
Evaluation of aortic wall strength in human immunodeficiency virus associated thoracic ascending aortic aneurysm: a pilot study
Highlight box
Key findings
• There was no difference in hydroxyproline concentration of aneurysmal aortic tissue and normal aortic tissue in human immunodeficiency virus (HIV) associated ascending aorta aneurysms.
What is known and what is new?
• Loss of elastin, smooth muscle damage with medial fibrosis and alterations of mucoid content are traditionally a trademark in the cause of aneurysms in HIV patients. No data is available on role of markers of collagen turnover in HIV associated ascending aortic aneurysms.
• We noted that the alteration in tensile strength in HIV associated ascending aortic aneurysms is not attributed solely to the amount of collagen concentration as measured by tissue hydroxyproline.
What is the implication, and what should change now?
• Factors apart from collagen degradation likely contribute to the pathophysiology of HIV associated ascending aortic aneurysms. Further studies are needed to elucidate the complex mechanisms in HIV associated ascending aortic aneurysms.
Introduction
Human immunodeficiency virus (HIV) is considered to be a global pandemic which is associated with a multisystem disease (1). The cardiovascular system is not spared. The relationship between HIV and the vascular system is unique in that it presents as aneurysms which have the propensity to dissect or rupture; and can also present as occlusive diseases (2). It is thought to be due to an inflammatory process where leucocytoclastic vasculitis converges with the vasa vasorum (3,4). HIV associated vasculopathy complicated by aneurysms usually tends to occur in advanced stages of the disease but the process of HIV vasculitis itself may present at any stage of the disease (3,5). Involvement of the large vessel in HIV vasculitis is uncommon and affects younger patients (2,6). There are other multiple risk factors that may contribute to thoracic ascending aortic aneurysm (TAAA) formation, being old age, hypertension, genetic disorders like Ehlers-Danlos and Marfan syndrome, and bicuspid aortic valve. Co-infections with syphilis and hepatitis are at higher risk of aortic aneurysm for HIV specific risk factors. People living with HIV have a 4-fold higher chance of aortic aneurysms compared to the uninfected controls (7).
Elastin and collagen are known mechanical components of the aorta in that they aid with tensile strength. Aneurysms develop due to weakness of the segmental wall of the artery (8). Loss of elastin, smooth muscle damage with medial fibrosis and alterations of mucoid content are traditionally a trademark in the cause of aneurysms in HIV patients (3,9). Surprisingly, collagen as a pivotal structural protein has been poorly studied as a cause of aneurysms. However, it has been shown that weakness in the aorta can be due to impaired collagen metabolism especially in rare conditions like Ehlers-Danlos (10). The data are limited however to abdominal aortic aneurysms (AAA). No data are available on HIV TAAA.
We hypothesized that there might be a difference in collagen quantity between aneurysmal and non-aneurysmal aortic walls as a surrogate marker for aortic wall strength and therefore we aimed to investigate collagen quantity in HIV infected patients on highly active antiretroviral therapy (HAART) presenting with TAAA.
Methods
This was a prospective pilot study, which consecutively enrolled 12 HIV infected patients, on HAART above the age of 18 years who presented for elective TAAA surgery from the period 2019–2021 at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) Cardiothoracic department. We excluded patients with known underlying cardiovascular diseases (e.g., Myocardial infarction, pericarditis) or connective tissue diseases (e.g., Marfan syndrome) and patients presenting for emergency surgery including aortic dissection (Figure 1).

Under the continuous data, hypertension was defined as systolic blood pressure ≥140 mmHg and or diastolic blood pressure ≥90 mmHg or currently using antihypertensive medication (7). Body mass index is defined according to the World Health Organization as underweight <18.5 kg/m2, normal weight 18.5–24.99 kg/m2, overweight 25–29.9 kg/m2 and obesity >30 kg/m2 (7). The patients underwent review and detailed echocardiography (Figure 2A,2B) by an experienced cardiologist. Aortic measurements were obtained as per standard guidelines on aorta measurements and chamber quantification (11). Demographic and clinical findings of these patients were recorded. Once an assessment of TAAA was made the same patients underwent Computed Tomography as per standard protocols (Figure 3) (12). Patient’s demography, clinical, laboratory, echocardiography and Computed Tomography angiography were captured on a Redcap data base and Microsoft Excel 2019.


The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics review board of University of the Witwatersrand (No. M200678). Informed consent was obtained from all the patients in this study.
Aortic sampling and analysis
Patients underwent thoracic aneurysmal aortic surgery as per indicated diagnosis and at surgery fresh aortic tissue sampling was done of the aneurysmal aorta, seemingly normal aorta 1cm downstream of diseased aorta and of the aortic valve leaflets. The specimens were taken to the School of Physiology, Witwatersrand University—stored frozen, to determine collagen content of the seemingly normal and aneurysmal aorta estimated by the hydroxyproline assay and the Department of Histopathology National Health Laboratory Service and National Institute for Occupational Health stored in formalin, for routine clinical histopathological assessment.
Hydroxyproline concentrations of tissue obtained from aneurysmal aorta, seemingly normal aorta and aortic valve leaflets were analysed. Samples of aortic tissue were stored frozen at −70 ℃ for tissue analysis. The tissues were weighed, sealed under vacuum, and hydrolysed with hydrochloric acid at 107 ℃ for 18 hours. Sealed glass tubes were opened and dry hydrolysed by blowing off hydrochloric acid under nitrogen. Determination of Hydroxyproline concentration was by the method of Stalder and Stegemann [1967] (13) where the reagents chloramine T and para-Dimethyl-amino-benzaldehyde were added and the samples placed in a water bath at 60 ℃ for 25 min. The change in colour represented the amount of hydroxyproline present, which was measured with a spectrophotometer at 550 nm against the standard curve. Standards using trans-4-hydroxyproline (µg/mL) were made. From the standard curves we determined the hydroxyproline concentration of samples in µg/mg (original weight of tissue hydrolysed) (14,15).
Statistical analyses
Statistical analysis was carried out using Microsoft excel 2019. Simple descriptive statistics was used to analyse demographic, clinical, echocardiographic, and computed tomography data. Continuous data was represented as median and interquartile range as the data was not normally distributed. The P value was set at P<0.05 to indicate statistical significance. Comparison between the normal aortic tissue and abnormal TAAA wall tissue with regards to hydroxyproline concentration was analysed via Wilcoxon two sample text (non-parametric test for paired data) because the data was not normally distributed. For comparisons between aneurysmal vs. normal aortic tissue of the 12 patient’s vs. 6 aortic valve leaflets data, a Kruskal-Wallis test was done [non-parametric anlysis of variance (ANOVA) test that extends the two samples Wilcoxon test in the situation where there are more than two groups]. Any missing imaging data or laboratory data were excluded from analysis.
Results
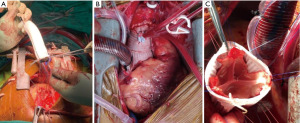
The clinical, demographic, echocardiographic and computed tomography findings are summarised in Table 1, Figure 2, and Figure 3. The study included 12 patients (75% females, median age of 49 years and all of African ethnicity). They were all HIV reactive on HAART, CD4 count 500 cells/µL and were virally suppressed. Hypertension was the most common comorbidity (66.6% of the patients). All patients had TAAA and 3 had concurrent aortic arch involvement. Ninety one percent (n=11) had severe aortic regurgitation with median ascending aortic size of 60 mm and preserved ejection fraction 51%. The common operative procedure was a composite graft for 6 (50%) patients (Figure 4 and Figure 5A,5B). Valve sparing aortic root replacement (Figure 5C) was done in two, sinus of Valsalva aneurysm repair and aortic valve replacement in one patient. Three patients had concomitant ascending aorta and aortic arch aneurysmal disease. Patient one received composite graft and frozen elephant trunk, patient 2 received interposition graft and frozen elephant trunk and in patient three valve sparring aortic root replacement and frozen elephant trunk was done.
Table 1
| Variable | Study patients (n=12) |
|---|---|
| Age (years) | 49 [47.5–57] |
| Gender, n [%] | |
| Female | 9 [75] |
| Male | 3 [25] |
| Body surface area (m2) | 1.7 [1.5–1.9] |
| Body mass index (kg/m2) | 26 [24–28] |
| Vitals | |
| Heart rate (bpm) | 71 [70–74] |
| Systolic blood pressure (mmHg) | 131 [117–150] |
| Diastolic blood pressure (mmHg) | 56 [47–68] |
| Co-morbidities, n [%] | |
| Hypertension | 8 [66.6] |
| Chronic kidney injury | 1 [8.3] |
| Medication, n [%] | |
| HAART (FDC) | 10 [83.3] |
| HAART (second line) | 2 [16.6] |
| Lab results | |
| Hb (g/dL) | 13 [11.9–14.3] |
| Urea (mmol/L) | 5.4 [4.9–6.7] |
| Creatinine (mmol/L) | 72 [67–83] |
| CRP (mg/L) | <10 |
| CD4 count (cells/μL) | 500 [457–568] |
| Viral load (copies/mL) | Lower than detectable |
| Echocardiography | |
| LVEDD (mm) | 59 [52.5–63.5] |
| LVESD (mm) | 45 [38–47] |
| LVEF (%) | 51 [47.5–57] |
| Severe aortic regurgitation, n [%] | 11 [91] |
| Aortic annulus (mm) | 21 [20.5–26] |
| Sinus of Valsalva (mm) | 49 [38.5–51] |
| Sino-tubular junction (mm) | 48 [45.5–56.5] |
| Ascending aorta (mm) | 58 [48–64.5] |
| CT angiogram | |
| Sinus of Valsalva (mm) | 45 [41.25–58.75] |
| Ascending aorta (mm) | 60 [58.5–62.5] |
| Aortic arch (mm) | 42 [30–59] |
| Descending thoracic aorta (mm) | 31.5 [27–39.5] |
Non-normally distributed data as medians [interquartile range]. bpm, beats per minute; HAART, highly active antiretroviral therapy; FDC, fixed dose combination; Hb, hemoglobin; CRP, C-reactive protein; LVEDD, left ventricular diastolic diameter; LVESD, left ventricular end systolic diameter; LVEF, left ventricular ejection fraction; CT, computed tomography.

Hydroxyproline concentration
Total of 33 samples were investigated from the 12 patients (14 aneurysmal, 13 seemingly normal aortic tissue and 6 aortic valve leaflets. We took two specimens of aneurysmal aortic tissue in two patients at different sites of the TAAA where macroscopically the aortic wall tissue looked weak. Two seemingly normal tissue samples were taken from one patient whom we managed to get seemingly normal tissue proximal and distal to the TAAA). For the rest of the patients one specimen for aneurysmal and one specimen for seemingly normal aortic tissue was sampled. Where patient had two samples, the mean of the two samples was taken. Hydroxyproline concentration of aneurysmal aortic tissue and normal aortic tissue was 19.40 (15.19–22.98) vs. 20.85 (15.55–25.83) µg/mg (P=0.82). Hydroxyproline concentration between aneurysmal vs normal aortic tissue vs aortic valve leaflets 19.40 (15.19–22.98) vs. 20.85 (15.55–25.83) vs. 19.09 (13.94–22.00) µg/mg (P=0.86) (Figure 6).

Histology
Eleven specimens from aneurysmal aortic tissues were sent to the National Health Laboratory Service and National Institute for Occupational Health histopathology department (Figure 4). The 12th specimen was from the patient with the ruptured sinus of Valsalva aneurysm, the aortic tissue was insufficient to be sent for histopathology only aortic leaflets were sent. Fragmentation of elastin fibres was seen in 50% (n=6) of the cases (Figure 7A). Infiltration of vasa vasorum by inflammatory cells was seen in 4 patients (Figure 7B), 6 showed atherosclerosis (Figure 7C), 3 granulomatous vasculitis (Figure 7D) (2 being necrotising granulomatous, despite the Ziehl-Neelsen stain negative for acid-fast bacilli), 2 cystic medial degeneration. From the patients that showed atherosclerosis on histology 5 out of 6 had concurrent diagnosis of hypertension and were older with median age of 55 years. No mention of collagen structural abnormalities was made. Six histology aortic valve leaflet results: 3 atheroma seen, 1 necrotising granulomatous vasculitis, 1 cystic myxoid degeneration, 1 normal aortic leaflet.

Discussion
The extracellular matrix of the aorta is a very important element of the tensile strength of the aorta. Any changes in its composition—mainly the collagen and elastin components—can lead to mechanical failure of the aortic wall which can represent as aneurysms that progress to aortic dissection or rupture (8). Our study is the first study to assess collagen quantity in terms of hydroxyproline concentration in TAAA patients with HIV. Our study population was young patients with median age of 49 years, this was comparable to a study of HIV-associated infective native aortic aneurysms (16). However, we had predominantly women (75%), which contrasts with a study in the first world country, where the males predominate (7). A possible explanation could be that in South Africa women are more likely to seek medical assistance and be diagnosed with HIV and take HAART than men (1). Also women are more likely to contract HIV due to biological differences (17).
Surprisingly there was no significant difference in hydroxyproline concentration of aneurysmal aortic tissue and normal aortic tissue. This is contrary to a study that observed 50% decrease in hydroxyproline content in AAA. They compared AAA with control group of autopsies of non-aneurysmal abdominal aortas not specific to HIV related groups (18).
Borges and colleagues (9) evaluated collagen in thoracic aneurysms compared with control of normal aortic tissue. They excluded connective tissue diseases as in our case because there are known reasons for aneurysms (i.e., Marfan syndrome = mutation in fibrilinin-1 gene). Sirius red stain was used for evaluation of collagen quantity which is comparable to hydroxyproline content. They found that there was decrease in collagen content in the thoracic aneurysms compared to the controls and this could be associated to the weakness of the aortic wall (9).
The insignificant difference in hydroxyproline concentration in our study between the two specimen groups is comparable to hydroxyproline concentration in a control group of normal aortic tissues in a study that looked at AAA (21±7 µg/mg) (18). This could support that collagen content has no bearing on the aortic tensile strength in HIV TAAA, but this finding needs to be interpreted with slight caution as our study lacked a normal control group specific to this population. Yet in another study by Pichamuthu et al. that compared hydroxyproline concentration in TAAA between patients with bicuspid aortic valve and tricuspid aortic valve (18.9±1.9 vs. 19.0±1.5 µg/mg; P=0.12), the hydroxyproline concentrations were comparable to our results of aneurysmal and normal aortic tissue in patients with HIV TAAA (8).
There have been reports that in the elderly (age 50 years or greater) HIV infected patients there is an increase in collagen and elastic fibres in the abdominal aorta compared to non-HIV infected patients (19). As we know HIV is a systemic disease, so the vascular remodelling is commenced by inflammation from the HIV itself and therefore there is activation of endothelial cells and expression of collagen (19). The aforementioned finding contrasts with the current study, whereby, in addition to a lack of change in collagen content we noted fragmentation of elastin fibres, this difference could be accounted for by the fact that we studied the ascending aorta which differs from the abdominal aorta in its collagen and elastin content, which would result in different reaction to injury by the two areas of the aorta (20) as well as different methodologies utilised in the two studies to measure the collagen and elastin content. Additionally, our sample size was too small to allow for meaningful subgroup comparisons between the young and the elderly patients with HIV.
The reason of no change in collagen content between the two groups (aneurysmal vs non-aneurysmal aortic tissue) in our study could lend support to the hypothesis that that it is not perhaps the quantity of collagen but the quality of the collagen that may be responsible for weakening of the aortic wall and resultant aneurysm formation in patients with HIV aortopathy. This may be analogous to alteration in the microstructure of the collagen in ageing (21,22).
Aortitis as evidenced by infiltration of the vasa vasorum by inflammatory cells was also observed in some of our cases, which is the classic sign of HIV aortopathy and is suggested to cause transmural ischemic necrosis. There have been reports of leucocytoclastic vasculitis converging with the vasa vasorum (3,20).
In addition to elastin fragmentation, atherosclerosis of the aneurysmal aorta was a common finding in the current study. It is suggested that the premature acceleration of atherosclerosis in HIV patients is from a sequela of (I) inflammation; (II) migration of monocyte and transformation to macrophages and foam cells; and (III) development of plaques with focal thickening of the intima through calcium dependent endoplasmic reticulum after apoptosis of the foam cells (23). Another contributing factor in accelerated atherosclerosis may also be from the second line protease inhibitors (24).
There is a known strong association of atherosclerosis with hypertension and age. In our study, we found out that 83% of the patients with atherosclerosis on histology had concurrent hypertension diagnosis and they were of older age. This could mean that the findings may be due to the comorbidities instead of HIV itself or a combination of all mentioned disease entities (25).
Out of the 6 aortic valve leaflets that were sent for histology, only one was normal, and the rest displayed atherosclerotic, necrotising granulomatous vasculitis and cystic myxoid degeneration disease process akin to the ascending aorta. Based on the aforementioned finding, together with a lack of difference in collagen content between the aortic leaflets and the ascending aorta one may conclude that aortic leaflets are a continuum of the adjacent aorta (26).
Study limitations
The limitation to the study is that of small sample size because the number of cases with this particular disease entity is quite low, however this study is the largest published so far. Another limitation is that there was no control group consisting of aortic tissue from normal living participants and this would nevertheless have been difficult to obtain due to obvious ethical considerations. Hence, all the specimens were taken from the HIV patient with TAAA delineating aneurysmal tissue from non-aneurysmal tissue. The findings of this study may not be extrapolated to other population groups due to biologic differences in age, gender, race, and genetic factors.
Conclusions
We found no difference in collagen concentration in aneurysmal vs. non-aneurysmal aortic tissue in HIV related TAAA. Therefore, the alteration in tensile strength in such patients with HIV associated TAAA is not attributed solely to the amount of collagen concentration. Further studies are needed to confirm this finding and unravel the pathophysiology of HIV related aortopathy.
Acknowledgments
The authors would like to thank Dr. Deepna Govind Lakhoo and Dr. Anita Gildenhuys, National Institute for Occupational Health (NIOH), Department of Anatomical Pathology, National Health Laboratory Service (NHLS), and University of the Witwatersrand, Johannesburg, South Africa for the histopathology micrographs. The authors would like to thank SA Heart Journal for publishing the abstract in their 2021 issue.
Funding: The last author was the recipient of Carnegie Post-Doctoral Fellowship grant.
Footnote
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-188/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-188/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-188/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics review board of University of the Witwatersrand (No. M200678). Informed consent was obtained from all the patients in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mabaso M, Makola L, Naidoo I, et al. HIV prevalence in South Africa through gender and racial lenses: results from the 2012 population-based national household survey. Int J Equity Health 2019;18:167. [Crossref] [PubMed]
- Ferfar Y, Savey L, Comarmond C, et al. Large-vessel vasculitis in human immunodeficiency virus-infected patients. J Vasc Surg 2018;67:1501-11. [Crossref] [PubMed]
- Pillay B, Ramdial PK, Naidoo DP. HIV-associated large-vessel vasculopathy: a review of the current and emerging clinicopathological spectrum in vascular surgical practice. Cardiovasc J Afr 2015;26:70-81. [Crossref] [PubMed]
- Meel R, Gonçalves R. Human Immunodeficiency Virus Associated Large Artery Disease. In: Shuhaiber J. editor. Aortic Aneurysm and Aortic Dissection. IntechOpen; 2020.
- Vega LE, Espinoza LR. Vasculitides in HIV Infection. Curr Rheumatol Rep 2020;22:60. [Crossref] [PubMed]
- Nair R, Robbs JV, Chetty R, et al. Occlusive arterial disease in HIV-infected patients: a preliminary report. Eur J Vasc Endovasc Surg 2000;20:353-7. [Crossref] [PubMed]
- Høgh J, Pham MHC, Knudsen AD, et al. HIV infection is associated with thoracic and abdominal aortic aneurysms: a prospective matched cohort study. Eur Heart J 2021;42:2924-31. [Crossref] [PubMed]
- Pichamuthu JE, Phillippi JA, Cleary DA, et al. Differential tensile strength and collagen composition in ascending aortic aneurysms by aortic valve phenotype. Ann Thorac Surg 2013;96:2147-54. [Crossref] [PubMed]
- de Figueiredo Borges L, Jaldin RG, Dias RR, et al. Collagen is reduced and disrupted in human aneurysms and dissections of ascending aorta. Hum Pathol 2008;39:437-43. [Crossref] [PubMed]
- Lindeman JH, Ashcroft BA, Beenakker JW, et al. Distinct defects in collagen microarchitecture underlie vessel-wall failure in advanced abdominal aneurysms and aneurysms in Marfan syndrome. Proc Natl Acad Sci U S A 2010;107:862-5. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Agarwal PP, Chughtai A, Matzinger FR, et al. Multidetector CT of thoracic aortic aneurysms. Radiographics 2009;29:537-52. [Crossref] [PubMed]
- Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta 1967;18:267-73. [Crossref] [PubMed]
- Brownlee M, Vlassara H, Kooney A, et al. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 1986;232:1629-32. [Crossref] [PubMed]
- Norton GR, Tsotetsi J, Trifunovic B, et al. Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation 1997;96:1991-8. [Crossref] [PubMed]
- Jönsson A, Ljungquist O, Sörelius K. HIV-associated infective native aortic aneurysms. APMIS 2023;131:3-12. [Crossref] [PubMed]
- Abbai NS, Wand H, Ramjee G. Biological factors that place women at risk for HIV: evidence from a large-scale clinical trial in Durban. BMC Womens Health 2016;16:19. [Crossref] [PubMed]
- Carmo M, Colombo L, Bruno A, et al. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2002;23:543-9. [Crossref] [PubMed]
- Oliveira MS, Torquato BGS, da Silveira LAM, et al. Evaluation of aortic changes in elderly people autopsied with acquired immunodeficiency syndrome. Surgical and Experimental Pathology. Surg Exp Pathol 2018;1:7. [Crossref]
- Maleszewski JJ. Inflammatory ascending aortic disease: perspectives from pathology. J Thorac Cardiovasc Surg 2015;149:S176-83. [Crossref] [PubMed]
- Jana S, Hu M, Shen M, et al. Extracellular matrix, regional heterogeneity of the aorta, and aortic aneurysm. Exp Mol Med 2019;51:1-15. [Crossref] [PubMed]
- Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface 2013;10:20121004. [Crossref] [PubMed]
- Shrestha S, Irvin MR, Grunfeld C, et al. HIV, inflammation, and calcium in atherosclerosis. Arterioscler Thromb Vasc Biol 2014;34:244-50. [Crossref] [PubMed]
- Kearns A, Gordon J, Burdo TH, et al. HIV-1-Associated Atherosclerosis: Unraveling the Missing Link. J Am Coll Cardiol 2017;69:3084-98. [Crossref] [PubMed]
- Agmon Y, Khandheria BK, Meissner I, et al. Independent association of high blood pressure and aortic atherosclerosis: A population-based study. Circulation 2000;102:2087-93. [Crossref] [PubMed]
- Agmon Y, Khandheria BK, Meissner I, et al. Aortic valve sclerosis and aortic atherosclerosis: different manifestations of the same disease? Insights from a population-based study. J Am Coll Cardiol 2001;38:827-34. [Crossref] [PubMed]


