
Transcatheter aortic valve replacement in patients with preoperative ascending aortic diameter ≥45 mm
Highlight box
Key findings
• Transfemoral transcatheter aortic valve replacement (TAVR) can be performed safely in patients with preoperative ascending aortic (AA) diameter ≥45 mm. The mid-term survival did not differ from that in patients with AA diameter <45 mm.
What is known and what is new?
• Early clinical trials regarding TAVR have excluded patients with significant AA dilatation.
• The mid-term survival appears not to be affected by the presence of AA ≥45 mm in patients undergoing transfemoral TAVR, and the adverse aortic events are rare.
What is the implication, and what should change now?
• The AA dilatation should not be considered as contraindication for transfemoral TAVR. However, further studies are required to evaluated the long-term results.
Introduction
Ascending aorta (AA) dilatation occurs frequently in patients with aortic stenosis (AS) who are candidates for transcatheter aortic valve replacement (TAVR) (1,2). This pathology has been considered as a risk factor for TAVR treatment, especially for the transfemoral route (3). Considering that the indications for TAVR continue to expand (4), and the transfemoral route is the first-line approach for patients undergoing TAVR (5), there is a severe evidence gap with regard to the outcome of patients with concomitant ascending aortic dilatation.
Previous landmark TAVR clinical trials have excluded patients with significant ascending aortic dilatation (6,7). However, several recently published studies reported that concomitant ascending aortic dilation did not increase perioperative complications, nor did it appear to affect the mid-term survival and the incidence of adverse aortic events such as aortic dissection (1,8-10). For patients undergoing surgical aortic valve replacement (SAVR), current guidelines recommend concomitant aortic repair or replacement if the diameter of AA exceeds 45 mm (11). However, it is unclear whether this cut-off value remains clinically significant in patients undergoing TAVR. The aim of the present study is to evaluate the safety of transfemoral TAVR, as well as the mid-term survival and the fate of AA in patients with preoperative ascending aortic diameter ≥45 mm. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-324/rc).
Methods
Study population
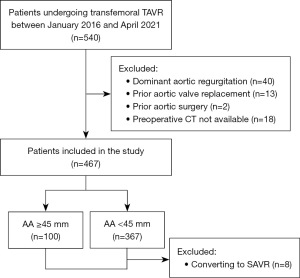
We retrospectively evaluated all patients who underwent transfemoral TAVR from January 2016 to April 2021. Patients with dominant aortic regurgitation, previous aortic valve replacement, and previous aortic surgery were excluded (Figure 1). In addition, patients without available preoperative computed tomography (CT) were also excluded. Electronic medical records were reviewed to obtain patient demographics, echocardiographic and CT data, perioperative information (in-hospital events and echocardiographic data before discharge), and follow-up data (clinical outcomes and follow-up echocardiographic data). Follow-up assessment of clinical outcomes and echocardiography were conducted at 1, 3, 6, 12 months after the procedure, and annually thereafter. Echocardiographic parameters included in the present study were left ventricular ejection fraction, aortic valve velocity and pressure gradient, preoperative aortic regurgitation or postoperative paravalvular aortic insufficiency. The CT data were analyzed by a dedicated core laboratory that included personnel who were blinded to the patient information at our center. When multiple echocardiographic or CT assessments were performed, the latest data were used. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Fuwai Hospital (No. 2022-1829), and informed consent was obtained from all patients.
Surgical procedure
All transfemoral TAVR procedures were conducted in accordance with guidelines using standard techniques. All TAVR candidates were evaluated by the multidisciplinary heart team of Fuwai Hospital. All patients received TAVR under fluoroscopic guidance and local and/or general anesthesia. The right femoral artery was used for valve-stent delivery, and the left femoral artery was punctured for coronary angiography. A temporary pacing lead was inserted through the right internal jugular vein. Standard transcatheter heart valve implantation techniques were used according to the size and morphological characteristics of the valves. The types of transcatheter heart valve included Venus-A (Venus MedTech, Hangzhou, China) in 302 (64.7%), Taurus One (Peijia Medical, Suzhou, China) in 35 (7.5%), VitaFlow (MicroPort, Shanghai, China) in 68 (14.6%), Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) in 16 (3.4%), and Edwards SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA) in 46 (9.9%). Individualized antithrombotic strategy was used according to the patients’ conditions.
Follow-up
Follow-up data were collected from the electronic medical record and telephone interview with patients or their family members. The primary endpoint was all-cause mortality during the follow-up. Secondary endpoints were the occurrence of the aortic dissection and/or rupture during the transfemoral TAVR procedure, as well as during the follow-up.
Statistical analyses
Non-normally distributed variables as median (interquartile range). Categorical data were expressed as counts and proportions. Student’s t-test was used for normally distributed variables and non-parametric tests were used for non-normally distributed variables. The χ2 test was used for categorical variables. Univariable and multivariable Cox proportional hazards regression were used to determine hazard ratios (HRs) and 95% confidential intervals (CIs). A backward variable selection approach with a significance level <0.2 was performed, and variables with P value of <0.2 in univariable analysis were included in the multivariable regression analysis. Kaplan-Meier survival curves were constructed between patients with preoperative AA ≥45 mm and AA <45 mm, and log-rank test was used to compare the difference in overall survival. Statistical analyses were performed using the Statistical Package for Social Sciences, version 23.0 (SPSS, Inc., Chicago, IL, USA).
Results
A total of 467 patients were included. These patients were divided into two groups based on the CT-measured preoperative AA diameter. One hundred patients (21.4%) presented preoperative AA ≥45 mm. In particular, 34 patients had AA ≥50 mm, with seven patients having AA ≥55 mm. The other 367 patients (78.6%) had preoperative AA <45 mm. Baseline characteristics were summarized in Table 1. The median age was 73 years for patients with AA ≥45 mm and 75 years for patients with AA <45 mm (P=0.021). The rate of patients with bicuspid aortic valve (BAV) was significantly higher in patients with AA ≥45 mm (61.0% vs. 21.0%, P<0.001). In addition, patients with AA <45 mm appeared to have higher rates of hypertension, diabetes, history coronary artery disease, prior coronary artery intervention, and prior coronary artery bypass grafting (P<0.05). Patients with AA <45 mm had higher Society of Thoracic Surgery (STS) score compared with those with AA ≥45 mm (4.1% vs. 3.9%, P=0.033).
Table 1
| Variables | AA ≥45 mm (n=100) | AA <45 mm (n=367) | P value |
|---|---|---|---|
| Age (years) | 73 [69–77] | 75 [70–80] | 0.021 |
| Male | 63 (63.0) | 207 (56.4) | 0.236 |
| Body mass index (kg/m2) | 23.7 [21.1–26.7] | 24.1 [22.1–27.2] | 0.188 |
| STS score (%) | 3.9 [3.4–5.0] | 4.1 [3.7–5.1] | 0.033 |
| NYHA class III/IV | 89 (89.0) | 327 (89.1) | 0.977 |
| Smoking | 33 (33.0) | 118 (32.2) | 0.872 |
| Serum creatinine (mg/dL) | 1.0 [0.9–1.2] | 1.0 [0.9–1.3] | 0.740 |
| Hypertension | 48 (48.0) | 237 (64.6) | 0.003 |
| Diabetes mellitus | 16 (16.0) | 104 (28.3) | 0.012 |
| Dyslipidemia | 72 (72.0) | 292 (79.6) | 0.106 |
| History of coronary artery disease | 32 (32.0) | 189 (51.5) | 0.001 |
| History of cerebrovascular disease | 17 (17.0) | 61 (16.6) | 0.928 |
| Peripheral artery disease | 27 (27.0) | 122 (33.2) | 0.235 |
| Prior coronary artery intervention | 6 (6.0) | 62 (16.9) | 0.006 |
| Prior coronary artery bypass grafting | 0 | 19 (5.2) | 0.018 |
| Bicuspid aortic valve | 61 (61.0) | 77 (21.0) | <0.001 |
| Baseline echo characteristics | |||
| Left ventricular ejection fraction (%) | 60 [43.5–65] | 60 [51–65] | 0.115 |
| Peak aortic valve velocity (m/s) | 4.7 [4.3–5.2] | 4.7 [4.3–5.2] | 0.304 |
| Maximum aortic valve pressure gradient (mmHg) | 88.4 [74.0–108.2] | 88.4 [74.0–108.2] | 0.325 |
| Moderate-to-severe aortic regurgitation | 22 (22.0) | 89 (24.3) | 0.639 |
Values are presented as n (%) or median [interquartile range]. AA, ascending aorta; STS, Society of Thoracic Surgery; NYHA, New York Heart Association.
Procedural details are shown in Table 2. The in-hospital mortality rate was 1.1%. The incidences of other in-hospital events were similar between two groups. There was no iatrogenic injury to the AA in the entire study cohort. Only one patient (0.2%) in AA <45 mm group experienced retrograde type B aortic dissection in the descending aorta, which was managed conservatively. Post-procedure echocardiography showed no significant difference in the peak velocity, maximum pressure gradient, and paravalvular aortic insufficiency between two groups.
Table 2
| Variables | AA ≥45 mm (n=100) | AA <45 mm (n=367) | P value |
|---|---|---|---|
| Valve type implanted | 0.155 | ||
| Balloon-expandable | 9 (9.0) | 53 (14.4) | |
| Self-expandable | 91 (91.0) | 314 (85.6) | |
| In-hospital events | |||
| All-cause mortality | 0 | 5 (1.4) | 0.590 |
| Conversion to surgery | 2 (2.0) | 6 (1.6) | 0.682 |
| Stroke | 1 (1.0) | 1 (0.3) | 0.383 |
| Permanent pacemaker | 13 (13.0) | 32 (8.7) | 0.198 |
| Myocardial infarction | 0 | 1 (0.3) | >0.99 |
| Coronary obstruction | 0 | 1 (0.3) | >0.99 |
| Major vascular complication | 2 (2.0) | 11 (3.0) | 0.744 |
| New requirement for dialysis | 0 | 1 (0.3) | >0.99 |
| Implantation of second valve | 10 (10.0) | 42 (11.4) | 0.684 |
| Post-procedure echo characteristics | |||
| Left ventricular ejection fraction (%) | 60 [47.3–65] | 60 [55–65] | 0.331 |
| Peak aortic valve velocity (m/s) | 2.3 [2.1–2.8] | 2.4 [2.0–2.7] | 0.346 |
| Maximum aortic valve pressure gradient (mmHg) | 21.2 [17.6–30.9] | 23.0 [16.0–29.2] | 0.355 |
| Paravalvular aortic insufficiency | 0.595 | ||
| None or mild | 95 (95.0) | 353 (96.2) | |
| Moderate or severe | 5 (5.0) | 14 (3.8) |
Values are presented as n (%) or median [interquartile range]. AA, ascending aorta.
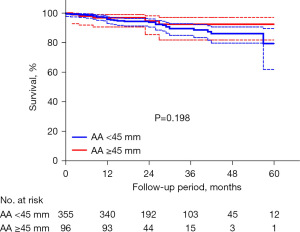
A total of 451 patients (96.6%) were included in the follow-up study. Baseline characteristics of these patients were shown in Table S1. Eight patients (1.7%) who were lost during the follow-up and 8 patients (1.7%) who were converted to SAVR were excluded (Table S2). The median follow-up was 19 [16–34] months in patients with AA ≥45 mm and 27 [15–37] months in patients with AA <45 mm (P=0.152). No statistical difference was found between two groups regarding the overall survival (92.5%±3.5% vs. 78.3%±6.8%, P=0.198) (Figure 2). Only one patient in AA <45 mm group (with a preoperative AA of 39 mm) experienced type A aortic dissection 10 months after the procedure. The median AA diameter measured by transthoracic echocardiography (TTE) remained relatively stable in both groups during a median follow-up of 12 months {AA ≥45 mm group: 45 [42–48] vs. 46 [44–48] mm, P=0.805; AA <45 mm group: 35 [32–38] vs. 35 [31–38] mm, P=0.260}. No aortic growth rate more than 5 mm/years was found in any patient.
In multivariable analysis, AA ≥45 mm was not independent predictor for all-cause mortality. Instead, preoperative serum creatinine (HR =2.39; 95% CI: 1.41–4.05; P=0.001), history of cerebrovascular disease (HR =2.55; 95% CI: 1.29–5.05; P=0.007), and moderate-to-severe paravalvular aortic insufficiency (HR =3.57; 95% CI: 1.07–11.91; P=0.039) were identified as independent risk factors (Table 3).
Table 3
| Variables | Death (n=43) | Survival (n=408) | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age (years) | 77 [71–82] | 75 [69–79] | 1.02 (0.97–1.06) | 0.487 | – | – | |
| Male | 27 (62.8) | 233 (57.1) | 1.23 (0.66–2.29) | 0.571 | – | – | |
| Body mass index (kg/m2) | 23.7 [21.1–26.7] | 24.0 [22.0–27.1] | 0.99 (0.92–1.06) | 0.706 | – | – | |
| NYHA class III/IV | 37 (86.0) | 364 (89.2) | 1.49 (0.63–3.55) | 0.366 | – | – | |
| Smoking | 16 (37.2) | 126 (30.9) | 1.24 (0.67–2.31) | 0.494 | – | – | |
| Serum creatinine (mg/dL) | 1.2 [0.9–1.5] | 1.0 [0.9–1.2] | 2.24 (1.37–3.67) | 0.001 | 2.39 (1.41–4.05) | 0.001 | |
| Hypertension | 31 (72.1) | 247 (60.5) | 1.43 (0.73–2.79) | 0.298 | – | – | |
| Diabetes mellitus | 17 (39.5) | 101 (24.8) | 1.72 (0.93–3.18) | 0.083 | – | – | |
| Dyslipidemia | 36 (83.7) | 318 (77.9) | 1.45 (0.65–3.27) | 0.367 | – | – | |
| History of coronary artery disease | 28 (65.1) | 188 (46.1) | 1.77 (0.94–3.32) | 0.077 | – | – | |
| History of cerebrovascular disease | 14 (32.6) | 61 (15.0) | 2.37 (1.25–4.49) | 0.008 | 2.55 (1.29–5.05) | 0.007 | |
| Peripheral artery disease | 18 (41.9) | 127 (31.1) | 1.41 (0.78–2.59) | 0.270 | – | – | |
| Prior coronary artery intervention | 6 (14.0) | 61 (15.0) | 0.78 (0.33–1.85) | 0.570 | – | – | |
| Prior coronary artery bypass grafting | 3 (7.1) | 16 (3.9) | 2.04 (0.63–6.61) | 0.235 | – | – | |
| Bicuspid aortic valve | 8 (18.6) | 127 (31.1) | 0.65 (0.30–1.40) | 0.267 | – | – | |
| Baseline echo characteristics | |||||||
| Left ventricular ejection fraction (%) | 59 [47–65] | 60 [48.5–65] | 0.99 (0.97–1.01) | 0.323 | – | – | |
| Peak aortic valve velocity (m/s) | 4.5 [4.2–5.2] | 4.7 [4.3–5.2] | 0.76 (0.49–1.17) | 0.207 | – | – | |
| Maximum aortic valve pressure gradient (mmHg) | 81.0 [70.6–108.2] | 88.4 [74.0–108.2] | 0.99 (0.98–1.01) | 0.359 | – | – | |
| Moderate-to-severe aortic regurgitation | 14 (32.6) | 96 (23.5) | 1.60 (0.85–3.04) | 0.148 | – | – | |
| Preoperative AA ≥45 mm | 5 (11.6) | 91 (22.3) | 0.55 (0.22–1.39) | 0.206 | – | – | |
| Valve type implanted | 0.47 (0.14–1.51) | 0.204 | – | – | |||
| Balloon-expandable | 3 (7.0) | 58 (14.2) | |||||
| Self-expandable | 40 (93.0) | 350 (85.8) | |||||
| Post-procedure echo characteristics | |||||||
| Left ventricular ejection fraction (%) | 60 [53.5–64] | 60 [53–65] | 0.98 (0.95–1.01) | 0.194 | – | – | |
| Maximum aortic valve velocity (m/s) | 2.3 [1.9–2.8] | 2.3 [2.0–2.7] | 1.02 (0.57–1.85) | 0.937 | – | – | |
| Maximum aortic valve gradient (mmHg) | 21.2 [14.4–30.9] | 21.2 [16.0–29.2] | 1.01 (0.98–1.04) | 0.609 | – | – | |
| Moderate-to-severe paravalvular aortic insufficiency | 3 (7.1) | 13 (3.2) | 2.68 (0.82–8.75) | 0.102 | 3.57 (1.07–11.91) | 0.039 | |
Values are presented as n (%) or median [interquartile range]. HR, hazard ratio; CI, confidential interval; NYHA, New York Heart Association; AA, ascending aorta.
Discussion
There are several possible mechanisms by which injuries to the AA can develop during transfemoral TAVR: stiff wire interaction in the AA, intimal disruption created by transcatheter heart valve injury to the aortic wall, balloon valvuloplasty injury, or post-dilation balloon interaction with the aorta (12,13). Useini et al. (3) reported that in patients with ascending aortic dilatation for whom transfemoral TAVR might be contraindicated or not feasible, transapical TAVR was a safe method and showed promising early and mid-term outcomes. However, their study did not specify in which situation the patients with ascending aortic dilatation were considered as having high interventional risk and deemed to be unsuitable for transfemoral route.
In fact, during the past decade, improvement in endovascular guidewire techniques has significantly decreased complications associated with transfemoral TAVR (14,15), even in a vulnerable dilatated AA. The result of the present study demonstrated a very low risk (0.2%) of intraprocedural adverse aortic events, and no injury to the AA was found. In Rylski et al.’s study (1), transfemoral route was also the preferred approach in patients with dilatated AA (accounting for 78%), and the risk of intraprocedural adverse aortic events was very low (1%). Several other studies also reported a safe transfemoral TAVR in this particular patient group (8,16).
The results of the present study demonstrate a comparable mid-term survival in patients with AA ≥45 mm who underwent TAVR. Several recently published studies from high-volume centers also reported similar results (1,8-10,16) (Table 4). The present study focused on the patients with AA ≥45 mm because this is the recommended cut-off value for concomitant aortic surgery in patients undergoing SAVR according to the current guidelines (11), while in most previous studies inclusion criteria for AA dilatation was defined as AA ≥40 mm. These results suggest that maintaining a conservative approach to concomitant AA dilatation might be an option in patients undergoing TAVR. To the best of our knowledge, only one study by Ochiai et al. (17) demonstrated a higher mid-term mortality rate in patients with dilatated AA (≥40 mm). However, the incidence of adverse aortic events was not reported in the study.
Table 4
| First author (year of publication) | Sample size | Inclusion criteria for dilatated AA | Prevalence of dilatated AA | Intraprocedural aortic events | Follow-up period | Survival | Adverse aortic event |
|---|---|---|---|---|---|---|---|
| Ochiai et al. (17), 2020 | 1,426 | ≥40 mm | 13.7% | NA | 391 days (median) | 65.5% | NA |
| Rylski et al. (1), 2014 | 457 | ≥40 mm | 22% | 1% | 14 months (median) | 86.7% (1-year survival) | None |
| Ancona et al. (8), 2019 | 680 | >40 mm | 15% | NA | 498 days (mean) | 85.2% | NA |
| Lv et al. (10), 2019 | 134 | >40 mm | 59% | NA | 27 months (median) | NA (one death during follow-up) | None |
| He et al. (9), 2019 | 208 | ≥45 mm | 21.2% | NA | NA (up to 5 years) | NA | None |
| An (current case), 2023 | 467 | ≥45 mm | 21.4% | 0.2% | 19 months (median) | 92.5% | None |
TAVR, transcatheter aortic valve replacement; AA, ascending aorta; NA, not appliable.
Previous studies regarding the changes of AA diameters after TAVR are limited. Lv et al. (10) reported a very slight decrease in AA diameter (40.7 to 40.6 mm) based on 1-year CT follow-up, and suggested that TAVR could prevent a further progression of aortic diameter by correcting hemodynamic derangements. He et al. (9) reported a mild dilatation rate of AA (0.3±0.8 mm/years) based on CT follow-up. In Rylski et al.’s study (1), the proximal AA diameter was assessed with TTE, and it remained stable during a median follow-up of 14 months. In the present study, also measured by TTE, the AA diameter remained stable during a median follow-up of 12 months. However, due to the limitation of TTE in measuring AA, this result should be interpreted with caution.
Several limitations should be acknowledged. First, risk factors for adverse aortic events could not be analyzed because of the very small number of these incidents. Second, the cut-off value of 45 mm for AA is arbitrary, although it is the recommended value for concomitant aortic surgery in patients undergoing SAVR according to the guidelines. In addition, in patients with aneurysmatic AA (≥55 mm), the safety of TAVR requires further study, although in the present study, seven patients with AA ≥55 mm experienced uneventful procedure and the prognosis was satisfactory. Third, as mentioned above, TTE has inherent limitations in the evaluation of AA diameters. Although CT is more accurate, only about one third of the patients underwent postoperative CT exam in the present study. Therefore, changes of the AA diameters during the follow-up should be interpreted with caution. Fourth, as the indications for TAVR continue to expand to younger and lower-risk patients, the long-term impact of AA dilation requires more attention. Further studies comparing the outcomes of TAVR, SAVR, and SAVR plus AA repair/replacement (Wheat’s procedure) in patients with dilated AA would be helpful. In addition, the present study focused on the baseline characteristics and perioperative data. Other factors, such as medication, blood pressure control, and family history of adverse aortic events, were not considered. Finally, the retrospective and observational nature of the study may bring out bias. Future studies with a larger number of patients and longer follow-up may further clarify the effect of AA dilatation on the clinical outcome following TAVR.
Conclusions
Transfemoral TAVR can be performed safely in patients with preoperative AA ≥45 mm with a low intraprocedural risk. The mid-term survival appears not to be affected by the presence of AA ≥45 mm, and the adverse aortic events are rare.
Acknowledgments
We would like to thank Dr. Jun An for his help in polishing our paper.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-324/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-324/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-324/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-324/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Fuwai Hospital (No. 2022-1829) and informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rylski B, Szeto WY, Bavaria JE, et al. Transcatheter aortic valve implantation in patients with ascending aortic dilatation: safety of the procedure and mid-term follow-up†. Eur J Cardiothorac Surg 2014;46:228-33; discussion 233. [Crossref] [PubMed]
- Kerneis C, Pasi N, Arangalage D, et al. Ascending aorta dilatation rates in patients with tricuspid and bicuspid aortic stenosis: the COFRASA/GENERAC study. Eur Heart J Cardiovasc Imaging 2018;19:792-9. [Crossref] [PubMed]
- Useini D, Beluli B, Christ H, et al. Transapical transcatheter aortic valve implantation in patients with aortic diseases. Eur J Cardiothorac Surg 2021;59:1174-81. [Crossref] [PubMed]
- Rahhab Z, El Faquir N, Tchetche D, et al. Expanding the indications for transcatheter aortic valve implantation. Nat Rev Cardiol 2020;17:75-84. [Crossref] [PubMed]
- Biasco L, Ferrari E, Pedrazzini G, et al. Access Sites for TAVI: Patient Selection Criteria, Technical Aspects, and Outcomes. Front Cardiovasc Med 2018;5:88. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a andomized controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Ancona MB, Moroni F, Chieffo A, et al. Impact of Ascending Aorta Dilation on Mid-Term Outcome After Transcatheter Aortic Valve Implantation. J Invasive Cardiol 2019;31:278-81. [PubMed]
- He YX, Fan JQ, Zhu QF, et al. Ascending aortic dilatation rate after transcatheter aortic valve replacement in patients with bicuspid and tricuspid aortic stenosis: A multidetector computed tomography follow-up study. World J Emerg Med 2019;10:197-204. [Crossref] [PubMed]
- Lv WY, Zhao ZG, Li SJ, et al. Progression of the Ascending Aortic Diameter After Transcatheter Aortic Valve Implantation: Based on Computed Tomography Images. J Invasive Cardiol 2019;31:E234-41. [PubMed]
- Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022;146:e334-482. [Crossref] [PubMed]
- Langer NB, Hamid NB, Nazif TM, et al. Injuries to the Aorta, Aortic Annulus, and Left Ventricle During Transcatheter Aortic Valve Replacement: Management and Outcomes. Circ Cardiovasc Interv 2017;10:e004735. [Crossref] [PubMed]
- Walther T, Hamm CW, Schuler G, et al. Perioperative Results and Complications in 15,964 Transcatheter Aortic Valve Replacements: Prospective Data From the GARY Registry. J Am Coll Cardiol 2015;65:2173-80. [Crossref] [PubMed]
- Ugwu JK, Ndulue JK, Sherif KA, et al. Safety of Transcatheter Aortic Valve Replacement in Patients with Aortic Aneurysm: A Propensity-Matched Analysis. Cardiol Ther 2022;11:143-54. [Crossref] [PubMed]
- Denimal T, Delhaye C, Piérache A, et al. Feasibility and safety of transfemoral transcatheter aortic valve implantation performed with a percutaneous coronary intervention-like approach. Arch Cardiovasc Dis 2021;114:537-49. [Crossref] [PubMed]
- Kobayashi A, Lazkani M, Moualla S, et al. Impact of aortic aneurysms in trans-catheter aortic valve replacement: A single center experience. Indian Heart J 2018;70:S303-8. [Crossref] [PubMed]
- Ochiai T, Yoon SH, Sharma R, et al. Prevalence and Prognostic Impact of Ascending Aortic Dilatation in Patients Undergoing TAVR. JACC Cardiovasc Imaging 2020;13:175-7. [Crossref] [PubMed]


