardiovascular outcomes in the setting of CABG using IMA. The current study sought to characterize SAS-IMAF in patients receiving CABG with IMA. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-211/rc).
Methods
Study population
The current study retrospectively analyzed 677 consecutive patients with a history of CABG who were hospitalized due to acute coronary syndrome (ACS) or stable ischemic heart disease (SIHD) at National Cerebral and Cardiovascular Center (January 1st, 2005 to October 31st, 2020). ACS included ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) according to the third universal definition of myocardial infarction (MI). In addition, unstable angina pectoris (UAP) which did not show any elevation of cardiac enzyme was also included into the current analysis (6). SIHD was defined as documentation of ischemic heart disease in the absence of recent acute events (7). Of these, the following patients were excluded: those who had received CABG without using IMA (n=67) and those without any evaluation of subclavian artery (n=230). As a consequence, the remaining 380 patients were included into the current analysis (Figure S1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the National Cerebral and Cardiovascular Center (research project No. R21053). Informed consent was not obtained due to the retrospective and observational analysis of hospitalized patients.
Definition of SAS and SAS-IMAF
At the index of coronary angiography (CAG), subclavian artery angiography was concomitantly conducted to evaluate the severity of SAS including its percent diameter stenosis and pressure gradient. Percent diameter stenosis of SAS was measured by quantitative CAG analysis (QAngio® XA, Medis, Leiden, the Netherlands). Pressure gradient was measured by recording arterial pressure through manual pullback of the 5 French Judkins Right 3.5 or 4.0 catheter from the subclavian artery to the aortic artery. In the case of SAS exhibiting very severe stenosis or its occlusion, two 5 French Judkins Right catheters were positioned at the aortic artery and the distal site of subclavian artery, respectively. Then, pressure gradient was measured. SAS was defined as a lesion which fulfilled the following two criteria: percent diameter stenosis ≥50% and pressure gradient ≥15 mmHg (8,9). SAS-IMAF was defined as the presence of myocardial ischemia caused by SAS. The presence of myocardial ischemia was evaluated by electrocardiogram (ECG), single photon emission computed tomography, coronary flow reserve using transthoracic echocardiography or steal phenomenon on angiography.
Therapeutic management and antithrombotic therapy
Following diagnostic coronary and subclavian artery angiography, interventional cardiologists decided therapeutic management. In the case of percutaneous coronary intervention (PCI), all procedural decisions including device selection, the use of mechanical support and adjunctive pharmacotherapy were made according to the discretion of the individual PCI operator. When endovascular treatment (EVT) for SAS or surgery was considered to be required, heart team discussion with cardiac surgeon and neurosurgeon was undergone to select appropriate revascularization therapy. With regard to antithrombotic therapy, loading of dual antiplatelet therapy (DAPT, 200 mg aspirin + 300 mg clopidogrel or 20 mg prasugrel) was performed prior to primary PCI. After the completion of the procedure, DAPT with its approved maintenance dose in Japan (100 mg/day aspirin + 75 mg/day clopidogrel or 3.75 mg/day prasugrel) was continued for at least 1 year for ACS or 6 months for SIHD. The selection and duration of DAPT after EVT or surgery was conducted by each physician’s discretion. In patients with atrial fibrillation, anticoagulation agent (vitamin K antagonist or direct oral anticoagulant) was added according to the Japanese Circulation Society guideline (10).
Outcomes
The primary outcome was defined as the occurrence of major adverse cardiovascular events (MACE) which consisted of a composite of cardiac death, non-fatal MI and non-fatal ischemic stroke. Ischemic stroke was defined as lacunar infarction, atherothrombotic brain infarction or cardioembolic infarction. The secondary outcome was defined as the occurrence of each component of primary outcome (cardiac death, non-fatal MI and non-fatal ischemic stroke). These outcomes were firstly obtained through reviewing the medical records. If needed, questionnaire was conducted by mail or telephonic follow-up. A clinical event committee consisting of two cardiologists (N.T. and Y.K.) and another referee (M.F.) in case of disagreement adjudicated all events based on the aforementioned original source documents of outcomes.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation and compared using the t-test if data were normally distributed. Categorical variables were compared using the Fisher exact test or the Chi-square test as appropriate. The Kaplan-Meier method was used to estimate survival curves for primary and secondary outcomes, and the log-rank test was used to assess differences between patients with and without SAS-IMAF. Unadjusted hazard ratios (HRs) for primary and secondary outcomes were calculated by a univariate Cox proportional hazards model. Adjusted HRs were calculated by a multivariate Cox proportional hazards model with a P value <0.15. All P values <0.05 were considered statistically significant. To conduct propensity score matching (PSM) analysis for balancing the baseline characteristics of patients with and without SAS-IMAF, we obtained propensity score by using multivariable logistic regression models, with the depending variable of SAS-IMAF and following covariates: age, gender, kidney function, left ventricular ejection fraction (LVEF), ACS and the use of statin. The settings of PSM were variable-rate (one-to-many) matching which is reported as well-removing bias method (11-13), and caliper of 0.25 to balance the patients with and without SAS-IMAF. After obtaining the matched group patients with and without SAS-IMAF, we performed Cox regression analysis and obtained HRs with 95% confidence interval (CI) (14). R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) and MatchIt version 4.3.0. All analyses were performed with JMP version 13.0.0 (SAS Institute, Cary, NC, USA).
Results
Prevalence and characteristics of SAS-IMAF
In the current study, SAS-IMAF was observed in 5.5% (21/380) of study subjects (Figure 1). Table 1 summarizes clinical demographics. Patients with SAS-IMAF were more likely to have a history of hemodialysis (42.9% vs. 10.3%, P<0.001), stroke (52.4% vs. 19.5%, P<0.001) and lower extremity artery disease (LEAD, 66.7% vs. 19.8%, P<0.001). In addition, they more frequently had chronic kidney disease (76.2% vs. 57.1%) and a lower LVEF (42.8%±15.3% vs. 48.5%±12.8%), but these comparisons did not meet statistical significance (P=0.09 and 0.05, respectively). Of note, patients with SAS-IMAF presented ACS (71.4% vs. 37.9%) rather than SIHD (28.6% vs. 62.1%, P=0.002). The averaged duration from CABG to coronary events was 9.0±6.3 years (P=0.86). The frequency of the use of left and bilateral IMA was 63.7% and 32.1%, respectively (P=0.09). With regard to anastomosis designs of IMA, left IMA was anastomosed to LAD alone in 55.5% of study subjects (P=0.55), followed by its anastomosis to multiple native coronary arteries including LAD in 30.0% of them (P=0.88, Table 1). Detailed clinical and angiographical characteristics of SAS-IMAF were summarized by Table 2. Most of SAS (90.5%, 19/21) was located at the left subclavian artery, and one patient (4.8%, 1/21) had multiple SASs at both subclavian arteries. Percent diameter stenosis and averaged pressure gradient of SAS were 78.2%±19.0% and 41.3±17.5 mmHg, respectively. Over 60% of SAS had visible calcification on angiography. Of particular interests, there were nine SAS-IMAF patients who had already received maintenance hemodialysis, and 88.9% of them had arteriovenous access at the same side of IMA graft (Table 2), which suggested arteriovenous access as a potential cause of coronary steal phenomenon through SAS in these cases.

Table 1
| Variables | Overall (n=380) | SAS-IMAF (+) (n=21) | SAS-IMAF (−) (n=359) | P value |
|---|---|---|---|---|
| Age (years) | 72.5±8.5 | 74.4±6.7 | 72.4±8.6 | 0.28 |
| Female | 59 (15.5) | 1 (4.8) | 58 (16.2) | 0.16 |
| Hypertension | 331 (87.1) | 20 (95.2) | 311 (86.6) | 0.25 |
| Dyslipidemia | 313 (82.4) | 16 (76.2) | 297 (82.7) | 0.44 |
| Type 2 DM | 224 (58.9) | 13 (61.9) | 211 (58.8) | 0.78 |
| Smoking | 49 (12.9) | 5 (23.8) | 44 (12.3) | 0.13 |
| CKD | 221 (58.2) | 16 (76.2) | 205 (57.1) | 0.09 |
| Hemodialysis | 46 (12.1) | 9 (42.9) | 37 (10.3) | <0.001 |
| Previous MI | 140 (36.8) | 9 (42.9) | 131 (36.5) | 0.56 |
| Previous stroke | 81 (21.3) | 11 (52.4) | 70 (19.5) | <0.001 |
| LEAD | 85 (22.4) | 14 (66.7) | 71 (19.8) | <0.001 |
| LVEF (%) | 48.2±13.0 | 42.8±15.3 | 48.5±12.8 | 0.05 |
| Clinical diagnosis of ACS/SIHD | 0.002 | |||
| ACS | 151 (39.7) | 15 (71.4) | 136 (37.9) | |
| STEMI | 20 (5.3) | 0 | 20 (5.6) | |
| NSTEMI | 49 (12.9) | 5 (23.8) | 44 (12.3) | |
| UAP | 82 (21.6) | 10 (47.6) | 72 (20.1) | |
| SIHD | 229 (60.3) | 6 (28.6) | 223 (62.1) | |
| AP | 93 (24.5) | 3 (14.3) | 90 (25.1) | |
| SMI | 136 (35.8) | 3 (14.3) | 133 (37.0) | |
| Characteristics of CABG using IMA | ||||
| Duration from CABG (years) | 9.0±6.3 | 9.2±5.4 | 9.0±6.4 | 0.86 |
| The use of IMA | 0.09 | |||
| LIMA | 242 (63.7) | 17 (81.0) | 225 (62.7) | |
| RIMA | 16 (4.2) | 1 (4.8) | 15 (4.2) | |
| Both | 122 (32.1) | 3 (14.3) | 119 (33.1) | |
| Anastomosis designs of IMA | ||||
| Isolated anastomosis of LIMA to LAD | 211 (55.5) | 13 (61.9) | 198 (55.2) | 0.55 |
| Anastomosis of LIMA to multiple native coronary arteries including LAD | 114 (30.0) | 6 (28.6) | 108 (30.1) | 0.88 |
| Isolated anastomosis of RIMA to LAD | 32 (8.4) | 1 (4.8) | 31 (8.6) | 0.53 |
| Anastomosis of RIMA to multiple native coronary arteries including LAD |
9 (2.4) | 1 (4.8) | 8 (2.2) | 0.46 |
| Anastomosis of IMA to RCA/LCX | 14 (3.7) | 0 | 14 (3.9) | 0.36 |
Data are presented as mean ± standard deviation or n (%). SAS-IMAF, subclavian artery stenosis related internal mammary artery failure; DM, diabetes mellitus; CKD, chronic kidney disease; MI, myocardial infarction; LEAD, lower extremity artery disease; LVEF, left ventricular ejection fraction; ACS, acute coronary syndrome; SIHD, stable ischemic heart disease; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-STEMI; UAP, unstable angina pectoris; AP, angina pectoris; SMI, silent myocardial ischemia; CABG, coronary artery bypass grafting; IMA, internal mammary artery; LIMA, left IMA; RIMA, right IMA; LAD, left anterior descending artery; RCA, right coronary artery; LCX, left circumflex artery.
Table 2
| Variables | SAS-IMAF (n=21) |
|---|---|
| Characteristics of SAS | |
| Location | |
| Right | 1 (4.8) |
| Left | 19 (90.5) |
| Both | 1 (4.8) |
| Site of SAS | |
| Ostial | 10 (47.6) |
| Non-ostial | 11 (52.4) |
| Angiographic features | |
| %DS | 78.2±19.0 |
| PG (mmHg) | 41.3±17.5 |
| Calcification | 14 (66.7) |
| Suggestive findings of myocardial ischemia/necrosis | |
| Steal phenomenon on angiography | 8 (38.1) |
| Ischemic change of ECG | 5 (23.8) |
| Reversible perfusion defect on SPECT | 6 (28.6) |
| Abnormal CFR (<2.0) using TTE | 2 (9.5) |
| Features related to hemodialysis | |
| Maintenance hemodialysis | 9 (42.9) |
| AV access at the same side of IMA graft | 8 (38.1) |
Data are presented as n (%) or mean ± standard deviation. SAS-IMAF, subclavian artery stenosis related internal mammary artery failure; DS, diameter stenosis; PG, pressure gradient; ECG, electrocardiogram; SPECT, single photon emission computed tomography; TTE, transthoracic echocardiography; AV, arteriovenous; IMA, internal mammary artery.
Therapeutic management of SAS-IMAF
Summary of revascularization and medical therapies are shown in Table 3. In patients with SAS-IMAF, despite SAS as a culprit lesion causing ACS/SIHD, PCI was firstly selected in 47.6% (10/21) of them to treat stenosis within native coronary arteries, which was significantly lower than those without SAS-IMAF (47.6% vs. 79.9%, P<0.001). The remaining 52.4% (11/21) of SAS-IMAF subjects received revascularization of SAS, which included EVT (n=10) and bypass surgery (n=1). Importantly, mechanical support was more frequently used in patients with SAS-IMAF (14.3% vs. 3.6%, P=0.02). Following these initial therapies, 33.3% (7/21) of SAS-IMAF patients required additional revascularization procedure (vs. 0.3%, P<0.001), which was mainly for SAS causing ACS/SIHD. The detailed timing of initial and additional revascularization procedures in patients with SAS-IMAF was summarized in Table S1.
Table 3
| Variables | Overall (n=380) | SAS-IMAF (+) (n=21) | SAS-IMAF (−) (n=359) | P value |
|---|---|---|---|---|
| Initial revascularization strategy, n (%) | ||||
| PCI | 297 (78.2) | 10 (47.6) | 287 (79.9) | <0.001 |
| Re-CABG | 6 (1.6) | 0 | 6 (1.7) | 0.55 |
| Revascularization for SAS | 11 (2.9) | 11 (52.4) | – | – |
| EVT | 10 (2.6) | 10 (47.6) | – | – |
| Bypass surgery | 1 (0.3) | 1 (4.8) | – | – |
| Mechanical support | 16 (4.2) | 3 (14.3) | 13 (3.6) | 0.02 |
| Additional revascularization procedure, n (%) | ||||
| Frequency | 8 (2.1) | 7 (33.3) | 1 (0.3) | <0.001 |
| Re-CABG | 1 (0.3) | 0 | 1 (0.3) | 0.81 |
| Revascularization for SAS | 7 (1.8) | 7 (33.3) | – | – |
| EVT | 6 (1.6) | 6 (28.6) | – | – |
| Bypass surgery | 1 (0.3) | 1 (4.8) | – | – |
| Medication at discharge, n (%) | ||||
| DAPT | 258 (67.9) | 16 (76.2) | 242 (67.4) | 0.53 |
| β-blocker | 307 (80.8) | 18 (85.7) | 289 (80.5) | 0.56 |
| ACE inhibitor or ARB | 213 (56.1) | 7 (33.3) | 206 (57.4) | 0.03 |
| Statin | 292 (76.8) | 15 (71.4) | 277 (77.2) | 0.55 |
SAS-IMAF, subclavian artery stenosis related internal mammary artery failure; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; EVT, endovascular treatment; DAPT, dual antiplatelet therapy; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.
There were no significant differences in the use of DAPT (76.2% vs. 67.4%, P=0.53), β-blocker (85.7% vs. 80.5%, P=0.56), and statin (71.4% vs. 77.2%, P=0.55), whereas angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB) was less frequently used in patients with SAS-IMAF (33.3% vs. 57.4%, P=0.03, Table 3). In the current study, on-treatment low-density lipoprotein-cholesterol (LDL-C), systolic blood pressure and diastolic blood pressure levels were obtained in 84.2% (320/380) of study subjects. These risk controls did not differ between those with and without SAS-IMAF (Table S2).
Cardiovascular outcomes of SAS-IMAF
The follow-up period was from January 1st, 2005 to October 31st, 2021. During the observational period (median =4.9 years, interquartile range: 1.8 to 5.0 years), there were 50 MACE, 29 cardiac death, 7 non-fatal MI and 21 non-fatal ischemic stroke (Table S3). SAS-IMAF was associated with a 5.82-fold (95% CI: 2.31–14.65, P<0.001) greater likelihood of experiencing MACE (Figure 2, Table 4). Even after adjusting for age and gender (Model 1), and other covariates including medication use (DAPT, β-blocker, ACE inhibitor or ARB, and statin) (Model 2), SAS-IMAF was still an independent predictor of MACE (Model 1: HR 5.34, 95% CI: 2.10–13.57, P<0.001; Model 2: HR 4.04, 95% CI: 1.44–11.38, P=0.008; Table 4 and Table S4). Furthermore, increased risks of non-fatal MI and non-fatal ischemic stroke were observed in patients with SAS-IMAF (non-fatal MI: HR 7.45, 95% CI: 1.36–40.94, P=0.02; non-fatal ischemic stroke: HR 6.68, 95% CI: 2.17–20.52, P<0.001), whereas SAS-IMAF did not predict cardiac death (Figure 3A-3C, Tables S5-S7). On multivariate analysis, SAS-IMAF still continued to predict the occurrence of non-fatal ischemic stroke (HR 7.72, 95% CI: 2.33–25.58, P<0.001, Table S7). In 320 patients with on-treatment LDL-C, systolic blood pressure and diastolic blood pressure levels, multivariate analyses adjusting this risk controls consistently demonstrated the association of SAS-IMAF with the occurrence of MACE (HR 5.55, 95% CI: 1.54–20.07, P=0.009; Table S8). PSM analysis was conducted to further analyze the relationship of SAS-IMAF with the occurrence of MACE. This analysis matched 21 and 53 patients with and without SAS-IMAF, respectively, and were well matched (Table S9). Even in this PSM cohort, SAS-IMAF continued to predict the occurrence of MACE significantly (HR 2.67, 95% CI: 1.06–6.73, P=0.038; Figure S2, Table S10). The occurrence of MACE was further evaluated in subjects exhibiting ACS and SIHD, respectively (Figure 4A,4B). In those with ACS, SAS-IMAF was associated with a greater frequency of MACE (P<0.001). By contrast, the occurrence of this outcome did not differ in SIHD subjects with and without SAS-IMAF (P=0.46). The details of patients with SAS-IMAF are summarized in Table S11.
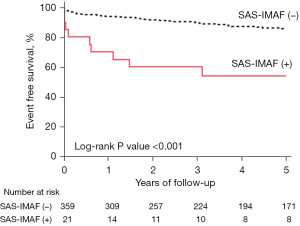
Table 4
| Variables | Univariate analysis | Multivariate analysis (Model 1) | Multivariate analysis (Model 2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |||
| SAS-IMAF | 5.82 | 2.31–14.65 | <0.001 | 5.34 | 2.10–13.57 | <0.001 | 4.04 | 1.44–11.38 | 0.008 | ||
| Age ≥75 years | 1.60 | 0.88–2.92 | 0.12 | 1.57 | 0.85–2.90 | 0.15 | 1.20 | 0.59–2.43 | 0.61 | ||
| Female | 0.57 | 0.22–1.50 | 0.25 | 0.60 | 0.23–1.62 | 0.32 | 0.78 | 0.26–2.34 | 0.66 | ||
| Hypertension | 1.38 | 0.52–3.68 | 0.51 | – | – | – | – | – | – | ||
| Dyslipidemia | 0.72 | 0.35–1.50 | 0.39 | – | – | – | – | – | – | ||
| Type 2 DM | 1.16 | 0.63–2.13 | 0.64 | – | – | – | – | – | – | ||
| CKD | 2.26 | 1.16–4.41 | 0.02 | – | – | – | 1.77 | 0.83–3.80 | 0.14 | ||
| Previous MI | 1.70 | 0.94–3.10 | 0.08 | – | – | – | 1.20 | 0.57–2.54 | 0.62 | ||
| Previous stroke | 1.31 | 0.66–2.59 | 0.45 | – | – | – | – | – | – | ||
| LVEF <40% | 2.69 | 1.42–5.10 | 0.002 | – | – | – | 2.40 | 1.09–5.25 | 0.03 | ||
| ACS | 3.49 | 1.86–6.53 | <0.001 | – | – | – | 4.38 | 2.06–9.32 | <0.001 | ||
| Duration from CABG ≥10 years | 1.22 | 0.67–2.23 | 0.51 | – | – | – | – | – | – | ||
| LIMA to LAD | 1.28 | 0.52–3.16 | 0.59 | – | – | – | – | – | – | ||
| DAPT | 1.01 | 0.52–1.96 | 0.99 | – | – | – | 0.76 | 0.35–1.66 | 0.49 | ||
| β-blocker | 0.56 | 0.28–1.10 | 0.09 | – | – | – | 0.54 | 0.24–1.23 | 0.14 | ||
| ACE inhibitor or ARB | 0.83 | 0.46–1.50 | 0.54 | – | – | – | 0.94 | 0.45–1.96 | 0.88 | ||
| Statin | 0.59 | 0.31–1.13 | 0.11 | – | – | – | 0.53 | 0.24–1.15 | 0.11 | ||
Model 1: adjusted by age and gender. Model 2: adjusted by age, gender, kidney function, MI history, LVEF, ACS, DAPT, β-blocker, ACE inhibitor or ARB, and statin. MACE, major adverse cardiovascular events; HR, hazard ratio; CI, confidence interval; SAS-IMAF, subclavian artery stenosis related internal mammary artery failure; DM, diabetes mellitus; CKD, chronic kidney disease; MI, myocardial infarction; LVEF, left ventricular ejection fraction; ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; LIMA, left internal mammary artery; LAD, left anterior descending artery; DAPT, dual antiplatelet therapy; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.


Discussion
It has not been fully evaluated how SAS affects cardiovascular outcomes in patients who received CABG using IMA. The main findings from the current study are (I) 5.5% of patients with CABG using IMA exhibited ACS or SIHD caused by SAS-IMAF; (II) those with SAS-IMAF more likely had a history of hemodialysis, stroke, LEAD, and more frequently presented ACS; (III) despite the presence of SAS causing ACS or SIHD, PCI was first selected to treat stenosis within native coronary artery in 47.6% of SAS-IMAF patients, which resulted in undergoing additional revascularization therapy for SAS in 33.3% of SAS-IMAF; and (IV) SAS-IMAF significantly elevated a risk of MACE even after adjusting clinical characteristics. These findings suggest SAS-IMAF as a profound disease substrate which substantially worsens cardiovascular outcomes in patients who received CABG using IMA.
The current study elucidated that SAS-IMAF was a manifestation of systemic atherosclerosis. As shown in the aforementioned analysis, a concomitance of stroke and LEAD was more frequently observed in subjects with SAS-IMAF. Of note, around two-thirds of them concomitantly had LEAD. These extensive propagations of atherosclerosis could be caused by a higher frequency of hemodialysis. In general, atherosclerotic involvement of polyvascular beds has long been associated with heightened cardiovascular risks (15-17). In particular, LEAD has been reported as an independent predictor of SAS (8) and an accelerated atheroma progression (18). Moreover, patients requiring hemodialysis harbour a considerably increased risk of atherosclerotic cardiovascular events. The atherosclerotic involvement of systemic arteries and concomitant atherogenic profile in SAS-IMAF may be one of contributors to more frequent occurrence of MACE and ischemic stroke.
We observed that a significant relationship of SAS-IMAF with MACE consistently existed even after a multivariate Cox proportional hazards model and propensity score-matched analyses. It could be argued that SAS-IMAF itself may reflect an atherosclerotic phenotype harbouring its greater disease activity. A published study reported that SAS was identified in 1.9–7.1% of study subjects, and smoking status, a higher level of systolic blood pressure, a lower level of high-density lipoprotein and LEAD were associated with the presence of SAS (8). Whether specific mechanism exists through the formation and progression of SAS has not been fully investigated yet. However, these findings indicate that the formation of SAS is mainly driven by a variety of atherogenic risk factors. Its pathophysiological aspect could be a malignant substrate associated with future occurrence of MACE.
Recently, increasing attentions have focused on minimally invasive approaches of CABG (19). Minimally invasive direct coronary artery bypass (MIDCAB) surgery and its robotic-assisted one have been shown to reduce the length of hospital stay and surgery-related complications while presenting similar clinical efficacy compared to conventional CABG (20,21). These more advanced CABG procedures may improve cardiovascular outcomes in patients with SAS-IMAF. Future studies are warranted to elucidate whether MIDCAB surgery could affect clinical course in patients with and without SAS.
The selection of appropriate revascularization therapies is crucial to mitigate myocardial ischemia/necrosis in the setting of SAS-IMAF. In the current study, PCI for native coronary artery stenosis was performed in 47.6% of SAS-IMAF subjects, although culprit/target lesion was SAS but not native coronary artery stenosis. As a result, revascularization for SAS itself was required in 33.3% of them. This time delay for identification of SAS and adoption of EVT or surgical procedure may affect worse cardiovascular outcomes in those with SAS-IMAF. Table S1 presents the detailed timing of initial and additional revascularization procedures in patients with SAS-IMAF. The timing of revascularization procedures and its selection varied in each individual. Future studies are warranted to standardize selection and timing of therapeutic approach in patients with SAS-IMAF.
As shown in Figure 4 and Table S11, SAS-IMAF affected cardiovascular outcomes in ACS but not SIHD subjects. Of note, while 7 cases of 15 SAS-IMAF subjects with ACS required additional revascularization therapy, most of subjects presenting SIHD were treated by revascularization for SAS. In addition, none of them did not receive another revascularization therapy. These observations suggest difficulties to evaluate subclavian artery and have mutual discussion between interventionalist and surgeons in the setting of ACS. More actions are needed for interventionalists to improve their awareness toward the importance of SAS in patients with a history of CABG using IMA.
Our observations support clinical importance of pre- and post-operative evaluation of SAS in patients who are scheduled for CABG as well as those with a history of CABG using IMA. Mechanistically, the proximal portion of left subclavian artery is more susceptible to flow-limiting disease than other supra-aortic vessels due to its anatomical structure, which underscores the screening of especially left subclavian artery prior to CABG (22,23). Bilateral blood pressure measurement is an easily applicable approach which helps to identify the presence of unilateral SAS (24). Doppler ultrasound is a non-invasive approach to evaluate SAS. However, in the real-world clinical practice, all of patients who has received CABG using IMA do not necessarily receive Doppler ultrasound for follow-up evaluation of subclavian artery. Another issue of Doppler ultrasound is inter- and intra-observer variabilities (25). It is required for evaluation of subclavian artery in patients who have received CABG. More standardized evaluation of subclavian artery with Doppler ultrasound is clinically needed as well. Subclavian artery angiography is another approach which can be conducted during pre-operative CAG. In particular, when patients with a history of CABG using IMA present ACS or SIHD, subclavian artery angiography concomitantly with CAG should be always considered (26). The other important consideration is arteriovenous access for hemodialysis. By using ipsilateral IMA for CABG, the formation of SAS definitely increases a risk of SAS-IMAF in patients receiving hemodialysis (27). In this situation, it is needed to consider the use of contralateral IMA or other grafts through heart-team discussion.
Study limitations
Several caveats should be noted. Firstly, this was a retrospective observational study, but not prospective randomized one. Therefore, management of SAS-IMAF was not standardized but selected according to each physician’s discretion. This may be a potential bias. Secondly, approximately one-third patients with myocardial ischemia after CABG was excluded because subclavian artery angiography was not conducted. These might affect current findings. Thirdly, pre-operative evaluation of subclavian artery was not necessarily conducted in all of study subjects. Therefore, it remains unknown whether SAS already existed at the index of CABG. Fourthly, therapeutic management including procedures and medication use was mainly selected by each attending physician, which may be a potential bias. Lastly, the current study analyzed patients from 2005 to 2020. During this period, guidelines for coronary revascularization, anti-thrombotic and lipid-lowering therapies has changed, which may affect cardiovascular outcomes in the study subjects.
Conclusions
The current study revealed that 5.5% of patients with CABG using IMA exhibited ACS or SIHD caused by SAS-IMAF. PCI was first performed for native coronary artery in 47.6% of SAS-IMAF patients, which required additional revascularization therapy for SAS in 33.3% of them. Furthermore, SAS-IMAF was associated with a significantly elevated prospective risk of cardiovascular events. These findings highlight the importance of meticulous evaluation about subclavian artery in patients with a history of CABG using IMA who presented ACS or SIHD.
Acknowledgments
We would like to acknowledge Miss Yuko Yoshioka and Miss Emi Kanai for their excellent assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-211/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-211/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-211/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-211/coif). Y.K. serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2021 to August 2023, and has received research support from Nipro and Abbott, and honoraria from Nipro, Abbott, Kowa, Amgen, Sanofi, Astellas, Takeda and Daiichi-Sankyo. T.S. reports research funding from Canon Medical Systems Corporation, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research protocol was approved by the ethics committee of the National Cerebral and Cardiovascular Center (research project No. R21053). Informed consent for publication was not obtained due to the retrospective and observational analysis of hospitalized patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149-56. [Crossref] [PubMed]
- Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest 1974;66:436-8. [Crossref] [PubMed]
- FitzGibbon GM, Keon WJ. Coronary subclavian steal: a recurrent case with notes on detecting the threat potential. Ann Thorac Surg 1995;60:1810-2. [Crossref] [PubMed]
- Crowley SD, Butterly DW, Peter RH, et al. Coronary steal from a left internal mammary artery coronary bypass graft by a left upper extremity arteriovenous hemodialysis fistula. Am J Kidney Dis 2002;40:852-5. [Crossref] [PubMed]
- Tomura N, Fujino M, Kataoka Y, et al. Heart Team Intervention for Calcified Left Main Coronary Disease and Jeopardized Left Internal Mammary Artery Graft. Case Rep Cardiol 2022;2022:7712888. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231-64. [Crossref] [PubMed]
- Santucci A, Riccini C, Cavallini C. Treatment of stable ischaemic heart disease: the old and the new. Eur Heart J Suppl 2020;22:E54-9. [Crossref] [PubMed]
- Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol 2004;44:618-23. [Crossref] [PubMed]
- Rigatelli G, Rigatelli G. Simultaneous preoperative brachiocephalic angiography and coronary angiography to prevent coronary-subclavian steal syndrome in coronary surgery candidates. Heart Surg Forum 2005;8:E175-7. [Crossref] [PubMed]
- Nakamura M, Kimura K, Kimura T, et al. JCS 2020 Guideline Focused Update on Antithrombotic Therapy in Patients With Coronary Artery Disease. Circ J 2020;84:831-65. [Crossref] [PubMed]
- Cepeda MS, Boston R, Farrar JT, et al. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol 2003;158:280-7. [Crossref] [PubMed]
- Gu XS, Rosenbaum PR. Comparison of Multivariate Matching Methods: Structures, Distances, and Algorithms. Journal of Computational and Graphical Statistics. 1993;2:405-20.
- Ming K, Rosenbaum PR. Substantial gains in bias reduction from matching with a variable number of controls. Biometrics 2000;56:118-24. [Crossref] [PubMed]
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710-8. [Crossref] [PubMed]
- Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350-7. [Crossref] [PubMed]
- Bhatt DL, Peterson ED, Harrington RA, et al. Prior polyvascular disease: risk factor for adverse ischaemic outcomes in acute coronary syndromes. Eur Heart J 2009;30:1195-202. [Crossref] [PubMed]
- Gutierrez JA, Mulder H, Jones WS, et al. Polyvascular Disease and Risk of Major Adverse Cardiovascular Events in Peripheral Artery Disease: A Secondary Analysis of the EUCLID Trial. JAMA Netw Open 2018;1:e185239. [Crossref] [PubMed]
- Hussein AA, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol 2011;57:1220-5. [Crossref] [PubMed]
- Gaudino M, Andreotti F, Kimura T. Current concepts in coronary artery revascularisation. Lancet 2023;401:1611-28. [Crossref] [PubMed]
- Davierwala PM, Verevkin A, Bergien L, et al. Twenty-year outcomes of minimally invasive direct coronary artery bypass surgery: The Leipzig experience. J Thorac Cardiovasc Surg 2023;165:115-127.e4. [Crossref] [PubMed]
- Piperata A, Busuttil O, Jansens JL, et al. A Single Center Initial Experience with Robotic-Assisted Minimally Invasive Coronary Artery Bypass Surgery (RA-MIDCAB). J Pers Med 2022;12:1895. [Crossref] [PubMed]
- Labropoulos N, Nandivada P, Bekelis K. Prevalence and impact of the subclavian steal syndrome. Ann Surg 2010;252:166-70. [Crossref] [PubMed]
- Zimmerman NB. Occlusive vascular disorders of the upper extremity. Hand Clin 1993;9:139-50. [Crossref] [PubMed]
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation 2011;124:e54-130. [PubMed]
- Scicchitano P, De Palo M, Parisi G, et al. Doppler Ultrasound Selection and Follow-Up of the Internal Mammary Artery as Coronary Graft. Biomedicines 2022;11:66. [Crossref] [PubMed]
- Cua B, Mamdani N, Halpin D, et al. Review of coronary subclavian steal syndrome. J Cardiol 2017;70:432-7. [Crossref] [PubMed]
- Gaudino M, Serricchio M, Luciani N, et al. Risks of using internal thoracic artery grafts in patients in chronic hemodialysis via upper extremity arteriovenous fistula. Circulation 2003;107:2653-5. [Crossref] [PubMed]


