Effects of percutaneous closure of atrial septal defects via the right internal jugular vein
Highlight box
Key findings
• Percutaneous closure of atrial septal defects (ASDs) via the right internal jugular vein (RIJV) is a safe and effective therapeutic approach. It is indicated for various cases and presents several advantages, particularly in young patient. The short-term clinical results are satisfactory.
What is known and what is new?
• Percutaneous ASD closure is the preferred treatment for patients with suitable ASDs anatomy. The safety and effectiveness of transcatheter closure have been established.
• Reports on transesophageal echocardiography-guided percutaneous closure of ASDs via the RIJV are limited.
What is the implication, and what should change now?
• The aim of this study is to discuss a safe and effective therapeutic approach of percutaneous trans-jugular vein closure of ASDs.
Introduction
Atrial septal defects (ASDs) are among the most common congenital heart defects. Transcatheter femoral closure has been introduced as a treatment option for ASDs with suitable anatomy (1,2), following the satisfactory clinical results achieved by King et al. (3). Currently, this method is widely used in clinical practice (4-7). However, it carries the risk of radiation-related injury and has age requirements (8,9). Transthoracic ASD closure is a modified approach designed to circumvent age limitations and minimize radiation-related injuries. Nonetheless, this method still requires a 1–2 cm incision in the chest wall and is performed in the thoracic region. The minimal invasiveness of the procedure has been questioned (10,11). Percutaneous closure of ASD via the right internal jugular vein (RIJV) has recently gained attention as a potential solution to overcome the limitations of the aforementioned methods and has shown promising clinical outcomes (12). Although studies exist on the efficacy of transesophageal echocardiography (TEE)-guided percutaneous closure of ASD via the RIJV (13), the number of research samples is limited. Therefore, the aim of this study was to evaluate the clinical experience and early-term outcomes of patients treated with percutaneous closure of ASD via the RIJV. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-282/rc).
Methods
In this study, we conducted a cross-sectional study and analyzed the effects of TEE-guided ASD device closure via the RIJV in 103 patients with different types of ASD treated at the Second Hospital of Jilin University between July 2015 to July 2022. Demographic data and clinical information were recorded and TEE was performed for all candidates undergoing percutaneous closure. The follow-up time ranges from 3 months to 1 year. Similarly, clinical information and echocardiography data (pericardial effusion, thrombosis and residual shunt) were collected. We arranged wo independent partners to include eligible cases to avoid potential selection bias and information bias and the specialist evaluating the imaging data is trained or credentialed.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of the Second Hospital of Jilin University [No. 2022(264)] and individual consent for this retrospective analysis was waived.
Among the 103 patients included in this study, 25 were male (24.3%) and 78 were female (75.7%). The mean age of patients was 36±18 years (range, 3–74 years), and the mean BMI was 22.46±4.56 kg/m2 (range, 1.17–32.81 kg/m2).
The inclusion criteria were as follows: (I) ASD with a diameter of ≥5 mm, with a distance of ≥5 mm between the defect edge and the inferior vena cava, pulmonary vein, and mitral valve; (II) atrial septal stretch diameter greater than or equal to the diameter of the left side of the occluder; (III) no contradictions in the diagnosis based on preoperative blood biochemical examination; (IV) diagnosis of ASD made via TTE without the presence of other heart diseases requiring surgery.
The exclusion criteria were as follows: (I) infectious diseases within 2 weeks before surgery; (II) presence of a mural thrombus found in the heart cavity; (III) hemolytic disease or coagulation dysfunction; (IV) patients with severe pulmonary hypertension or Eisenmenger syndrome; (V) patients with decompensated heart failure.
Device choice
The size of the ASD was defined as the maximum diameter of the defect. The size of the occluder device (manufactured by Lifetech Scientific, Shenzhen, China) was determined based on the size of the ASD, typically being 4–6 mm larger than the maximum defect size observed on TEE.
Percutaneous closure via the RIJV was initially attempted in all patients with suitable anatomy. In cases where the RIJV approach was unsuccessful, patients were transferred for transthoracic ASD closure.
Surgical technique
Informed consent was obtained from all patients before the procedures. The patients were positioned supine and in the horizontal position. All procedures were conducted via the RIJV under general anesthesia, guided by TEE. The defect was visualized thoroughly to accurately demonstrate its anatomy.
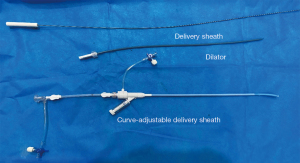
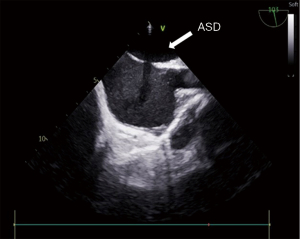
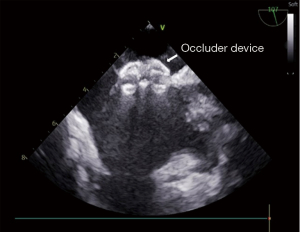
All patients received a heparin dose of 1 mg/kg. The guidewire and one Fustar curve-adjustable delivery sheath (manufactured by Lifetech Scientific, Shenzhen, China) were inserted. The pictures of device have been added as Figure 1. The guidewire was inserted via the RIJV, approximately to the distance of the right nipple. Subsequently, the guidewire was retracted, and a dilator was used to facilitate sheath insertion. The tip of the sheath was adjusted to approximately 90° to ensure its vertical positioning relative to the level of ASDs and in the middle of the ASDs. The sheath was then rotated to allow the tip to pass through the ASD site. The appropriate size of the occluder was finally selected based on the TEE results. Morphology of the ASD has been added as Figure 2. The occluder was delivered through the sheath. The left disk of the occluder was opened and fully released first. After confirming the complete adherence of the left disk to the septum, the right disk of the occluder was released. Once both disks of the occluder were fully opened, a push-and-pull test was performed to assess the stability of the occluder. TEE was used to verify the proper positioning and satisfactory shape of the occluder, ensuring no residual shunt or valve regurgitation. Morphology of the ASD occlude has been added as Figure 3. Following the removal of the delivery sheath and neutralization of heparin, the pressure was applied to the puncture site for approximately 20 min to prevent potential bleeding. The patient was then transferred to the intensive care unit (ICU) after extubation in the operating room. Postoperative routine antibiotics were administered for 24 h, and oral aspirin at a dose of 3 mg/kg was prescribed for 6 months starting from the day after the surgery.
For patients we failed via the RIJV access, we would change to a small incision in the chest.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics are presented as mean ± standard deviation (SD), and measurement data are presented as mean ± SD or n (%). Nonparametric rank sum tests were used to compare tricuspid regurgitation before and after surgery, while paired sample t-tests were used to compare left atrial (LA) size before and after surgery. A one-sided test of P<0.05 was considered to indicate statistically significant differences.
Results
Surgical procedure
Here, 97 out of 103 (94.2%) patients successfully underwent percutaneous closure of ASD via the RIJV (Table 1). The mean procedure time was 34.48±13.06 min. Postoperatively, complications occurred in four patients, comprising three patients with residual shunt and one patient with atrial fibrillation. The residual shunts disappeared in all patients during the 3-month follow-up. One patient developed postoperative atrial fibrillation, but the rhythm returned to normal sinus rhythm with medication. No new cases of pulmonary or systemic vein flow obstructions, aortic erosion, left ventricular dysfunction, or complete atrioventricular block were reported during the follow-up period. In six patients, percutaneous closure of ASD via the RIJV was unsuccessful. Five of these patients were transferred for trans-thoracic ASD closure, which yielded satisfactory results, and one patient refused further surgical treatment. None of the patients required a thoracotomy or a full median sternotomy. The arrangements of the procedure in the operation room are shown in Figure 4.
Table 1
| Characteristics | Mean ± SD or n (%) |
|---|---|
| Gender | |
| Male | 25 (24.3) |
| Female | 78 (75.7) |
| BMI (kg/m2) | 22.46±4.56 |
| Age (years) | 36±18 |
| Defect diameter (mm) | 15.45±5.82 |
| Device size (mm) | 21.92±5.74 |
| Left ventricular ejection fraction (%) | 65.95±5.73 |
| Rims | |
| Good | 90 (87.4) |
| Deficient | 13 (12.6) |
| Single-hole type | 99 (96.1) |
| Porous type | 4 (3.9) |
| Tricuspid regurgitation | 3.07±1.79 |
| Size of LA (mm) | 30.93±5.12 |
BMI, body mass Index; LA, left atrium; SD, standard deviation.

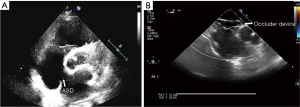
In our series, aortic rim deficiency was observed in 10 instances (9.7%). Nine out of 10 (90%) patients successfully underwent percutaneous closure of ASD via the RIJV. The preoperative and postoperative echocardiography images are shown in Figure 5A,5B.
Postoperative data
The procedure characteristics and outcome of percutaneous closure of ASD via the RIJV are presented in Table 2.
Table 2
| Characteristics | Mean ± SD or n (%) |
|---|---|
| Procedure time (min) | 34.48±13.06 |
| Duration of postoperative stay in the ICU (h) | 13.71±16.89 |
| Duration of postoperative hospitalization (day) | 3.29±1.45 |
| Complications | |
| Residual shunt | 3 (2.9) |
| Atrial fibrillation | 1 (1.0) |
ICU, intensive care unit; SD, standard deviation.
No occurrences of 30-day mortality were observed. The mean postoperative stay in the ICU was 13.71±16.89 h (range, 0–144 h). No blood loss occurred during the operation, and blood transfusion was not required.
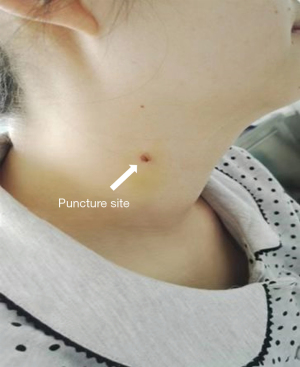
The mean postoperative hospitalization (from surgical intervention to discharge) was 3.29±1.45 days. All patients had no noticeable surgical scar, and they expressed satisfaction with the surgical wound.
All patients were discharged in good clinical condition without any extracardiac organ impairment or limitations to physical activity. The puncture site all recovered and patients are satisfied with the scar (Figure 6).
Echocardiography data
Tricuspid valve regurgitation significantly decreased (P<0.001) before and after surgery. Additionally, the size of the left atrium decreased (P=0.003) compared with the preoperative size (Table 3). A flow diagram to state the number of individuals at each stage (Figure 7).
Table 3
| Variables | Tricuspid valve regurgitation (cm2) | Left atrium size (mm) |
|---|---|---|
| Preoperative, mean | 3.0 | 30.93 |
| Postoperative, mean | 1.4 | 29.96 |
| t | – | 2.014 |
| P | <0.001 | 0.003 |
Nonparametric rank sum tests were used for analyzing tricuspid regurgitation before and after surgery. Paired sample t-tests were used to compare the left atrial size before and after surgery.

Follow-up
All patients were discharged within one day and underwent a follow-up period ranging from 3 months to 1 year. During this period, no related complications were detected by electrocardiography or cardiac ultrasound. Normal cardiopulmonary function was restored in all patients, and significant improvements in growth and development were observed.
Discussion
ASDs are among the most common congenital heart defects, and various surgical techniques have been reported for their treatment. Percutaneous ASD closure has emerged as the primary treatment due to its advantages of minimal incision, reduced trauma, and faster recovery (14). It includes transthoracic ASD, transfemoral vein, and trans-jugular vein closures. Transthoracic ASD closure involves a 1–2 cm chest wall incision and is performed in the thoracic region. Similarly, transfemoral vein closure has certain disadvantages. The transcatheter technique requires X-ray guidance, which can be harmful to both patients and doctors despite the use of lead aprons (15). Additionally, the use of a contrast medium during surgery may elicit hypersensitivity reactions and result in renal impairment. Moreover, the use of a long delivery system makes the operation highly challenging and intricate, leading to increased duration and complexity.
Due to the limitations of transthoracic ASD and transfemoral vein closures, trans-jugular vein ASD closure has been introduced and applied in our center since 2015. Our experience has demonstrated several advantages of trans-jugular vein ASD closure compared with other techniques. First, trans-jugular guided occlusion uses a shorter and more direct transmission system, reducing the surgical operation path (16). The tip of the sheath is adjustable from 0 to 160°, allowing for accurate placement of the occluder perpendicular to the plane of the ASD. The delivery system is shorter and more flexible, facilitating easier handling. Second, direct access to the ASD without the need for a guidewire or dilator simplifies the operation, enhances control, improves safety, and increases surgical accuracy (13). It also helps to shorten the operation time. Third, trans-jugular vein ASD closure employs transesophageal or transthoracic echocardiography to guide the entire surgical process, providing clear imaging and enabling a comprehensive display of the occluder morphology, adjacent structures, and the defect. The application of TEE eliminates X-ray radiation damage and considerably reduces psychological distress for patients.
Due to the aforementioned advantages, trans-jugular vein occlusion has become the preferred occlusion method in our center. Its safety and effectiveness have been affirmed, as evidenced by successful operations in 97 out of 103 patients. The surgical outcomes and short-term follow-up results were satisfactory. As has been reported by Gao et al. (13), in all 19 patients, the ASDs were successfully closed and the occluder was confirmed to have a stable position and good shape, with no residual shunt. The number of patients in this paper is limited. For the patients who failed via the RIJV, we would switch to trans-thoracic ASD closure. In our series, percutaneous closure of ASD via the RIJV was unsuccessful in 6 patients. Five of these patients were transferred for trans-thoracic ASD closure, which yielded satisfactory results, and one patient refused further surgical treatment. Nevertheless, long-term follow-up is required to evaluate the prolonged effects.
The diameter of the internal jugular vein is larger than that of the femoral vein, allowing for the use of a larger sheath. This makes even infants younger than 1 year old a suitable candidate for ASD closure. In our study, the youngest patient was 3 years old, and the diameter of the femoral vein was 3–4 mm (17), which cannot ensure successful implantation of the occluder. However, we were able to safely access the jugular vein. Therefore, in our experience, jugular vein closure can be safely applied in younger patients and those with thinner veins.
In patients with ASD, tricuspid valve regurgitation and enlargement of both the left and right atria are commonly observed. Enlargement of the left atrium can cause further electrical and structural remodeling, leading to functional impairment of the left atrium and a decline in left ventricular function, ultimately resulting in severe heart failure (18-20). Reducing the size of the left atrium and TI can effectively mitigate remodeling and improve patient prognosis. In our study, we compared preoperative sizes of the left atrium and areas of TI with postoperative follow-up data. We observed a decrease in LA diameter and tricuspid valve regurgitant flow compared with preoperative measurements. The significant decrease in the size of the left atrium after occlusion can be attributed to the disappearance of the left-to-right shunt following surgery (21). Furthermore, the areas of TI were significantly reduced compared with pre-operative data, which can be attributed to the decreased volume load on the right heart after the closure of the defect. These findings support the hypothesis that jugular vein closure promotes positive remodeling of the heart.
Conclusions
Percutaneous closure of ASDs via the RIJV has been widely implemented in our center. This procedure enables surgeons to perform percutaneous occlusion of ASDs under echocardiographic guidance using an adjustable delivery sheath. It is indicated for various cases and presents several advantages, including a short operation path, procedural accuracy, minimal trauma, and quick recovery, particularly in young patients with large ASDs. The short-term clinical results are satisfactory, but long-term follow-up is warranted to assess the prolonged outcomes.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-282/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-282/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-282/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-282/coif). H.W. reports that the study was funded by the Science and Technology Agency of Jilin Province. The other authors report that this study was funded by the Health Commission of Jilin Province. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of the Second Hospital of Jilin University [No. 2022(264)] and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bashir F, Hameed M, Hanif B, et al. Outcomes and complications of percutaneous device closure in adults with secundum atrial septal defect. J Pak Med Assoc 2022;72:385-9. [PubMed]
- Coskun S, Adina KA, Tiryakioglu SK, et al. Effect of percutaneous closure on atrium and appendage functions in patients with secundum atrial septal defects. Int J Cardiovasc Imaging 2023;39:1289-97. [Crossref] [PubMed]
- King TD, Thompson SL, Steiner C, et al. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 1976;235:2506-9. [Crossref] [PubMed]
- Jones TK, Latson LA, Zahn E, et al. Results of the U.S. multicenter pivotal study of the HELEX septal occluder for percutaneous closure of secundum atrial septal defects. J Am Coll Cardiol 2007;49:2215-21. [Crossref] [PubMed]
- Astarcioglu MA, Kalcik M, Sen T, et al. Ceraflex versus Amplatzer occluder for secundum atrial septal defect closure. Multicenter clinical experience. Herz 2015;40:146-50. [Crossref] [PubMed]
- Almanla A, Charafeddine F, Abutaqa M, et al. Transcatheter Closure of Atrial Septal Defects: Comparable Experience and Outcomes Between Developing and Developed Countries. Pediatr Cardiol 2019;40:610-5. [Crossref] [PubMed]
- Manolis AS, Koulouris S, Rouska E, et al. Simplified percutaneous closure of patent foramen ovale and atrial septal defect with use of plain fluoroscopy: Single operator experience in 110 consecutive patients. Indian Heart J 2018;70:24-31. [Crossref] [PubMed]
- Sitefane F, Malekzadeh-Milani S, Villemain O, et al. Reduction of radiation exposure in transcatheter atrial septal defect closure: How low must we go? Arch Cardiovasc Dis 2018;111:189-98. [Crossref] [PubMed]
- Hiremath G, Meadows J, Moore P. How Slow Can We Go? 4 Frames Per Second (fps) Versus 7.5 fps Fluoroscopy for Atrial Septal Defects (ASDs) Device Closure. Pediatr Cardiol 2015;36:1057-61. [Crossref] [PubMed]
- Wan L, Yu BT, Wu QC, et al. Transthoracic closure of atrial septal defect and ventricular septal defect without cardiopulmonary bypass. Genet Mol Res 2015;14:3760-6. [Crossref] [PubMed]
- Yi K, Guo X, You T, et al. Standard median sternotomy, right minithoracotomy, totally thoracoscopic surgery, percutaneous closure, and transthoracic closure for atrial septal defects in children: A protocol for a network meta-analysis. Medicine (Baltimore) 2019;98:e17270. [Crossref] [PubMed]
- Meier B. Percutaneous Closure of Atrial Septal Defects: Contraindications Are Hard to Find These Days. JACC Cardiovasc Interv 2015;8:607-8. [Crossref] [PubMed]
- Gao M, Li Z, Zhao Y, et al. Analysis of the therapeutic effect of transesophageal echocardiography-guided percutaneous device closure of atrial septal defects via the right internal jugular vein in children. Echocardiography 2019;36:1357-63. [Crossref] [PubMed]
- Tanaka S, Imamura T, Narang N, et al. Practical Therapeutic Management of Percutaneous Atrial Septal Defect Closure. Intern Med 2022;61:15-22. [Crossref] [PubMed]
- Aubry P, Brochet E, Verdonk C, et al. 3-D transoesophageal echocardiography guidance in percutaneous closure of three distant atrial septal defects. Eur Heart J Cardiovasc Imaging 2015;16:1045. [Crossref] [PubMed]
- Xie S, Fang J, Yang C, et al. Percutaneous trans-jugular vein closure of atrial septal defect with steerable introducer under echocardiographic guidance. J Thorac Dis 2015;7:1850-3. [PubMed]
- Álvarez JML, Quevedo OP, Cabrera LS, et al. Vascular ultrasound in pediatrics: utility and application of location and measurement of jugular and femoral vessels. J Med Ultrason (2001) 2018;45:469-77. [PubMed]
- Qiu D, Peng L, Ghista DN, et al. Left Atrial Remodeling Mechanisms Associated with Atrial Fibrillation. Cardiovasc Eng Technol 2021;12:361-72. [Crossref] [PubMed]
- Sunderland N, Maruthappu M, Nagendran M. What size of left atrium significantly impairs the success of maze surgery for atrial fibrillation? Interact Cardiovasc Thorac Surg 2011;13:332-8. [Crossref] [PubMed]
- Ikenouchi T, Inaba O, Takamiya T, et al. The impact of left atrium size on selection of the pulmonary vein isolation method for atrial fibrillation: Cryoballoon or radiofrequency catheter ablation. Am Heart J 2021;231:82-92. [Crossref] [PubMed]
- Monfredi O, Luckie M, Mirjafari H, et al. Percutaneous device closure of atrial septal defect results in very early and sustained changes of right and left heart function. Int J Cardiol 2013;167:1578-84. [Crossref] [PubMed]