
Takotsubo syndrome: unravelling the enigma of the broken heart syndrome?—a narrative review
Introduction
Background
Takotsubo syndrome (TTS), also known as stress-induced cardiomyopathy or broken heart syndrome is a unique and intriguing condition that has gained recognition in recent years. TTS mimics the symptoms of acute coronary syndrome (ACS) but is characterized by distinct cardiac abnormalities, often triggered by emotional or physical stressors (1). This syndrome is marked by transient left ventricular dysfunction.
Rationale and knowledge gap
However, there are numerous aspects within its pathophysiology that remain incompletely understood, and its treatment primarily relies on international consensus and expert recommendations without the support of randomized clinical trials.
Objective
In this updated review, we considered recent advances in our understanding of this complex clinical entity with a special focus on addressing a wide and updated spectrum of topics including epidemiology, physiopathology, diagnosis, clinical manifestations, treatment, and prognosis. We tried to not only cover the core aspects of TTS but also incorporate diverse perspectives, cutting-edge research, and emerging insights. Furthermore, we identified areas where consensus is lacking and where further investigation is needed, thus not only summarizing existing knowledge but also highlighting and suggesting directions for future research. We present this article in accordance with the Narrative Review reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-283/rc).
Methods
We conducted a search of the PubMed database for this narrative review, covering the period from 1990 to June 2023. The search strategy details are provided in Table 1. This review encompasses original research, review articles, and expert consensus documents in English.
Table 1
| Items | Specification |
|---|---|
| Date of search | 01/05/2023 to 15/06/2023 |
| Databases and other sources searched | PubMed |
| Search terms used | See Table S1 for details |
| Timeframe | 1990–2023 |
| Inclusion and exclusion criteria | Inclusion criteria: original articles, reviews, and expert consensus; articles in English. Exclusion criteria: Single case reports and articles not in English or not translated into English |
| Selection process | The selection process was conducted independently by both authors, who reached a consensus in a final meeting |
Discussion
Definition
TTS is an acute cardiac condition characterized by a form of transient regional wall motion abnormalities (RWMA) and left ventricular dysfunction. The name of the disease is derived from the similarity between the shape of the left ventricle (LV) and a Japanese fishing pot trap used for octopus. The clinical symptoms of TTS can be nearly identical to those of ACS (1-3).
Epidemiology
TTS has been increasingly recognized across the world since it was first reported by Japanese cardiologists in 1990 (4). TTS represents approximately 1–3% of all and 5–6% of female patients presenting with suspected ACS (5,6). In the United States, TTS accounts for 0.02% of hospitalizations (7). Most TTS patients are women (up to 90%), with a mean age of 65–70 years (8,9), and 80% are older than 50 years (7). Due to the increasing diagnostic awareness on the condition, male patients are increasingly being diagnosed with TTS, particularly following a physical event (10). TTS is not limited to adult patients and has also been described in children, even in a premature neonate born (11,12).
Although TTS was first reported in Japan, data on potential racial differences are inconsistent, and large-scale studies in this regard are lacking. TTS seems to be less common in African-Americans and Hispanics, while most of the cases reported in the United States are Caucasians (7,13,14). It is worth noting that the prevalence of TTS in men appears to be higher in Japan (10). Differences in clinical features and hospital outcomes can be observed between TTS patients in Japan and Europe. Japanese patients tend to be older, more frequently male, and experience physical triggering factors more often. However, it is important to note that ethnicity itself does not influence the prognosis for TTS patients. The poorer in-hospital outcomes among Japanese patients are primarily attributed to the higher incidence of physical triggers (15). African-American TTS patients have been reported to have more frequent in-hospital complications, such as respiratory failure and stroke and require mechanical ventilation, as compared with Caucasians and Hispanics (16). Notably, the prevalence of neoplasms in patients with TTS is high, up to 12–17% in some registries. Cancer, either history or active, could be associated with an increased risk of adverse events in TTS (17,18).
There are some conflicting results regarding possible seasonal variations in TTS. However, several studies from northern and southern hemispheres have shown a pattern of seasonal variation in TTS that is reversed compared with acute myocardial infarction (AMI), with peaks during summer (19-22).
Furthermore, with respect to the prevalence of TTS, it is quite probable that in some patients subclinical TTS go unnoticed, especially in centres that do not have the capability for percutaneous coronary intervention.
Pathogenesis and pathophysiology
The precise pathogenesis and pathophysiology of TTS remain unknown. Additionally, the relationship between the underlying mechanisms and the anatomical manifestations of left ventricular ballooning also remains unsettled. The strong connection with episodes of sudden, unexpected, stress, major physical illness, or trauma, has led to the widespread use of terms like “stress cardiomyopathy” or “broken heart syndrome”. In fact, the majority of research endeavours have focused on exploring the possible involvement of catecholamine surges in the development of this condition (23). Yet, in a significant proportion of patients (up to 30%), it is not possible to identify a potential stress trigger (1). In this regard, numerous pathophysiological pathways have been suggested to play a role in the development of TTS (Figure 1), and each of them has been the focus of extensive research efforts (23-26). Nevertheless, it is important to humbly acknowledge that the fundamental underlying pathophysiology of TTS remains unclear and continues to be a subject of ongoing academic discussion. Below, we present the main current pathophysiological hypotheses.

Sympathetic hyperactivity
Although the precise underlying pathophysiological mechanisms of TTS remain unclear, the significance of sympathetic hyperactivity is widely recognized (1). In response to stress, the cognitive centers of the brain activate the hypothalamic-pituitary-adrenal (HPA) axis, leading to an increased release of (nor)epinephrine. This phenomenon is referred to as HPA gain (27). Some studies demonstrated significantly elevated levels of plasma catecholamines and stress-related circulating neuropeptides during the acute phase of TTS compared to patients with ST-elevation myocardial infarction (STEMI) (28,29). However, a recent meta-analysis did not confirm the presence of excessively high catecholamine levels (30). Taking into account the short half-life of catecholamines, it is possible that their plasma concentration is significantly elevated at the onset of TTS but soon normalizes or remains only moderately increased during the first days, as indicated by Madias (30). Nonetheless, elevated levels of norepinephrine have been detected in the coronary sinus of TTS patients, indicating an increased release of myocardial catecholamines (31). Furthermore, the administration of beta-agonists and catecholamines to patients can induce various patterns of ballooning and clinical features resembling TTS (32). Providing further evidence for the involvement of adrenergic stimulation in TTS, Ueyama et al. showed that alpha- and beta-receptor blockage reduced the severity of LV apical ballooning induced by immobilization in rodents (33). Additionally, in a more recent study, the introduction of various catecholamines resulted in different RWMA patterns via an afterload-dependent mechanism (34). In addition to the excess of catecholamines, microneurographic investigations have offered direct proof of heightened sympathetic overactivity by detecting elevated sympathetic nerve activity in patients with TTS (35). Moreover, myocardial 123I-metaiodobenzylguanidine scintigraphy has revealed functional alterations in presynaptic sympathetic neurotransmission in TTS patients (36). Even after LV function recovery, all these abnormalities can endure for months and are thought to contribute to an interstitial mononuclear inflammatory response and occasional contraction band necrosis, which are the pathological hallmark of TTS (32,37). Similarly, although the circumferential pattern of RWMA often extends beyond a single coronary artery distribution, frequently correlates with the distribution of myocardial sympathetic nerve terminals (38). Overall, an increased sympathetic stimulation and increased tissue catecholamine levels due to impaired reuptake appear to play a critical role in TTS. In this regard, some authors proposed that elevated levels of circulating epinephrine prompt a shift in the internal pathway of signal transmission within ventricular cardiomyocytes, transitioning from G(s) protein to G(i) protein signaling via the beta(2)-adrenoceptor. While this transition safeguards against the proapoptotic consequences of intense beta(1)-adrenoceptor activation, it also has a detrimental impact on contractility. This impact seems most pronounced in the apical myocardium, which possesses the highest density of beta-adrenoceptors (39,40). However, the precise underlying mechanism of TTS remains elusive, leading to the open discussion of other pathophysiological theories.
Coronary spasm
The hypothesis of multivessel coronary spasm, resulting from sympathetic hyperactivity, has been suggested as a potential mechanism in TTS (1). Mental stress is known to induce endothelial dysfunction via endothelin-A receptors (41). Raynaud syndrome and migraines have also been linked with TTS, providing additional evidence of the importance of vasomotor dysfunction as underlying mechanisms (42). Consistent with this hypothesis, a study examining the flow-mediated dilation of the brachial artery in individuals with TTS showed significant and transient (after several weeks) endothelial dysfunction (43,44). In this regard, Angelini et al. proposed that TTS onset is likely driven by vasospasm and preexisting endothelial dysfunction as all the non-vasospasm theories do not explain the TTS non-apical variants (45). Nevertheless, using intracoronary acetylcholine testing, one study found a predisposition to coronary vasospasm in only 21% of TTS patients (46). Some concern persists that acetylcholine testing is not reliable; however, this objection could be attributable to improper timing of testing (45).
Myocardial bridging and long left anterior descending coronary artery
Myocardial bridging has also been suggested as a possible contributor to the development of TTS (47). Nonetheless, larger studies have shown no disparity in the occurrence of myocardial bridging between patients with TTS and matched controls (48,49). Likewise, a long left anterior descending coronary artery with a recurrent branch has been suggested to be implicated in some cases of TTS although this finding has not been corroborated in subsequent studies (50,51).
Left ventricular outflow tract obstruction (LVOTO)
Several studies have hinted at the potential significance of LVOTO in contributing to the development of TTS. LVOT obstruction can result in a marked increase in apical intraventricular pressure and wall stress, potentially predisposing the left ventricular apex to stunning. However, there are conflicting data, and even the variable, but by no means universal, prevalence of LVOTO in TTS patients can raise doubts about its consistency (52-55).
Acute coronary atherothrombosis
The mechanism of myocardial stunning in TTS has also been suggested to involve angiographically silent plaque rupture, thrombosis, and the consequent transient ischemia followed by swift reperfusion (56,57). Nevertheless, as mentioned earlier, TTS patients typically exhibit RWMA that extend beyond a single coronary artery distribution. Studies utilizing optical coherence tomography have reported a significant prevalence of atherosclerotic plaques and thin cap fibroatheromas in individuals with TTS. However, no evidence of ruptured plaques, active erosions, calcified nodules or intracoronary thrombi has been detected in the vast majority of these patients, indicating a lack of causal association between plaque complication and TTS (49,58).
Microvascular dysfunction
The majority of TTS patients have coronary arteries that appear normal or show non-significant coronary artery disease. Consequently, there has been growing interest in considering the role of coronary microcirculation (resistance vessels) as a pivotal factor in the pathogenesis of TTS. Coronary microcirculation regulates coronary blood flow in response to neural, metabolic, and mechanical influences, and its dysfunction compromises myocardial perfusion (59). Notably, catecholamines primarily induce vasoconstriction in the coronary microvascular system, where α1-receptors are predominantly found (60). Furthermore, several studies using transthoracic Doppler echocardiography have demonstrated a decreased coronary flow reserve (CFR) in the early stage of TTS, further indicating microvascular dysfunction as a potential mechanism (61-63). Nevertheless, a recent analysis indicated that despite poorer LV systolic function, the reduction in acute CFR was less severe in TTS as compared to control patients with AMI. This suggests that other mechanisms may also be involved in the development of RWMA in TTS (64). Nonetheless, by employing the thrombolysis in myocardial infarction frame-count (TFC) method, numerous investigations have revealed widespread TFC irregularities, pointing to elevated resistance in the coronary microvasculature in these patients (6,65-66). Consistent with this, intravenous administration of adenosine temporarily enhanced not only myocardial perfusion, but also the wall motion score index, and left ventricular ejection fraction during the acute phase of TTS (67). Additionally, impaired coronary vasomotion in response to acetylcholine challenge has been demonstrated in women who previously experienced TTS (68). Likewise, post-TTS patients have exhibited high vascular reactivity and reduced endothelial function in response to sudden psychological stress (69). Furthermore, Rivero et al. assessing the index of microvascular resistance, showed the presence of acute microvascular damage with temporal evolution toward resolution that correlated with myocardial stunning recovery (25). Recently, in a mouse model of coronary microvascular dysfunction, a study demonstrated the significant role of disruptions in myocardial perfusion in the development of TTS (70). The impact of vascular Kv1.5 channels, which connect coronary blood flow to myocardial metabolism, in wild-type, Kv1.5−/−, and TgKv1.5−/− mice following transaortic constriction, was examined. The results revealed that transaortic constriction caused systolic apical ballooning and lower LV apex myocardial blood flow in Kv1.5−/− mice, while other groups did not exhibit these effects. However, increasing myocardial blood flow through the use of chromonar, or inducing smooth muscle expression of Kv1.5 channels in TgKv1.5−/− mice, restored perfusion and normalized ventricular function between the apex and base (70). These interesting findings highlight the contribution of flow regulation abnormalities between the LV apex and base on the pathophysiology of TTS and also emphasize the potential of restoring normal perfusion to recover ventricular function.
Estrogen deprivation, hormonal and reproductive factors
The high prevalence of TTS in postmenopausal women strongly suggests a potential hormonal factor at play. It has been observed that a reduction in estrogen levels following menopause increases the vulnerability to TTS (71). Estrogens play a role in regulating endothelial nitric oxide synthase activity, which influences vasomotor tone (72). Additionally, in perimenopausal women, estrogens dampen the sympathetic reaction to psychological stress and decrease the vasoconstriction induced by catecholamines (73,74). The demonstration of estrogen supplementation in animal studies reducing the occurrence of TTS when faced with emotional stress (75,76) has prompted researchers to posit that reproductive factors might be involved in the initiation of TTS. However, there is a scarcity of human data exploring the influence of hormonal and reproductive factors on TTS development. One intriguing study, for instance, analyzed estradiol, progesterone, luteinizing hormone, and follicle-stimulating hormone levels in women with TTS, AMI, and those in good health. This study observed elevated estradiol levels in TTS patients compared to both control groups (77). In another small study, the reproductive traits of female TTS patients were compared to those of female AMI subjects and healthy women. The results revealed that women with TTS were more likely to report a history of irregular menstrual cycles, menopausal symptoms, higher parity, and the use of hormone replacement therapy when compared to both AMI patients and healthy female controls (78). These hormonal and reproductive factors undoubtedly play a role in the pathophysiology of TTS and could have therapeutic implications yet to be clarified.
Genetic predisposition
Regarding genetic predisposition, several investigations have noted an association between the TTS phenotype and ß1-AR gene polymorphisms (79-81). Highlighting the significance of G protein-coupled receptor signaling desensitization and downregulation in TTS, a susceptibility factor in TTS patients has been identified as an L41Q polymorphism of GRK5, one of the more prevalent isoforms in the heart (82). However, the association of the GRK5 polymorphism in TTS is also subject to ongoing debate (83,84). Eitel et al. (85) found promising preliminary results in the first genome-wide association study conducted with a cohort of TTS patients. They identified 18 genomic regions comprising leading single nucleotide polymorphisms (SNPs) that were reinforced by SNPs exhibiting strong linkage disequilibrium (85). Nevertheless, a hereditary pattern following multiple generations in a Mendelian fashion has not been documented for TTS, and further intensive research efforts are necessary to evaluate the potential genetic factors contributing to this syndrome (1).
Brain-heart axis
Psychological stress serves as the central trigger for TTS in many patients, also in the presence of a physical illness, as it may also induce psychological stress (86,87). Patients with TTS are more prone to present preexisting psychiatric conditions (1). Upregulated levels of some microRNA (16 and 26a) have been described in patients with depression and anxiety (88,89). In this regard, in a rodent model where microRNA 16 and microRNA 26a were overexpressed, the administration of exogenous epinephrine resulted in apical wall motion abnormalities (86,87). This mechanism aligns with the myocardium’s inclination to develop TTS when subjected to stress and may also explain the relatively elevated occurrence of previous psychiatric conditions in those affected. Acute neurological injury may cause transient left ventricular systolic dysfunction in 20% to 30% of patients, highlighting the intricate interaction between the brain and the heart: the so called “neurocardiogenic stunning” (90). Patients with TTS exhibit altered neuronal connectivity in various stress-associated limbic regions. Particularly, in the hippocampus, amygdala, cingulate gyrus, and insula, which play crucial roles in regulating emotional responses and the autonomic nervous system (91). Moreover, positron emission tomography scans using 18F-fluorodeoxyglucose have shown heightened activity in the amygdala long before the development of TTS (91). This might suggest that some individuals, in the presence of potential triggers, might have a diminished capacity to respond adequately. This, in turn, could disturb the balance in the nervous system, resulting in myocardial damage resembling what is observed after some acute neurological insults (92). Coupled with impaired neural innervation, this heightened reaction to catecholamines may increase vulnerability to TTS. The brain-heart axis remains a burning topic that requires further exploration and may hide the key to further understand the pathophysiology of TTS.
Metabolic and energetics alterations
Scally et al. recently described the presence of metabolic and energetic impairment in the acute phase of TTS, which is followed by a prolonged and incomplete recovery (93). One preclinical study has also demonstrated an increase in myocardial glucose uptake alongside a decrease in the available glycolysis metabolites, leading to a reduced generation of Krebs cycle intermediates (94). Indeed, one study demonstrated that TTS exhibits a distinct metabolic profile compared to AMI, with a reduced capacity for myocardial energy production during the acute phase (95). It remains uncertain whether this decline stems from a state of heightened myocardial metabolism resulting in exhaustion and metabolite loss, or if it arises from myocardial metabolic impairment causing enzymatic inhibition in the glycolytic, β-oxidative, or pentose phosphate pathways (94). Interestingly, there is a study exploring myocardial energetics in patients with acute TTS through phosphorus magnetic resonance spectroscopy showing profound cardiac energetic impairment with incomplete resolution over 4 months (96). The unusual, extended alteration in myocardial metabolism could clarify why TTS patients persist in experiencing symptoms and encounter recurrent events even when left ventricular function appears to have recovered (97).
Inflammatory mechanism
TTS is distinguished by the presence of increased inflammation in the myocardium due to macrophage infiltration, changes in the distribution of monocyte subsets, and elevated systemic proinflammatory cytokine levels (93). These changes were observed to persist for a minimum of 5 months, suggesting the presence of a low-grade chronic inflammatory condition (93). Moreover, postmortem analysis of hearts from acute phase TTS patients revealed that the present macrophages were predominantly of the proinflammatory M1 type, rather than the reparative M2 type (98). Furthermore, the presence of M1 macrophages and the continued existence of the intermediate (CD14++CD16+) monocyte subset during a 5-month observation period firmly suggest a more proinflammatory and less regenerative state, in contrast to comparable stages seen in AMI patients (99). However, it is still uncertain whether this inflammatory activation is a contributing factor or, conversely, a result of TTS. Nevertheless, these findings provide a potential explanation for the low-grade chronic inflammatory condition that might underlie the evolution of acute TTS into a long-term heart failure syndrome.
Long-term abnormalities: a syndrome that persists over time or exists prior to the current condition?
Some authors have extensively studied the heart failure phenotype of TTS in predominantly symptomatic patients (97). This phenotype is characterized by a preserved LV ejection fraction, compromised cardiac energy status, limitations in physical activity (lower peak Vo2 and an elevated Ve/Vco2 slope during cardiopulmonary exercise testing), diminished counterclockwise rotation of the apical myocardium during systole along with changes in torsion and twist, and potentially microscopic fibrosis (93,97). Furthermore, long-term elevation of cardiac biomarkers such as B-type natriuretic peptide persists at a mild level (98). The enduring myocardial abnormalities over time prompt the question of whether these issues predated the initial TTS episode. This has led the authors themselves to wonder if this suggests the possibility of preexisting abnormalities that contribute to the predisposition for recurrent Takotsubo events and even suggest the presence of an undiagnosed and subtle preexisting cardiomyopathy that becomes apparent during an acute stressful event (3).
Classifications of TTS
Classification based on clinical presentation
We accept so far two different categories of TTS patients: primary and secondary forms (100). Patients experiencing primary TTS typically seek medical attention for acute cardiac symptoms. It is important to note that these individuals may or may not have easily identifiable stress triggers, which are commonly emotional in nature. While there may be other underlying medical conditions that might contribute to their susceptibility TTS, these conditions are not considered the main cause of the surge in catecholamine levels. However, a significant number of TTS cases occur in patients who are already admitted to the hospital for another (medical, surgical, anaesthetic, obstetric, neurological, or psychiatric) condition. In these individuals, the abrupt activation of the sympathetic nervous system, or an increase in catecholamine levels, triggers TTS as a complication of the primary condition or its treatment. Such cases should be classified as secondary TTS. In this scenario, the management approach should address, not only the TTS itself and its cardiac complications, but also the underlying condition that triggered the syndrome. When TTS patients are divided into primary and secondary forms, different clinical presentations are observed, including frequent chest pain with vegetative symptoms in primary patients as compared with palpitations, syncope, and heart failure/shock more frequent in secondary forms (101). Recently, a study showed that pheochromocytoma-induced cardiogenic shock (CS) should be suspected when TTS progresses to CS. This is especially relevant if there is an unusual echocardiographic pattern in TTS or if CS presents with severe cyclic blood pressure fluctuations, rapid hemodynamic decline, and elevated inflammatory markers (102). In this regard, we must highlight that TTS induced by physical factors exhibited higher short-term and long-term mortality. However, not all physical triggers affect patient prognosis equally. TTS patients whose physical trigger was hypoxia or neurological disorders were notably linked to elevated rates of adverse events and in-hospital mortality (103,104). Likely, variations in the short- and long-term outcomes for TTS patients arise from the combined influence of potential pathophysiological distinctions among different triggers and the individual prognoses of accompanying comorbidities. These findings further substantiate the notion that, within the category of TTS, there may be distinct, yet undiscovered clinical profiles, thereby emphasizing the concept that TTS is not always a benign condition.
Classification based on anatomical characteristics
While various anatomical variants of TTS have been described, four major types can be distinguished based on the distribution of RWMA. The most prevalent and broadly acknowledged presentation of TTS is the apical ballooning variant (Figure 2), often referred to as the typical TTS manifestation (1). Other “atypical” TTS types include midventricular (Figure 2), basal, and focal wall motion patterns. A fifth variant of TS has also been proposed recently where the mid-LV is hyperdynamic, but the apex and base are akinetic or hypokinetic (a reverse mid-ventricular Takotsubo) (105). Patients displaying an inverted, mid-ventricular, or basal pattern tend to be younger and frequently have an identifiable emotional or physical trigger when compared to TTS patients who exhibit the traditional apical pattern (1). Heart failure symptoms and notable mitral regurgitation are more commonly observed in the classical presentations while the association with subarachnoid hemorrhage appears to be more common in mid-ventricular forms (1). Recent data indicate that certain non-standard variants, especially the reverse pattern, may be associated with a poorer prognosis (100,106,107). In addition, to the four primary types of TTS, there are other morphological variants that have been identified, such as the biventricular type [involving both the apical LV region and the right ventricle (RV)] (10), the isolated RV type (108), and a global form (even if confirming global hypokinesia as a specific manifestation of TTS can be challenging due to the wide range of potential alternative causes) (1). Some extent of RV involvement occurs in up to one third of TTS patients, even if not immediately clinically apparent (1). The involvement of the RV has been linked to a greater occurrence of significant in-hospital cardiovascular events, which encompass heart failure, CS, and in-hospital mortality (8,100,108,109). Participation of the RV is more common in elderly patients with a lower initial left ventricular ejection fraction and is correlated with a heightened occurrence of substantial or bilateral pleural effusions (8,100,109).

Clinical, electrocardiographic, and imaging diagnosis
Clinical criteria for TTS
The clinical presentation of TTS can mimic AMI, making its diagnosis challenging. Thus, in patients with chest pain suggesting ACS, especially when intense physical or emotional stress or underlying illness is present, it is essential to consider TTS as a potential alternative diagnosis. Currently, invasive coronary angiography is considered the gold standard for evaluating the status of the coronary tree. Commonly, echocardiography is the initial choice for imaging in patients with suspected TTS to evaluate RWMA. However, in many cases these can be better disclosed by left ventriculography, that remains key for the diagnosis in these patients. Various diagnostic criteria have been proposed (Table 2), including the Mayo Clinic criteria (110), the Heart Failure Association diagnostic criteria (100), and the International Takotsubo Registry (InterTAK Registry) diagnostic criteria (1). All of them share a common essence, but it is worth noting that the InterTAK criteria recognize the possibility of significant coronary artery disease coexisting with TTS and also acknowledge that pheochromocytoma may act as a trigger for TTS. In order to better assess the probability of TTS and attempt to differentiate between patients with AMI, the Takotsubo International Registry has developed the InterTAK Diagnostic Score (111) (which gives seven criteria: female gender, emotional trigger, physical trigger, absence of ST-segment depression, psychiatric disorders, neurologic disorders, and QTc prolongation for 25, 24, 13, 12, 11, 9, and 6 points, respectively). This scoring system offers a user-friendly approach and can be swiftly computed at the bedside within an emergency context. Notably, it demonstrates a robust sensitivity in TTS diagnosis and exhibits a remarkable capacity to distinguish TTS from ACS with a high degree of specificity. When TTS was diagnosed in patients with a score of ≥50, approximately 95% of TTS cases were accurately identified. Conversely, when patients with a score of ≤31 were diagnosed with AMI, approximately 95% of those patients were correctly diagnosed (111).
Table 2
| Criteria set 1: Revised Mayo Clinic Criteria (110) |
| (I) Transient hypokinesis, akinesis, or dyskinesis of the left ventricular mid segments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial vascular distribution; a stressful trigger is often, but not always presenta |
| (II) Absence of obstructive coronary disease or angiographic evidence of acute plaque ruptureb |
| (III) New electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin |
| (IV) Absence of pheochromocytoma and myocarditis |
| Criteria set 2: Heart Failure Association-European Society of Cardiology Criteria (100) |
| (I) Transient regional wall motion abnormalities of LV or RV myocardium, which are frequently, but not always, preceded by a stressful trigger (emotional or physical) |
| (II) The regional wall motion abnormalities usuallyc extend beyond a single epicardial vascular distribution, and often result in circumferential dysfunction of the ventricular segments involved |
| (III) The absence of culprit atherosclerotic coronary artery disease, including acute plaque rupture, thrombus formation, and coronary dissection or other pathological conditions to explain the pattern of temporary LV dysfunction observed (e.g., hypertrophic cardiomyopathy, viral myocarditis) |
| (IV) New and reversible ECG abnormalities (ST-segment elevation, ST-segment depression, LBBBd, T-wave inversion, and/or QTc prolongation) during the acute phase (3 months) |
| (V) Significantly elevated serum natriuretic peptide (BNP or NT-proBNP) during the acute phase |
| (VI) Positive but relatively small elevation in cardiac troponin measured with conventional assay (i.e., disparity between the troponin level and the amount of dysfunctional myocardium present)e |
| (VII) Recovery of ventricular systolic function on cardiac imaging at follow-up (3–6 months)f |
| Criteria set 3: International Takotsubo Diagnostic Criteria (InterTAK Registry Diagnostic Criteria) (1) |
| (I) Patients show transientg left ventricular dysfunction (hypokinesia, akinesia, or dyskinesia) presenting as apical ballooning or midventricular, basal or focal wall motion abnormalities. Right ventricular involvement can be present. Besides these regional wall motion patterns, transitions between all types can exist. The regional wall motion abnormality usually extends beyond a single epicardial vascular distribution; however, rare cases can exist where the regional wall motion abnormality is present in the subtended myocardial territory of a single coronary artery (focal TTS)h |
| (II) An emotional, physical, or combined trigger can precede the takotsubo syndrome event, but this is not obligatory |
| (III) Neurologic disorders (e.g., subarachnoid haemorrhage, stroke/transient ischemic attack, or seizures) as well as pheochromocytoma may serve as triggers for takotsubo syndrome |
| (IV) New ECG abnormalities are present (ST-segment elevation, ST-segment depression, T-wave inversion, and QTc prolongation); however, rare cases exist without any ECG changes |
| (V) Levels of cardiac biomarkers (troponin and creatine kinase) are moderately elevated in most cases; significant elevation of brain natriuretic peptide is common |
| (VI) Significant coronary artery disease is not a contradiction in takotsubo syndrome |
| (VII) Patients have no evidence of infectious myocarditish |
| (VIII) Postmenopausal women are predominantly affected |
a, there are rare exceptions to these criteria such as those patients in whom the regional wall motion abnormality is limited to a single coronary territory. b, it is possible that a patient with obstructive coronary atherosclerosis may also develop ABS. However, this is very rare in our experience and in the published literature, perhaps because such cases are misdiagnosed as an acute coronary syndrome. c, acute, reversible dysfunction of a single coronary territory has been reported. d, left bundle branch block may be permanent after TTS but should also alert clinicians to exclude other cardiomyopathies. T-wave changes and QTc prolongation may take many weeks to months to normalize after recovery of LV function. e, Troponin-negative cases have been reported but are atypical. f, small apical infarcts have been reported. Bystander subendocardial infarcts have been reported, involving a small proportion of the acutely dysfunctional myocardium. These infarcts are insufficient to explain the acute regional wall motion abnormality observed. g, wall motion abnormalities may remain for a prolonged period of time or documentation of recovery may not be possible. For example, death before evidence of recovery is captured. h, cardiac magnetic resonance imaging is recommended to exclude infectious myocarditis and diagnosis confirmation of takotsubo syndrome. LV, left ventricle; RV, right ventricle; ECG, electrocardiography; LBBB, left bundle branch block; QTc, QT interval corrected for heart rate; BNP, B-type natriuretic peptide; NT-BNP, N-terminal pro-B-type natriuretic peptide; TTS, Takotsubo syndrome; InterTAK Registry, International Takotsubo Registry; ABS, apical ballooning syndrome.
Electrocardiographic patterns in TTS
Performing an electrocardiogram (ECG) is essential for patients experiencing chest pain or suspected TTS, with the utmost priority being the identification of individuals in need of urgent coronary angiography (2). Abnormal ECG with ST-segment elevation and/or T-wave inversion are described in most TTS patients (2,112). ST-segment elevation was previously reported in as many as 80% of TTS cases, but this percentage may be inflated due to the higher likelihood of these patients undergoing diagnostic coronary angiography (113,114). Actually, fewer than 50% of patients are reported to exhibit ST-segment elevation, according to some recent large registries (2,115). TTS also exhibits various features commonly seen in myocardial infarction. These may include transient Q waves and flattened T waves, which eventually return to normal (116). However, it is important to keep in mind that the ECG may be unremarkable or show nonspecific changes in some patients (114). Irrespective of the findings on the ECG, the majority of patients with TTS will experience QT interval prolongation, which typically returns to normal within 48 hours, even if T-wave inversion may persist for a longer duration (Figure 3) (1,28,114). In terms of ECG manifestations, QT interval prolongation, and T-wave inversion are more often reported in African-American women with TTS (117). A specific set of ECG criteria has been proposed to differentiate TTS from AMI. These criteria include the absence of abnormal Q waves, the absence of “reciprocal” ECG changes, the lack of ST elevation in lead V1, and the presence of ST elevation in lead aVR. According to some authors, these features have a sensitivity of 91% and specificity of 96% for the diagnosis of TTS (118). Interestingly, even ECG alterations observed in the initial two days of TTS presentation provide distinct and valuable indicators for clinicians to suspect the diagnosis (119).

Cardiac biomarkers in TTS
Laboratory tests show typically elevated Troponin I, Troponin T, and Creatinine kinase levels in TTS patients. Troponin levels typically reach their highest point within 24 hours, but these elevations are relatively minor when compared to the severe ECG changes, wall motion abnormalities, and potential hemodynamic instability that may ensue (2,8,110). In addition, during the acute phase of TTS, the concentrations of plasma natriuretic peptides (B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide) are almost always elevated, and significantly raised in TTS compared to AMI, frequently to extremely high levels that correlate more closely with the degree of ventricular strain derangement and RWMA. Natriuretic peptides levels peak within 48 hours of symptom onset and may persist elevated for some months (112). Currently, there is a need for further research to improve the specificity of acute and chronic biomarkers in diagnosing and predicting outcomes in TTS.
Imaging in TTS
Transthoracic echocardiography (TTE) is commonly the first imaging technique used during the acute phase of TTS. It typically reveals abnormal motion of the apical and mid-ventricular segments, which appears either aki- or dyskinetic compared to the basal segments, resembling an “apical ballooning” effect (120). Typically, the RWMA are not limited to one arterial territory. Initially, TTS leads to a reduction in LV systolic function, however, this dysfunction improves over time (110). Sequential TTE is employed during the recuperation period to track the recovery of LV systolic function (110). TTE is vital in detecting acute complications associated with TTS, including the formation of left ventricular thrombus (121), mitral regurgitation arising from leaflet tethering or papillary muscle dysfunction (122), dynamic LVOTO due to basal hyperkinesia (123) and in uncommon cases, ventricular rupture (124). Additionally, RV participation may be indicated by the reverse McConnell sign, indicative of biventricular ballooning (125). Speckle-tracking analysis reveals impaired circumferential strain in both longitudinal and radial directions, further characterizing the extent of myocardial dysfunction associated with TTS (125). Interestingly, in a recent paper investigating the utility of left atrial strain in this population, lower left atrial reservoir and pump strain values provided more accurate predictions for left ventricular end-diastolic pressure (LVEDP) than traditional echocardiographic indexes in the acute phase and were an independent predictor of adverse in-hospital outcomes (126).
As mentioned before, coronary angiography plays a crucial role in the diagnosis of TTS, especially in cases with ST-segment elevation. The initial diagnosis of TTS often involves coronary angiography and left ventriculography, which reveals, in typical cases, akinesis in the mid and apical segments of the LV and the characteristic hyperdynamic basal segments (2,127). Patients may exhibit either normal coronary arteries on angiography or a degree of atherosclerosis that does not match the extent of left ventricular dysfunction or RWMA (128,129). Interestingly, TTS patients who exhibit coronary slow flow at presentation experience a more severe clinical manifestation, with an elevated incidence of intrahospital complications and an unfavorable long-term clinical prognosis (130). Furthermore, invasive hemodynamic evaluation can be employed to assess LVEDP and the existence of a pressure gradient in the outflow tract. LVOTO is observed in about 20% of individuals with TTS and carries implications for treatment (2,131). Assessing LVEDP has been shown to be a reliable predictor of in-hospital complications (132). Remarkably, TTS patients exhibit changes in cardiac hemodynamics in the initial stages, yet there is limited knowledge about the ventricular abnormalities occurring in this condition (133,134). The hemodynamic shifts observed in the acute phase of TTS, as assessed by invasive monitoring of pressure-volume interactions, primarily involve diminished cardiac contractility within a shortened systolic duration, inefficient myocardial energy utilization, and prolonged active myocardial relaxation, while diastolic passive stiffness remains unchanged (135,136).
Cardiac computed tomography angiography may be an alternative to coronary angiography, and even more adequate, in cases with triggers like intracranial bleeding, severe septic shock, and advanced-stage malignancies, where invasive coronary angiography might not be deemed suitable. Also, cardiac computed tomography angiography is particularly attractive in frail patients and in individuals with a history of previous TTS, low clinical suspicion of ACS, or a documented coronary anatomy from a recent angiographic procedure (2).
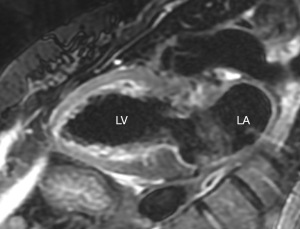
Cardiac magnetic resonance imaging (CMR) is a highly precise diagnostic tool for identifying TTS and can effectively rule out other related causes that may exhibit similar biochemical, echocardiographic, and angiographic characteristics, such as myocarditis or AMI (137). CMR is also useful in identifying complications not evident on other imaging modalities, particularly during the acute phase (2,120). In the acute phase, myocardial edema and the absence of late gadolinium-enhanced imaging (LGE) are the hallmark findings of TTS (Figure 4). Interestingly, myocardial edema has been shown to resolve over time (1,2,138-140). Its extension progressive recovery over time could suggest that the recovery of LV function requires a previous resolution of the edema (138). LGE can serve as a valuable discriminator aiding in the diagnosis of TTS, but some studies showed that LGE can also happen in the context of TTS (140). While patients with AMI have evidence of focal sub-endocardial or transmural LGE, those with myocarditis typically show mid-wall or sub-epicardial LGE distribution (137). Interestingly, in TTS patients LGE has been observed during the acute phase, exhibiting a transmural distribution at the junctions between the dyskinetic and hypercontractile segments. These observations are temporary and resolve in successive imaging, thereby confirming the diagnosis of TTS (141-143). Nonetheless, experimental evidence indicates that the delayed elimination of gadolinium in TTS can be attributed to heightened interstitial water content, which is linked to temporary myocardial edema, rather than irreversible myocardial necrosis or fibrosis (144). The occurrence of LGE during the acute phase of TTS has been linked to a higher likelihood of CS and a prolonged period for the resolution of wall motion abnormalities (120,145). Importantly, one study found that 35% of patients diagnosed with myocardial infarction with non-obstructive coronary arteries (MINOCA) were eventually identified as having TTS after undergoing a thorough evaluation using CMR, despite no apparent prior evidence of the disease on TTE or ventriculography (146). Furthermore, CMR can also identify ventricular thrombi that may not be visible on TTE (140,141). Lastly, CMR is the preferred method for evaluating the RV and, while it was initially believed that TTS primarily impacted the LV, as CMR imaging becomes more prevalent different investigations have shown RV involvement in up to 26–33% of TTS patients (140,147-149).
Management and medical therapy
Therapeutic approaches are applied following clinical observations and guidance from experts, but we should recognize the absence of recommendations grounded in empirical evidence for addressing this elusive condition (Figure 5). The immediate management of TTS patients should focus on ruling out and managing potential ACS, maintaining continuous ECG monitoring, and getting organized for emergent, urgent, or semi-urgent coronary angiography as needed (2,127). Acute management of TTS is restricted to supportive therapy in most cases. Nonetheless, acute complications such as acute heart failure and CS may necessitate aggressive therapeutic measures (2).

TTS complicated by acute heart failure
The management of acute heart failure follows established general guidelines. It involves a combination of diuretics when required, consideration of nitroglycerin to decrease LV filling pressures (except in patients with significant LVOTO), as well as the use of angiotensin II receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEI), and beta-blockers (BB) (2,127). Regarding the use of BB, some authors propose an additional benefit in TTS, by attenuating the impact of excessive catecholamines and potentially reducing the risk of cardiac rupture (2,116). However, their use should be cautious and avoided in patients with clearly established contraindications, especially in the presence of bradycardia and QT interval corrected for heart rate (QTc) >500 ms, because of the possible hazard of torsades de pointes arrhythmia associated with bradycardia (2).
TTS complicated by CS and LVOTO
Notably, CS has been reported in up to 10% of cases and substantially worsens the mortality rate in these patients (8,150). Treating CS in this context primarily relies on the existence of LVOTO. Consequently, before initiating any treatment, it is crucial to assess LVOT pressure gradients using either echocardiography or invasive angiography (2). As mentioned previously, given the heightened catecholamine levels, it is advisable to restrict the use of exogenous catecholamines to a minimum (91). Alternatively, levosimendan has been shown to have some benefit in these patients and therefore should be considered (151). In TTS patients with significant LVOTO, the administration of intravenous fluids and short-acting BB can potentially aid in ameliorating CS and resolving the LVOTO. However, it is advisable to refrain from using diuretics, nitroglycerin, inotropes, or implementing intra-aortic balloon pump. In patients with LVOTO and refractory CS, mechanical circulatory support must be considered (2,116,152). Interestingly, mechanical circulatory support for TTS-related CS is on the rise, with an increased trend in the adoption of venoarterial extracorporeal membrane oxygenation and Impella, while the utilization of intra-aortic balloon pumps has decreased (152,153). Currently, available clinical data support this approach with an excellent overall survival rate (152,153). However, it is essential to conduct prospective studies to assess the safety and effectiveness of various devices, along with determining the optimal timing for implementing mechanical circulatory support in this unique group of patients.
TTS complicated by life-threatening arrhythmias
Life-threatening arrhythmias, including severe bradyarrhythmias or ventricular tachycardia and ventricular fibrillation, have the potential to arise early during the course of TTS (2,154). Ventricular tachycardia or ventricular fibrillation can be observed in approximately 3.0–8.6% of cases and is related to significantly worse short- and long-term prognoses (154). These arrhythmic events tend to correlate with a lower left ventricular ejection fraction at the time of presentation and a higher incidence of conduction disturbances. A majority of severe ventricular tachyarrhythmic episodes coincide with a prolonged corrected QT interval exceeding 500 ms (2). For managing acute ventricular tachycardia, the approach involves magnesium sulfate and/or a short-acting BB. If there is sustained, pulseless ventricular tachycardia, direct current cardioversion might be necessary. Given the elevated prevalence of prolonged QT intervals in acute-phase TTS patients, the use of drugs that could potentially prolong the QT interval should be avoided or immediately discontinued, and the assessment of amiodarone or sotalol usage should be considered on a case-by-case basis. In cases where torsades de pointes with QT interval prolongation emerge associated with bradycardia, temporary pacing should be considered. Persistent high-grade atrio-ventricular block warrants permanent pacemaker implantation. Interestingly, during the acute phase, ventricular arrhythmias are observed in TTS patients, without subsequent recurrence among survivors of hospitalization. However, it is important to note that significant conduction disorders tend to persist during long-term follow-up (154).
TTS complicated by left ventricular thrombus
The presence of LV thrombus at the apex has been observed in some TTS patients, and it is important to exclude this diagnosis in individuals with severe LV systolic dysfunction, due to the potential risk of systemic embolization. To evaluate the presence of LV thrombus, TTE or CMR should be employed (2,155-157). The use of anticoagulation in TTS patients diagnosed with left ventricular thrombus is based on extrapolation from observational studies conducted in the AMI population (158). LV thrombus occurs in approximately 1% to 2% of TTS patients and, therefore, routine prophylactic anticoagulation is not advised (155-157). In TTS patients with LV thrombus, anticoagulation duration should be continued for at least 3 months or until LV function has resolved (155-157).
Long-term pharmacological treatment of TTS
The extended treatment for TTS entails the use of long-term heart failure therapy, including ACEI or ARBs, BB, and diuretics as needed, or until full recovery of LV function is achieved (2,127). In this way, and as some authors have rightly pointed out if the underlying condition were an irreversible cardiomyopathy rather than TTS, heart failure medication would have already been initiated as standard practice (116). Due to its specific pathophysiology and the aforementioned catecholamine surge, the interest and potential usefulness of BB in this scenario would appear evident. However, while in a large registry the use of ACEI or ARBs showed enhanced survival rates at the one-year follow-up and was additionally linked to a reduced occurrence of TTS recurrence (8), the use of BB therapy did not demonstrate any survival benefit or reduction in recurrences (159). Further investigation and validation are necessary to determine the significance of these findings, considering the low recurrence rate and conflicting results observed in other studies (160,161). It is important to emphasize that there is a lack of evidence regarding other widely used treatments for heart failure, such as sodium-glucose co-transporter 2 inhibitors or angiotensin receptor neprilysin inhibitors, which could potentially have a prognostic impact on patients with TTS (162). Additionally, for patients experiencing multiple, recurrent episodes of TTS triggered by emotional stressors or those with neuropsychiatric disorders, targeting the psychological response to emotionally stressful triggers may be a potential therapeutic approach (163). Patients with co-existing coronary artery disease or other well-established pre-existing indications should receive aspirin and statin therapy for secondary prevention (2). Antiplatelet therapy has no role in the remaining patients.
Prognosis
Once considered a harmless, self-limiting condition, TTS is currently recognized as having significant short- and long-term morbidity and mortality (3,115,164). In fact, the notion that TTS is a benign syndrome—due to the transient nature of LV dysfunction—may be actually misleading and underestimate the real risk of in-hospital complications. It is important to keep in mind that during the acute phase, TTS patients have a risk of developing hospital complications similar to those seen in patients with atherosclerotic ACS (115,116). Moreover, subclinical cardiac dysfunction has also been reported with long-lasting clinical consequences (97), and the recurrence rate is approximately 1% yearly (161,165). Conflicting reports exist about the long-term prognosis of TTS survivors. Comparisons of TTS outcomes with ACS and non-cardiovascular disease control groups show varying results, from TTS having similar all-cause mortality to the general population to outcomes as severe as AMI. These discrepancies might stem from methodological constraints, such as the absence of differentiation between in-hospital and post-discharge outcomes and the absence of appropriately representative control groups. Looi et al. recently compared post-discharge outcomes of TTS patients with matched ACS and non-CVD cohorts. Surviving TTS patients exhibited twice the mortality risk of matched controls from a contemporary population (166). However, patients with TTS prompted by emotional stress displayed a more advantageous short- and long-term prognosis (164,167). On the other hand, long-term mortality in patients with TTS triggered by medical conditions, or procedures, is three times higher compared to those elicited by emotional triggers (133,167). Along the same lines, the mortality rate of those TTS with neurological conditions was nearly sixfold greater than that seen in the remaining patients (164). While this is likely indicative of the extended-term mortality resulting from the underlying disease prognosis and linked comorbidities, additional research is indeed needed to acquire a more comprehensive insight. Of note, TTS patients present a 3% absolute 6-year risk of heart failure hospitalization, which is higher than that seen in age- and sex-matched individuals (168). Moreover, TTS patients may also develop atrial fibrillation. In this regard, previous studies have reported a prevalence of this arrhythmia ranging between 5% and 25% (169,170). Notably, TTS patients have a transient ischemic attack or stroke incidence of 1.7% per patient-year. Some authors suggest employing the CHA2DS2VASc score for improved prediction of cerebrovascular events and the initiation of suitable anticoagulation, even in the absence of atrial fibrillation (120,171).
TTS recurrence
TTS patients face the possibility of experiencing recurrences. The recurrence rate for TTS is estimated to be 1.8% per patient-year (8). Interestingly, a variable TTS pattern upon recurrence is observed in up to 20% of cases (8). However, there are conflicting results regarding potential predictors of TTS recurrence (162,172). Unfortunately, we lack evidence about the optimal treatment to prevent such recurrences. It is crucial to recognize that the triggers may also vary in cases of recurrence. Moreover, one study advocates that psychiatric and neurological conditions should be regarded as potential factors predisposing to recurrent episodes (173). Notably, a substantial portion of recurrences occurred many years following the index episode. Consequently, these findings highlight the importance of extended clinical monitoring for TTS patients and the identification of efficient prevention methods.
Gaps in knowledge and future directions
Currently, several key knowledge gaps persist in the understanding of this intriguing condition, underscoring the need for continued and deeper research. The complete elucidation of its pathophysiology remains elusive, and there is also an absence of comprehensive knowledge about the various phenotypes and their associated prognoses. The treatment approaches for both the acute phase and follow-up are still not fully established, and the specificity of acute and chronic biomarkers for diagnosing and prognosticating TTS remains uncertain. These critical aspects warrant dedicated focus through robust multicenter research initiatives. For instance, to the best of our knowledge, nowadays there are two ongoing randomized clinical trials, NACRAM (N-Acetylcysteine and Ramipril Takotsubo Syndrome Trial, ACTRN12616000781448) and BROKEN-SWEDE-HEART [Optimized Pharmacological Treatment for Broken Heart (Takotsubo) Syndrome, NCT04666454], currently investigating different treatment strategies for patients with TTS (174,175). This, therefore, underscores the need for immediate action, encompassing not only fresh research initiatives addressing these aspects but also the implementation of randomized clinical trials. These trials would assess the effectiveness of existing treatment strategies (i.e., BB) while also delving into innovative approaches that draw from historical insights and emerging pathophysiological understandings of this captivating condition.
Strengths and limitations
One of the primary strengths of this review lies in its comprehensive nature. It not only provides an up-to-date analysis of the field but also does so while retaining the context of significant prior research and consensus. This approach ensures that readers gain a holistic understanding of TTS. Furthermore, the review identifies gaps in knowledge and outlines future directions for research, thereby contributing to the ongoing advancement of our understanding in this area. While this review offers valuable insights into TTS, it is not without limitations. The primary limitation is that, despite our best efforts to encompass the most relevant research, the field of TTS is continually evolving. Thus, there may be newer findings that were not included in this review. Additionally, the assessment of the quality and impact of prior research may have a subjective element and may vary among different readers. However, every effort has been made to provide a balanced, informative, and rigorous perspective on the topic.
Conclusions
TTS is an intriguing and increasingly recognized cardiovascular condition with a distinct phenotype. It manifests clinically as transient ventricular dysfunction without culprit obstructive coronary artery disease, often closely resembling ACS. The excessive release of catecholamines in the presence of stressful triggers is believed to be a major contributing factor, although the precise underlying mechanism remains poorly understood. It is crucial to note that TTS is not a benign condition because it can be associated with substantial short and long-term adverse event rates, comparable to those seen in ACS patients. Acute treatment primarily focuses on ruling out AMI and providing supportive standard care for subsequent LV dysfunction. Existing research suggests a potential role for ACEI and ARBs in the treatment of TTS with the aim of improving long-term mortality. However, further research is necessary to provide clinicians with a deeper understanding of this elusive syndrome leading to an effective, evidence-based, specific treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-283/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-283/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-283/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ghadri JR, Wittstein IS, Prasad A, et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur Heart J 2018;39:2032-46. [Crossref] [PubMed]
- Ghadri JR, Wittstein IS, Prasad A, et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur Heart J 2018;39:2047-62. [Crossref] [PubMed]
- Singh T, Khan H, Gamble DT, et al. Takotsubo Syndrome: Pathophysiology, Emerging Concepts, and Clinical Implications. Circulation 2022;145:1002-19. [Crossref] [PubMed]
- Sato H, Tateishi H, Uchida T, et al. Tako-Tsubo–like left ventricular dysfunction due to multivessel coronary spasm. In: Kodama K, Haze K, Hori M, editors. Clinical aspect of myocardial injury: from ischemia to heart failure (in Japanese). Tokyo: Kagakuhyoronsha Publishing Co.; 1990:56-64.
- Prasad A, Dangas G, Srinivasan M, et al. Incidence and angiographic characteristics of patients with apical ballooning syndrome (takotsubo/stress cardiomyopathy) in the HORIZONS-AMI trial: an analysis from a multicenter, international study of ST-elevation myocardial infarction. Catheter Cardiovasc Interv 2014;83:343-8. [Crossref] [PubMed]
- Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol 2004;94:343-6. [Crossref] [PubMed]
- Deshmukh A, Kumar G, Pant S, et al. Prevalence of Takotsubo cardiomyopathy in the United States. Am Heart J 2012;164:66-71.e1. [Crossref] [PubMed]
- Templin C, Ghadri JR, Diekmann J, et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med 2015;373:929-38. [Crossref] [PubMed]
- Schneider B, Athanasiadis A, Stöllberger C, et al. Gender differences in the manifestation of tako-tsubo cardiomyopathy. Int J Cardiol 2013;166:584-8. [Crossref] [PubMed]
- Aizawa K, Suzuki T. Takotsubo cardiomyopathy: Japanese perspective. Heart Fail Clin 2013;9:243-7. x. [Crossref] [PubMed]
- Berton E, Vitali-Serdoz L, Vallon P, et al. Young girl with apical ballooning heart syndrome. Int J Cardiol 2012;161:e4-6. [Crossref] [PubMed]
- Otillio JK, Harris JK, Tuuri R A. 6-year-old girl with undiagnosed hemophagocytic lymphohistiocytosis and takotsubo cardiomyopathy: a case report and review of the literature. Pediatr Emerg Care 2014;30:561-5. [Crossref] [PubMed]
- Nascimento FO, Larrauri-Reyes MC, Santana O, et al. Comparison of stress cardiomyopathy in Hispanic and non-Hispanic patients. Rev Esp Cardiol (Engl Ed) 2013;66:67-8. [Crossref] [PubMed]
- Regnante RA, Zuzek RW, Weinsier SB, et al. Clinical characteristics and four-year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol 2009;103:1015-9. [Crossref] [PubMed]
- Imori Y, Kato K, Cammann VL, et al. Ethnic comparison in takotsubo syndrome: novel insights from the International Takotsubo Registry. Clin Res Cardiol 2022;111:186-96. [Crossref] [PubMed]
- Franco E, Dias A, Koshkelashvili N, et al. Distinctive Electrocardiographic Features in African Americans Diagnosed with Takotsubo Cardiomyopathy. Ann Noninvasive Electrocardiol 2016;21:486-92. [Crossref] [PubMed]
- Núñez-Gil IJ, Vedia O, Almendro-Delia M, et al. Erratum to "Takotsubo syndrome and cancer, clinical and prognostic implications, insights of RETAKO" [Med Clin (Barc). 2020;155(12):521-528]. Med Clin (Barc) 2022;159:155. Erratum for Med Clin (Barc) 2020;155:521-8. [PubMed]
- Cammann VL, Sarcon A, Ding KJ, et al. Clinical Features and Outcomes of Patients With Malignancy and Takotsubo Syndrome: Observations From the International Takotsubo Registry. J Am Heart Assoc 2019;8:e010881. [Crossref] [PubMed]
- Summers MR, Dib C, Prasad A. Chronobiology of Tako-tsubo cardiomyopathy (apical ballooning syndrome). J Am Geriatr Soc 2010;58:805-6. [Crossref] [PubMed]
- Manfredini R, Citro R, Previtali M, et al. Summer preference in the occurrence of takotsubo cardiomyopathy is independent of age. J Am Geriatr Soc 2009;57:1509-11. [Crossref] [PubMed]
- Song BG, Oh JH, Kim HJ, et al. Chronobiological variation in the occurrence of Tako-tsubo cardiomyopathy: experiences of two tertiary cardiovascular centers. Heart Lung 2013;42:40-7. [Crossref] [PubMed]
- Looi JL, Lee M, Grey C, et al. Seasonal variation in Takotsubo syndrome compared with myocardial infarction: ANZACS-QI 16. N Z Med J 2018;131:21-9. [PubMed]
- Lyon AR, Citro R, Schneider B, et al. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;77:902-21. [Crossref] [PubMed]
- Gutiérrez-Barrios A, Rivero F, Noval-Morillas I, et al. Feasibility of absolute coronary blood flow and microvascular resistance quantification in tako-tsubo cardiomyopathy. Rev Esp Cardiol (Engl Ed) 2020;73:94-5. [Crossref] [PubMed]
- Rivero F, Cuesta J, García-Guimaraes M, et al. Time-Related Microcirculatory Dysfunction in Patients With Takotsubo Cardiomyopathy. JAMA Cardiol 2017;2:699-700. [Crossref] [PubMed]
- Alfonso F, Núñez-Gil IJ, Hernández R. Optical coherence tomography findings in Tako-Tsubo cardiomyopathy. Circulation 2012;126:1663-4. [Crossref] [PubMed]
- Akashi YJ, Nef HM, Lyon AR. Epidemiology and pathophysiology of Takotsubo syndrome. Nat Rev Cardiol 2015;12:387-97. [Crossref] [PubMed]
- Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539-48. [Crossref] [PubMed]
- Núñez-Gil IJ, Bernardo E, Feltes G, et al. Platelet function in Takotsubo cardiomyopathy. J Thromb Thrombolysis 2015;39:452-8. [Crossref] [PubMed]
- Madias JE. Blood norepinephrine/epinephrine/dopamine measurements in 108 patients with takotsubo syndrome from the world literature: pathophysiological implications. Acta Cardiol 2021;76:1083-91. [Crossref] [PubMed]
- Kume T, Kawamoto T, Okura H, et al. Local release of catecholamines from the hearts of patients with tako-tsubo-like left ventricular dysfunction. Circ J 2008;72:106-8. [Crossref] [PubMed]
- Abraham J, Mudd JO, Kapur NK, et al. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol 2009;53:1320-5. [Crossref] [PubMed]
- Ueyama T, Kasamatsu K, Hano T, et al. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of 'tako-tsubo' cardiomyopathy. Circ J 2002;66:712-3. [Crossref] [PubMed]
- Redfors B, Ali A, Shao Y, et al. Different catecholamines induce different patterns of takotsubo-like cardiac dysfunction in an apparently afterload dependent manner. Int J Cardiol 2014;174:330-6. [Crossref] [PubMed]
- Vaccaro A, Despas F, Delmas C, et al. Direct evidences for sympathetic hyperactivity and baroreflex impairment in Tako Tsubo cardiopathy. PLoS One 2014;9:e93278. [Crossref] [PubMed]
- Burgdorf C, von Hof K, Schunkert H, et al. Regional alterations in myocardial sympathetic innervation in patients with transient left-ventricular apical ballooning (Tako-Tsubo cardiomyopathy). J Nucl Cardiol 2008;15:65-72. [Crossref] [PubMed]
- Verberne HJ, van der Heijden DJ, van Eck-Smit BL, et al. Persisting myocardial sympathetic dysfunction in takotsubo cardiomyopathy. J Nucl Cardiol 2009;16:321-4. [Crossref] [PubMed]
- Zaroff JG, Rordorf GA, Ogilvy CS, et al. Regional patterns of left ventricular systolic dysfunction after subarachnoid hemorrhage: evidence for neurally mediated cardiac injury. J Am Soc Echocardiogr 2000;13:774-9. [Crossref] [PubMed]
- Lyon AR, Rees PS, Prasad S, et al. Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med 2008;5:22-9. [Crossref] [PubMed]
- Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation 2012;126:697-706. [Crossref] [PubMed]
- Spieker LE, Hürlimann D, Ruschitzka F, et al. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation 2002;105:2817-20. [Crossref] [PubMed]
- Scantlebury DC, Prasad A, Rabinstein AA, et al. Prevalence of migraine and Raynaud phenomenon in women with apical ballooning syndrome (Takotsubo or stress cardiomyopathy). Am J Cardiol 2013;111:1284-8. [Crossref] [PubMed]
- Vasilieva E, Vorobyeva I, Lebedeva A, et al. Brachial artery flow-mediated dilation in patients with Tako-tsubo cardiomyopathy. Am J Med 2011;124:1176-9. [Crossref] [PubMed]
- Naegele M, Flammer AJ, Enseleit F, et al. Endothelial function and sympathetic nervous system activity in patients with Takotsubo syndrome. Int J Cardiol 2016;224:226-30. [Crossref] [PubMed]
- Angelini P, Uribe C, Tobis JM. Pathophysiology of Takotsubo Cardiomyopathy: Reopened Debate. Tex Heart Inst J 2021;48:e207490. [Crossref] [PubMed]
- Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001;38:11-8. [Crossref] [PubMed]
- Migliore F, Maffei E, Perazzolo Marra M, et al. LAD coronary artery myocardial bridging and apical ballooning syndrome. JACC Cardiovasc Imaging 2013;6:32-41. [Crossref] [PubMed]
- Stiermaier T, Desch S, Blazek S, et al. Frequency and significance of myocardial bridging and recurrent segment of the left anterior descending coronary artery in patients with takotsubo cardiomyopathy. Am J Cardiol 2014;114:1204-9. [Crossref] [PubMed]
- Eitel I, Stiermaier T, Graf T, et al. Optical Coherence Tomography to Evaluate Plaque Burden and Morphology in Patients With Takotsubo Syndrome. J Am Heart Assoc 2016;5:e004474. [Crossref] [PubMed]
- Ibáñez B, Navarro F, Farré J, et al. Asociación del síndrome take-tsubo con la arteria coronaria descendente anterior con extensa distribución por el segmento diafragmático Rev Esp Cardiol 2004;57:209-16. [Tako-tsubo syndrome associated with a long course of the left anterior descending coronary artery along the apical diaphragmatic surface of the left ventricle]. [Crossref] [PubMed]
- To AC, Kay P, Khan AA, et al. Coronary artery anatomy and apical sparing in apical ballooning syndrome: implications for diagnosis and aetiology. Heart Lung Circ 2010;19:219-24. [Crossref] [PubMed]
- To AC, Khan AA, Kay P, et al. Resting systolic anterior motion of mitral valve apparatus: association with apical ballooning syndrome. Circ Heart Fail 2008;1:84-5. [Crossref] [PubMed]
- Merli E, Sutcliffe S, Gori M, et al. Tako-Tsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echocardiogr 2006;7:53-61. [Crossref] [PubMed]
- Akashi YJ, Goldstein DS, Barbaro G, et al. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008;118:2754-62. [Crossref] [PubMed]
- Looi JL, Gabriel R, Khan A, et al. Left ventricular morphology and response to beta-adrenergic stimulation in apical ballooning syndrome. Eur Heart J Cardiovasc Imaging 2012;13:510-6. [Crossref] [PubMed]
- Ibanez B, Navarro F, Cordoba M, et al. Tako-tsubo transient left ventricular apical ballooning: is intravascular ultrasound the key to resolve the enigma? Heart 2005;91:102-4. [Crossref] [PubMed]
- Alfonso F, Cárdenas A, Ibáñez B. Mid-ventricular Tako-Tsubo cardiomyopathy with structurally normal coronary arteries confirmed by optical coherence tomography. J Invasive Cardiol 2013;25:E214-5. [PubMed]
- Haghi D, Roehm S, Hamm K, et al. Takotsubo cardiomyopathy is not due to plaque rupture: an intravascular ultrasound study. Clin Cardiol 2010;33:307-10. [Crossref] [PubMed]
- Vitale C, Rosano GM, Kaski JC. Role of Coronary Microvascular Dysfunction in Takotsubo Cardiomyopathy. Circ J 2016;80:299-305. [Crossref] [PubMed]
- Cohen RA, Shepherd JT, Vanhoutte PM. Prejunctional and postjunctional actions of endogenous norepinephrine at the sympathetic neuroeffector junction in canine coronary arteries. Circ Res 1983;52:16-25. [Crossref] [PubMed]
- Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary microcirculation in patients with takotsubo-like left ventricular dysfunction. Circ J 2005;69:934-9. [Crossref] [PubMed]
- Rigo F, Sicari R, Citro R, et al. Diffuse, marked, reversible impairment in coronary microcirculation in stress cardiomyopathy: a Doppler transthoracic echo study. Ann Med 2009;41:462-70. [Crossref] [PubMed]
- Meimoun P, Malaquin D, Sayah S, et al. The coronary flow reserve is transiently impaired in tako-tsubo cardiomyopathy: a prospective study using serial Doppler transthoracic echocardiography. J Am Soc Echocardiogr 2008;21:72-7. [Crossref] [PubMed]
- Meimoun P, Clerc J, Vincent C, et al. Non-invasive detection of tako-tsubo cardiomyopathy vs. acute anterior myocardial infarction by transthoracic Doppler echocardiography. Eur Heart J Cardiovasc Imaging 2013;14:464-70. [Crossref] [PubMed]
- Kurisu S, Inoue I, Kawagoe T, et al. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J Am Coll Cardiol 2003;41:743-8. [Crossref] [PubMed]
- Khalid N, Iqbal I, Coram R, et al. Thrombolysis In Myocardial Infarction Frame Count in Takotsubo Cardiomyopathy. Int J Cardiol 2015;191:107-8. [Crossref] [PubMed]
- Galiuto L, De Caterina AR, Porfidia A, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J 2010;31:1319-27. [Crossref] [PubMed]
- Patel SM, Lerman A, Lennon RJ, et al. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy). Eur Heart J Acute Cardiovasc Care 2013;2:147-52. [Crossref] [PubMed]
- Martin EA, Prasad A, Rihal CS, et al. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol 2010;56:1840-6. [Crossref] [PubMed]
- Dong F, Yin L, Sisakian H, et al. Takotsubo syndrome is a coronary microvascular disease: experimental evidence. Eur Heart J 2023;44:2244-53. [Crossref] [PubMed]
- Ueyama T, Kasamatsu K, Hano T, et al. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann N Y Acad Sci 2008;1148:479-85. [Crossref] [PubMed]
- Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res 2002;53:597-604. [Crossref] [PubMed]
- Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab 1999;84:606-10. [Crossref] [PubMed]
- Sung BH, Ching M, Izzo JL Jr, et al. Estrogen improves abnormal norepinephrine-induced vasoconstriction in postmenopausal women. J Hypertens 1999;17:523-8. [Crossref] [PubMed]
- Ueyama T, Hano T, Kasamatsu K, et al. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J Cardiovasc Pharmacol 2003;42:S117-9. [Crossref] [PubMed]
- Ueyama T, Ishikura F, Matsuda A, et al. Chronic estrogen supplementation following ovariectomy improves the emotional stress-induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ J 2007;71:565-73. [Crossref] [PubMed]
- Brenner R, Weilenmann D, Maeder MT, et al. Clinical characteristics, sex hormones, and long-term follow-up in Swiss postmenopausal women presenting with Takotsubo cardiomyopathy. Clin Cardiol 2012;35:340-7. [Crossref] [PubMed]
- Salmoirago-Blotcher E, Dunsiger S, Swales HH, et al. Reproductive History of Women With Takotsubo Cardiomyopathy. Am J Cardiol 2016;118:1922-8. [Crossref] [PubMed]
- Vriz O, Minisini R, Citro R, et al. Analysis of beta1 and beta2-adrenergic receptors polymorphism in patients with apical ballooning cardiomyopathy. Acta Cardiol 2011;66:787-90. [Crossref] [PubMed]
- Zaroff JG, Pawlikowska L, Miss JC, et al. Adrenoceptor polymorphisms and the risk of cardiac injury and dysfunction after subarachnoid hemorrhage. Stroke 2006;37:1680-5. [Crossref] [PubMed]
- Sharkey SW, Maron BJ, Nelson P, et al. Adrenergic receptor polymorphisms in patients with stress (tako-tsubo) cardiomyopathy. J Cardiol 2009;53:53-7. [Crossref] [PubMed]
- Spinelli L, Trimarco V, Di Marino S, et al. L41Q polymorphism of the G protein coupled receptor kinase 5 is associated with left ventricular apical ballooning syndrome. Eur J Heart Fail 2010;12:13-6. [Crossref] [PubMed]
- Figtree GA, Bagnall RD, Abdulla I, et al. No association of G-protein-coupled receptor kinase 5 or β-adrenergic receptor polymorphisms with Takotsubo cardiomyopathy in a large Australian cohort. Eur J Heart Fail 2013;15:730-3. [Crossref] [PubMed]
- Novo G, Giambanco S, Guglielmo M, et al. G-protein-coupled receptor kinase 5 polymorphism and Takotsubo cardiomyopathy. J Cardiovasc Med (Hagerstown) 2015;16:639-43. [Crossref] [PubMed]
- Eitel I, Moeller C, Munz M, et al. Genome-wide association study in takotsubo syndrome - Preliminary results and future directions. Int J Cardiol 2017;236:335-9. [Crossref] [PubMed]
- Couch LS, Fiedler J, Chick G, et al. Circulating microRNAs predispose to takotsubo syndrome following high-dose adrenaline exposure. Cardiovasc Res 2022;118:1758-70. [Crossref] [PubMed]
- Jaguszewski M, Osipova J, Ghadri JR, et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J 2014;35:999-1006. [Crossref] [PubMed]
- Baudry A, Mouillet-Richard S, Schneider B, et al. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 2010;329:1537-41. [Crossref] [PubMed]
- Rinaldi A, Vincenti S, De Vito F, et al. Stress induces region specific alterations in microRNAs expression in mice. Behav Brain Res 2010;208:265-9. [Crossref] [PubMed]
- Ancona F, Bertoldi LF, Ruggieri F, et al. Takotsubo cardiomyopathy and neurogenic stunned myocardium: similar albeit different. Eur Heart J 2016;37:2830-2. [Crossref] [PubMed]
- Templin C, Hänggi J, Klein C, et al. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur Heart J 2019;40:1183-7. [Crossref] [PubMed]
- Radfar A, Abohashem S, Osborne MT, et al. Stress-associated neurobiological activity associates with the risk for and timing of subsequent Takotsubo syndrome. Eur Heart J 2021;42:1898-908. [Crossref] [PubMed]
- Scally C, Abbas H, Ahearn T, et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019;139:1581-92. [Crossref] [PubMed]
- Godsman N, Kohlhaas M, Nickel A, et al. Metabolic alterations in a rat model of takotsubo syndrome. Cardiovasc Res 2022;118:1932-46. [Crossref] [PubMed]
- Nuñez-Gil IJ, Andrés M, Benito B, et al. Serum Metabolomic Analysis Suggests Impairment of Myocardial Energy Production in Takotsubo Syndrome. Metabolites 2021;11:439. [Crossref] [PubMed]
- Dawson DK, Neil CJ, Henning A, et al. Tako-Tsubo Cardiomyopathy: A Heart Stressed Out of Energy? JACC Cardiovasc Imaging 2015;8:985-7. [Crossref] [PubMed]
- Scally C, Rudd A, Mezincescu A, et al. Persistent Long-Term Structural, Functional, and Metabolic Changes After Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2018;137:1039-48. [Crossref] [PubMed]
- Neil C, Nguyen TH, Kucia A, et al. Slowly resolving global myocardial inflammation/oedema in Tako-Tsubo cardiomyopathy: evidence from T2-weighted cardiac MRI. Heart 2012;98:1278-84. [Crossref] [PubMed]
- Wilson HM, Cheyne L, Brown PAJ, et al. Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy. JACC Basic Transl Sci 2018;3:766-78. [Crossref] [PubMed]
- Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2016;18:8-27. [Crossref] [PubMed]
- Núñez-Gil IJ, Almendro-Delia M, Andrés M, et al. Secondary forms of Takotsubo cardiomyopathy: A whole different prognosis. Eur Heart J Acute Cardiovasc Care 2016;5:308-16. [Crossref] [PubMed]
- De Angelis E, Bochaton T, Ammirati E, et al. Pheochromocytoma-induced cardiogenic shock: A multicentre analysis of clinical profiles, management and outcomes. Int J Cardiol 2023;383:82-8. [Crossref] [PubMed]
- Uribarri A, Núñez-Gil IJ, Conty DA, et al. Short- and Long-Term Prognosis of Patients With Takotsubo Syndrome Based on Different Triggers: Importance of the Physical Nature. J Am Heart Assoc 2019;8:e013701. [Crossref] [PubMed]
- Cammann VL, Scheitz JF, von Rennenberg R, et al. Clinical correlates and prognostic impact of neurologic disorders in Takotsubo syndrome. Sci Rep 2021;11:23555. [Crossref] [PubMed]
- Bridgman PG, Chan CW. The fifth takotsubo variant. Echocardiography 2017;34:122-3. [Crossref] [PubMed]
- Angelini P. Reverse, or inverted, transient Takotsubo cardiomyopathy: terms and status of an open discussion. Tex Heart Inst J 2013;40:60-3. [PubMed]
- Elgendy AY, Elgendy IY, Mansoor H, et al. Clinical presentations and outcomes of Takotsubo syndrome in the setting of subarachnoid hemorrhage: A systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care 2018;7:236-45. [Crossref] [PubMed]
- Kagiyama N, Okura H, Kume T, et al. Isolated right ventricular takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging 2015;16:285. [Crossref] [PubMed]
- Ghadri JR, Cammann VL, Napp LC, et al. Differences in the Clinical Profile and Outcomes of Typical and Atypical Takotsubo Syndrome: Data From the International Takotsubo Registry. JAMA Cardiol 2016;1:335-40. [Crossref] [PubMed]
- Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408-17. [Crossref] [PubMed]
- Ghadri JR, Cammann VL, Jurisic S, et al. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur J Heart Fail 2017;19:1036-42. [Crossref] [PubMed]
- Yoshikawa T. Takotsubo cardiomyopathy, a new concept of cardiomyopathy: clinical features and pathophysiology. Int J Cardiol 2015;182:297-303. [Crossref] [PubMed]
- Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523-9. [Crossref] [PubMed]
- Hurst RT, Prasad A, Askew JW 3rd, et al. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging 2010;3:641-9. [Crossref] [PubMed]
- Salamanca J, García-Guimaraes M, Camacho-Freire SJ, et al. Spontaneous coronary artery dissection and Takotsubo syndrome: comparison of baseline clinical and angiographic characteristics and in-hospital outcomes. Coron Artery Dis 2021;32:509-16. [Crossref] [PubMed]
- Assad J, Femia G, Pender P, et al. Takotsubo Syndrome: A Review of Presentation, Diagnosis and Management. Clin Med Insights Cardiol 2022;16:11795468211065782. [Crossref] [PubMed]
- Qaqa A, Daoko J, Jallad N, et al. Takotsubo Syndrome in African American vs. Non-African American Women. West J Emerg Med 2011;12:218-23. [PubMed]
- Kosuge M, Ebina T, Hibi K, et al. Simple and accurate electrocardiographic criteria to differentiate takotsubo cardiomyopathy from anterior acute myocardial infarction. J Am Coll Cardiol 2010;55:2514-6. [Crossref] [PubMed]
- Looi JL, Wong CW, Lee M, et al. Usefulness of ECG to differentiate Takotsubo cardiomyopathy from acute coronary syndrome. Int J Cardiol 2015;199:132-40. [Crossref] [PubMed]
- Citro R, Lyon AR, Meimoun P, et al. Standard and advanced echocardiography in takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr 2015;28:57-74. [Crossref] [PubMed]
- de Gregorio C, Grimaldi P, Lentini C. Left ventricular thrombus formation and cardioembolic complications in patients with Takotsubo-like syndrome: a systematic review. Int J Cardiol 2008;131:18-24. [Crossref] [PubMed]
- Izumo M, Nalawadi S, Shiota M, et al. Mechanisms of acute mitral regurgitation in patients with takotsubo cardiomyopathy: an echocardiographic study. Circ Cardiovasc Imaging 2011;4:392-8. [Crossref] [PubMed]
- Peters MN, George P, Irimpen AM. The broken heart syndrome: Takotsubo cardiomyopathy. Trends Cardiovasc Med 2015;25:351-7. [Crossref] [PubMed]
- Akashi YJ, Tejima T, Sakurada H, et al. Left ventricular rupture associated with Takotsubo cardiomyopathy. Mayo Clin Proc 2004;79:821-4. [Crossref] [PubMed]
- Citro R, Okura H, Ghadri JR, et al. Multimodality imaging in takotsubo syndrome: a joint consensus document of the European Association of Cardiovascular Imaging (EACVI) and the Japanese Society of Echocardiography (JSE). Eur Heart J Cardiovasc Imaging 2020;21:1184-207. [Crossref] [PubMed]
- Iannaccone G, Graziani F, Del Buono MG, et al. Left atrial strain analysis improves left ventricular filling pressures non-invasive estimation in the acute phase of Takotsubo syndrome. Eur Heart J Cardiovasc Imaging 2023;24:699-707. [Crossref] [PubMed]
- Dias A, Núñez Gil IJ, Santoro F, et al. Takotsubo syndrome: State-of-the-art review by an expert panel - Part 2. Cardiovasc Revasc Med 2019;20:153-66. [Crossref] [PubMed]
- Kumar S, Chu M, Tu S, et al. Physiologic and compositional coronary artery disease extension in patients with takotsubo syndrome assessed using artificial intelligence: an optical coherence tomography study. Coron Artery Dis 2022;33:349-53. [Crossref] [PubMed]
- Jiménez-Méndez C, Cecconi A, Vera A, et al. Concomitant acute myocardial infarction and stress cardiomyopathy. Coron Artery Dis 2021;32:261-2. [Crossref] [PubMed]
- Montone RA, Galiuto L, Meucci MC, et al. Coronary slow flow is associated with a worse clinical outcome in patients with Takotsubo syndrome. Heart 2020;106:923-30. [Crossref] [PubMed]
- De Backer O, Debonnaire P, Gevaert S, et al. Prevalence, associated factors and management implications of left ventricular outflow tract obstruction in takotsubo cardiomyopathy: a two-year, two-center experience. BMC Cardiovasc Disord 2014;14:147. [Crossref] [PubMed]
- Del Buono MG, Montone RA, Meucci MC, et al. Left ventricular end-diastolic pressure predicts in-hospital outcomes in takotsubo syndrome. Eur Heart J Acute Cardiovasc Care 2021;10:661-7. [Crossref] [PubMed]
- Messas N, Trimaille A, Marchandot B, et al. Left ventricular mechanics in the acute phase of Takotsubo cardiomyopathy: distinctive ballooning patterns translate into different diastolic properties. Heart Vessels 2020;35:537-43. [Crossref] [PubMed]
- Medeiros K, O'Connor MJ, Baicu CF, et al. Systolic and diastolic mechanics in stress cardiomyopathy. Circulation 2014;129:1659-67. [Crossref] [PubMed]
- Stiermaier T, Reil JC, Sequeira V, et al. Hemodynamic Assessment in Takotsubo Syndrome. J Am Coll Cardiol 2023;81:1979-91. [Crossref] [PubMed]
- Salamanca J, Alfonso F. Novel Hemodynamic Insights in Takotsubo Syndrome. J Am Coll Cardiol 2023;81:1992-5. [Crossref] [PubMed]
- Eitel I, Behrendt F, Schindler K, et al. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J 2008;29:2651-9. [Crossref] [PubMed]
- Salamanca J, Cecconi A, Pozo E, et al. Temporal Resolution Pattern of Myocardial Edema in Patients With Takotsubo Syndrome. J Card Fail 2018;24:345-6. [Crossref] [PubMed]
- Lyon AR, Akashi YJ. Use of cardiac MRI to diagnose Takotsubo syndrome. Nat Rev Cardiol 2015;12:669. [Crossref] [PubMed]
- Bratis K. Cardiac Magnetic Resonance in Takotsubo Syndrome. Eur Cardiol 2017;12:58-62. [Crossref] [PubMed]
- Gunasekara MY, Mezincescu AM, Dawson DK. An update on cardiac magnetic resonance imaging in takotsubo cardiomyopathy. Curr Cardiovasc Imaging Rep 2020;13:17. [Crossref]
- Rolf A, Nef HM, Möllmann H, et al. Immunohistological basis of the late gadolinium enhancement phenomenon in tako-tsubo cardiomyopathy. Eur Heart J 2009;30:1635-42. [Crossref] [PubMed]
- Avegliano G, Huguet M, Costabel JP, et al. Morphologic pattern of late gadolinium enhancement in Takotsubo cardiomyopathy detected by early cardiovascular magnetic resonance. Clin Cardiol 2011;34:178-82. [Crossref] [PubMed]
- Nakamori S, Matsuoka K, Onishi K, et al. Prevalence and signal characteristics of late gadolinium enhancement on contrast-enhanced magnetic resonance imaging in patients with takotsubo cardiomyopathy. Circ J 2012;76:914-21. [Crossref] [PubMed]
- Naruse Y, Sato A, Kasahara K, et al. The clinical impact of late gadolinium enhancement in Takotsubo cardiomyopathy: serial analysis of cardiovascular magnetic resonance images. J Cardiovasc Magn Reson 2011;13:67. [Crossref] [PubMed]
- Sörensson P, Ekenbäck C, Lundin M, et al. Early Comprehensive Cardiovascular Magnetic Resonance Imaging in Patients With Myocardial Infarction With Nonobstructive Coronary Arteries. JACC Cardiovasc Imaging 2021;14:1774-83. [Crossref] [PubMed]
- Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 2011;306:277-86. [Crossref] [PubMed]
- Eitel I, Desch S, de Waha S, et al. Long-term prognostic value of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. Heart 2011;97:2038-45. [Crossref] [PubMed]
- Shetty S, Malik AH, Jayanna MB, et al. Impact of right ventricular impairment on morbidity and mortality in takotsubo syndrome-a meta-analysis of observational trials. Heart Fail Rev 2022;27:263-70. [Crossref] [PubMed]
- Almendro-Delia M, Núñez-Gil IJ, Lobo M, et al. Short- and Long-Term Prognostic Relevance of Cardiogenic Shock in Takotsubo Syndrome: Results From the RETAKO Registry. JACC Heart Fail 2018;6:928-36. [Crossref] [PubMed]
- Santoro F, Ieva R, Ferraretti A, et al. Safety and feasibility of levosimendan administration in takotsubo cardiomyopathy: a case series. Cardiovasc Ther 2013;31:e133-7. [Crossref] [PubMed]
- Napp LC, Westenfeld R, Møller JE, et al. Impella Mechanical Circulatory Support for Takotsubo Syndrome With Shock: A Retrospective Multicenter Analysis. Cardiovasc Revasc Med 2022;40:113-9. [Crossref] [PubMed]
- Mariani S, Richter J, Pappalardo F, et al. Mechanical circulatory support for Takotsubo syndrome: a systematic review and meta-analysis. Int J Cardiol 2020;316:31-9. [Crossref] [PubMed]
- Jesel L, Berthon C, Messas N, et al. Ventricular arrhythmias and sudden cardiac arrest in Takotsubo cardiomyopathy: Incidence, predictive factors, and clinical implications. Heart Rhythm 2018;15:1171-8. Erratum in: Heart Rhythm 2019;16:1598-9. [Crossref] [PubMed]
- Barrera-Ramirez CF, Jimenez-Mazuecos JM, Alfonso F. Apical thrombus associated with left ventricular apical ballooning. Heart 2003;89:927. [Crossref] [PubMed]
- Ding KJ, Cammann VL, Szawan KA, et al. Intraventricular Thrombus Formation and Embolism in Takotsubo Syndrome: Insights From the International Takotsubo Registry. Arterioscler Thromb Vasc Biol 2020;40:279-87. [Crossref] [PubMed]
- Santoro F, Stiermaier T, Tarantino N, et al. Left Ventricular Thrombi in Takotsubo Syndrome: Incidence, Predictors, and Management: Results From the GEIST (German Italian Stress Cardiomyopathy) Registry. J Am Heart Assoc 2017;6:e006990. [Crossref] [PubMed]
- Camaj A, Fuster V, Giustino G, et al. Left Ventricular Thrombus Following Acute Myocardial Infarction: JACC State-of-the-Art Review. J Am Coll Cardiol 2022;79:1010-22. [Crossref] [PubMed]
- Raposeiras-Roubín S, Núñez-Gil IJ, Jamhour K, et al. Long-term prognostic impact of beta-blockers in patients with Takotsubo syndrome: Results from the RETAKO Registry. Rev Port Cardiol 2023;42:237-46. [Crossref] [PubMed]
- Kim H, Senecal C, Lewis B, et al. Natural history and predictors of mortality of patients with Takotsubo syndrome. Int J Cardiol 2018;267:22-7. [Crossref] [PubMed]
- Lau C, Chiu S, Nayak R, et al. Survival and risk of recurrence of takotsubo syndrome. Heart 2021;107:1160-6. [Crossref] [PubMed]
- Ali A, Redfors B, Alkhoury J, et al. Sacubitril/valsartan decreases mortality in the rat model of the isoprenaline-induced takotsubo-like syndrome. ESC Heart Fail 2021;8:4130-8. [Crossref] [PubMed]
- Kato K, Lyon AR, Ghadri JR, et al. Takotsubo syndrome: aetiology, presentation and treatment. Heart 2017;103:1461-9. [Crossref] [PubMed]
- Ghadri JR, Kato K, Cammann VL, et al. Long-Term Prognosis of Patients With Takotsubo Syndrome. J Am Coll Cardiol 2018;72:874-82. [Crossref] [PubMed]
- Pelliccia F, Pasceri V, Patti G, et al. Long-Term Prognosis and Outcome Predictors in Takotsubo Syndrome: A Systematic Review and Meta-Regression Study. JACC Heart Fail 2019;7:143-54. [Crossref] [PubMed]
- Looi JL, Lee M, Webster MWI, et al. Postdischarge outcome after Takotsubo syndrome compared with patients post-ACS and those without prior CVD: ANZACS-QI 19. Open Heart 2018;5:e000918. [Crossref] [PubMed]
- Looi JL, Verryt T, McLeod P, et al. Type of Stressor and Medium-Term Outcomes After Takotsubo Syndrome: What Becomes of the Broken Hearted? (ANZACS-QI 59). Heart Lung Circ 2022;31:499-507. [Crossref] [PubMed]
- Butt JH, Bang LE, Rørth R, et al. Long-term Risk of Death and Hospitalization in Patients With Heart Failure and Takotsubo Syndrome: Insights From a Nationwide Cohort. J Card Fail 2022;28:1534-44. [Crossref] [PubMed]
- Stiermaier T, Santoro F, Eitel C, et al. Prevalence and prognostic relevance of atrial fibrillation in patients with Takotsubo syndrome. Int J Cardiol 2017;245:156-61. [Crossref] [PubMed]
- El-Battrawy I, Cammann VL, Kato K, et al. Impact of Atrial Fibrillation on Outcome in Takotsubo Syndrome: Data From the International Takotsubo Registry. J Am Heart Assoc 2021;10:e014059. [Crossref] [PubMed]
- Parodi G, Scudiero F, Citro R, et al. Risk Stratification Using the CHA(2)DS(2)-VASc Score in Takotsubo Syndrome: Data From the Takotsubo Italian Network. J Am Heart Assoc 2017;6:e006065. [Crossref] [PubMed]
- El-Battrawy I, Santoro F, Stiermaier T, et al. Incidence and Clinical Impact of Recurrent Takotsubo Syndrome: Results From the GEIST Registry. J Am Heart Assoc 2019;8:e010753. [Crossref] [PubMed]
- Kato K, Di Vece D, Cammann VL, et al. Takotsubo Recurrence: Morphological Types and Triggers and Identification of Risk Factors. J Am Coll Cardiol 2019;73:982-4. [Crossref] [PubMed]
- Ong GJ, Nguyen TH, Stansborough J, et al. The N-AcetylCysteine and RAMipril in Takotsubo Syndrome Trial (NACRAM): Rationale and design of a randomised controlled trial of sequential N-Acetylcysteine and ramipril for the management of Takotsubo Syndrome. Contemp Clin Trials 2020;90:105894. [Crossref] [PubMed]
- Omerovic E, James S, Erlinge D, et al. Rationale and design of BROKEN-SWEDEHEART: a registry-based, randomized, parallel, open-label multicenter trial to test pharmacological treatments for broken heart (takotsubo) syndrome. Am Heart J 2023;257:33-40. [Crossref] [PubMed]


