
Dipeptidyl peptidase-4 inhibitors versus sulfonylureas on the top of metformin in patients with diabetes and acute myocardial infarction
Highlight box
Key findings
• The use of metformin (MET) combined with dipeptidyl peptidase-4 (DPP4) inhibitors in acute myocardial infarction (AMI) patients with type 2 diabetes mellitus (DM) was associated with significantly reduced incidence of recurrent myocardial infarction (MI) than that of MET combined with sulfonylureas (SU) during 3-year follow-up.
What is known and what is new?
• While SU were associated with higher risk of cardiovascular events and all-cause mortality, both monotherapy and combination therapy involving DPP4 inhibitors have demonstrated neural or beneficial effects on cardiovascular outcomes.
• The potential long-term benefits or risks of MET combined with DPP4 inhibitors in patients with AMI and type 2 DM were not assessed effectively.
What is the implication, and what should change now?
• The combination of MET with DPP4 inhibitors may be considered for improving long-term cardiovascular outcomes in patients with AMI and type 2 DM.
Introduction
Diabetes mellitus (DM) is an important modifiable risk factor for cardiovascular (CV) disease. DM is very common among patients with acute myocardial infarction (AMI), and these patients showed a 2-fold higher mortality rate than in those with normoglycemia (1,2). The recent trials showed that both the amplitude of reduction in glycated hemoglobin (HbA1c) and the duration of the intensification of glycemic control are major factors that may influence CV outcome results (3). Previous studies demonstrated that intensive in-hospital glycemic control and peri-procedural glycemic control in patients undergoing percutaneous coronary intervention (PCI) are associated with improved clinical outcomes (4,5). However, the CV effects of different glucose-lowering agents and of more intensive glucose control remain a matter of controversy (6).
The recent guidelines recommend that metformin (MET) should be the first-line therapy followed by various options for second-line treatment if adequate glycemic control is not achieved despite MET monotherapy (7,8). Although the newer therapies have been rapidly introduced and the treatment guidelines for type 2 DM are regularly updated based on new evidence (7), sulfonylureas (SU) such as glimepiride and dipeptidyl peptidase-4 (DPP4) inhibitors are the most commonly used second-line glucose-lowering agents in many countries (9). Several studies have suggested that the use of SU is associated with an increased risk for CV outcomes and all-cause mortality (10,11), in contrast, the use of DPP4 inhibitors in monotherapy or in combination has been shown to have neutral or slightly beneficial effects on CV outcomes (12-14). However, there are few data about the head-to-head comparison trials of the effectiveness of the MET-DPP4 inhibitors combination and the MET-SU combination on CV outcomes in type 2 DM patients with high CV risk such as AMI. Of note, the potential long-term benefits or risks were not assessed effectively.
Therefore, we investigated the impact of MET combined with DPP4 inhibitors (MET + DPP4 inhibitors group) or SU (MET + SU group) on 3-year clinical outcomes in patients with AMI and type 2 DM. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-349/rc).
Methods
Study population
The study participants were recruited from the Korea AMI Registry (KAMIR)-National Institutes of Health (NIH) registry. The previous publications (15,16) provide a comprehensive overview of the KAMIR study design, and additional registry details are available on the KAMIR website (http://www.kamir.or.kr). Essentially, the KAMIR study is a prospective, multicenter, observational cohort trial intended to reflect the “real world” clinical practices in a cohort of Korean AMI patients. It has been ongoing since November 2005, with the primary goal of investigating the current epidemiology and clinical outcomes of AMI in the Korean population. Over the period from November 2011 to December 2015, a total of 13,104 patients AMI patients have been consecutively enrolled in the nationwide KAMIR-NIH registry. The flow chart illustrates the procedural framework of the present study (Figure 1). Among the entire population, 3,752 patients had known DM history irrespective of treatment or newly diagnosed DM on admission. After the exclusion of those who had undergone failed PCI, those who had received PCI with a device other than drug-eluting stents (DES), those with missing MET data, those taking other glucose-lowering agents except MET combined with DPP4 inhibitors or SU at discharge and during follow-up period, and those who had in-hospital death, a total of 469 patients who have used MET combined with DPP4 inhibitors or SU according to the physician’s discretion were classified into two groups; the MET + DPP4 inhibitors group (n=234) and the MET + SU group (n=235).
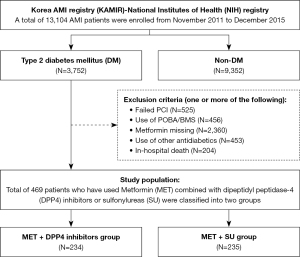
Data collection occurred through a web-based case report from at each collaborating center. The study protocol was approved by the Korea University Guro Hospital Institutional Review Board (IRB) under the #2016GR0740, and was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki (as revised in 2013). The other hospitals were informed and agreed with the study. Prior to enrollment, all patients furnished written informed consent. A comprehensive 3-year clinical follow-up was completed for all 469 participants, involving face-to-face interviews, telephone calls, or chart reviews.
PCI procedure and medical treatment
PCI was performed using a general standard PCI technique (17). PCI was proceeded through either the femoral or radial artery after an administration of unfractionated heparin (50–100 U/kg). All patients received loading doses of aspirin (200–300 mg) and other anti-platelets (ticagrelor 180 mg, prasugrel 60 mg or clopidogrel 300–600 mg as loading doses according to current guideline-based dual antiplatelet regimen) before PCI (18). DES were deployed after prior balloon angioplasty, and the use of anti-coagulation therapy during PCI was left to the discretion of the individual operator. Anti-ischemic therapy, comprising medications such as calcium channel blockers, beta-blockers, renin-angiotensin-aldosterone system (RAAS) inhibitors, and statins, was prescribed as deemed appropriate by the physician. The patients maintained dual anti-platelet therapy for at least one year.
Study definition and endpoint
DM was defined as either known DM for which patients received medical treatment (insulin or antidiabetics) or not, or newly diagnosed DM defined as an HbA1c level ≥6.5%, fasting plasma glucose (FPG) level ≥126 mg/dL (7.0 mmol/L), and/or random plasma glucose (RPG) level ≥200 mg/dL (11.1 mmol/L) according to the American Diabetes Association clinical practice recommendations (19).
The key combined primary endpoint was major adverse cardiac events (MACE) as defined as the composite of all-cause death, recurrent MI, and any repeat revascularization. The key secondary endpoints were the occurrence of any clinical events such as all-cause death, recurrent MI, any repeat revascularization including surgical coronary artery bypass graft (CABG) or repeat PCI, target lesion failure (TLF), stent thrombosis (ST), and re-hospitalization due to heart failure (HF). All deaths were considered to be cardiac in origin unless a non-cardiac origin was definitely documented. Recurrent MI was defined as recurrent symptoms with new ST-segment elevation or re-elevation of cardiac markers to at least twice the upper limit of normal. Target lesion revascularization (TLR) was defined as repeat PCI within the index procedure stent or 5 mm edge. Target vessel revascularization (TVR) was defined as any repeat PCI or surgical CABG of any segment in the target vessel. Any repeat revascularization was defined as any repeat PCI or CABG of target vessel or non-target vessel. TLF was defined as the composite of clinically driven TLR, recurrent MI or cardiac death related to the target vessel. All participants were required to visit the outpatient department of cardiology at the end of the first month and then every six months after the PCI procedure, as well as whenever angina-like symptoms occurred.
Statistical analysis
For continuous variables, differences between the two groups were evaluated using the unpaired t-test or Mann-Whitney rank test. Data were expressed as mean ± standard deviations. For discrete variables, differences were expressed as counts and percentages and analyzed with the χ2 or Fisher’s exact test between two groups. To adjust for any potential confounders, an inverse probability weighting (IPTW) analysis was performed using the logistic regression model. We tested all available variables that could be of potential relevance: age, sex (male), body mass index, Killip class on admission, left ventricular ejection fraction, cardiovascular risk factors (e.g., hypertension, dyslipidemia, heart failure, cerebrovascular disease, prior CABG, prior MI, and prior PCI), co-medication treatment (e.g., aspirin, other anti-platelets, calcium channel blockers, beta-blockers, RAAS inhibitors, and statins), angiographic and procedural characteristics (e.g., target vessel, number of diseased vessels, lesion type, and DES type). Various clinical outcomes up to 3 years were estimated by the Kaplan-Meier analysis, and differences between the groups were compared with the log-rank test before and after IPTW. Binary logistic regression analysis was used to assess the hazard ratio (HR) of the MET + DPP4 inhibitors group compared to the MET + SU group in the IPTW population. For all analyses, a two-sided P<0.05 was considered statistically significant. All data were processed with SPSS (version 20.0, SPSS-PC, Inc. Chicago, IL, USA).
Results
Baseline clinical characteristics of the patients are shown in Table 1. The baseline clinical characteristics were balanced between the two groups. The angiographic and procedural characteristics and medications at discharge are presented in Table 2. The prescription rate of clopidogrel (63.2% vs. 74.5%, P=0.009) was lower in the MET + DPP4 inhibitors group than in the MET + SU group. However, this intergroup difference was well balanced after IPTW adjustment.
Table 1
| Variables | Crude population | IPTW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Metformin and | P value | S.diff | Metformin and | P value | S.diff | ||||
| DPP4i (n=234) | SU (n=235) | DPP4i (n=468) | SU (n=468) | ||||||
| Sex, male | 171 (73.1) | 160 (68.1) | 0.236 | −0.60 | 325 (69.4) | 336 (71.8) | 0.430 | 0.28 | |
| Age, year | 64.2±10.8 | 65.6±11.6 | 0.198 | 0.12 | 64.6±10.5 | 64.5±11.6 | 0.824 | −0.01 | |
| Blood pressure, mmHg | |||||||||
| Systolic | 130±26 | 127±25 | 0.231 | −0.11 | 128±26 | 128±25 | 0.847 | −0.01 | |
| Diastolic | 79±16 | 77±15 | 0.077 | −0.16 | 78±15 | 78±15 | 0.914 | −0.01 | |
| Heart rate, beat per minutes | 80±19 | 78±18 | 0.185 | −0.12 | 79±19 | 79±18 | 0.853 | −0.01 | |
| Body mass index, kg/m2 | 24.2±3.6 | 24.1±2.9 | 0.743 | −0.03 | 24.1±3.4 | 24.0±2.9 | 0.601 | −0.03 | |
| LV ejection fraction, % | 51.4±10.7 | 50.7±11.4 | 0.464 | −0.07 | 51.2±10.7 | 50.8±11.2 | 0.605 | −0.03 | |
| Final diagnosis | |||||||||
| STEMI | 113 (48.3) | 112 (47.7) | 0.891 | −0.09 | 218 (46.5) | 215 (45.9) | 0.868 | −0.08 | |
| NSTEMI | 121 (51.7) | 123 (52.3) | 0.891 | 0.09 | 251 (53.5) | 253 (54.1) | 0.868 | 0.07 | |
| Killip class | |||||||||
| I | 173 (73.9) | 174 (74.0) | 0.978 | 0.01 | 356 (75.9) | 355 (75.9) | 0.985 | −0.01 | |
| II | 27 (11.5) | 25 (10.6) | 0.756 | −0.27 | 48 (10.2) | 50 (10.7) | 0.822 | 0.14 | |
| III | 17 (7.3) | 18 (7.7) | 0.871 | 0.14 | 30 (6.4) | 29 (6.2) | 0.893 | −0.09 | |
| IV | 17 (7.3) | 18 (7.7) | 0.871 | 0.14 | 35 (7.5) | 34 (7.3) | 0.900 | −0.08 | |
| History of patients | |||||||||
| Hypertension | 143 (61.1) | 155 (66.0) | 0.276 | 0.61 | 295 (63.0) | 296 (63.2) | 0.946 | 0.03 | |
| Dyslipidemia | 50 (21.4) | 35 (14.9) | 0.069 | −1.52 | 86 (18.4) | 90 (19.2) | 0.738 | 0.20 | |
| Prior CAD | |||||||||
| Myocardial infarction | 13 (5.6) | 23 (9.8) | 0.085 | 1.53 | 30 (6.4) | 35 (7.5) | 0.515 | 0.41 | |
| Angina pectoris | 21 (9.0) | 23 (9.8) | 0.763 | 0.27 | 40 (8.5) | 41 (8.8) | 0.899 | 0.08 | |
| Prior PCI | 20 (8.5) | 26 (11.1) | 0.360 | 0.80 | 41 (8.7) | 42 (9.0) | 0.900 | 0.08 | |
| Prior CABG | 1 (0.4) | 4 (1.7) | 0.372 | 1.24 | 2 (0.4) | 5 (1.1) | 0.287 | 0.74 | |
| Stroke | 17 (7.3) | 20 (8.5) | 0.617 | 0.44 | 41 (8.8) | 32 (6.8) | 0.273 | −0.69 | |
| Infarction | 2 (0.9) | 0 | 0.248 | −1.31 | 2 (0.4) | 0 | 0.499 | −0.92 | |
| Hemorrhage | 16 (6.8) | 20 (8.5) | 0.496 | 0.60 | 40 (8.5) | 32 (6.8) | 0.326 | −0.62 | |
| Smoking | |||||||||
| Currently | 93 (39.7) | 83 (35.3) | 0.322 | −0.72 | 169 (36.0) | 166 (35.5) | 0.857 | −0.09 | |
| Ex-smoker | 47 (20.1) | 49 (20.9) | 0.837 | 0.17 | 104 (22.2) | 100 (21.4) | 0.751 | −0.18 | |
| Serum glucose, mg/dL | 230±93 | 233±93 | 0.754 | 0.03 | 227±97 | 235±96 | 0.225 | 0.08 | |
| HbA1c, % | 7.8±1.5 | 7.8±1.6 | 0.984 | 0.00 | 8.0±1.7 | 7.9±1.6 | 0.408 | −0.06 | |
Data are presented as n (%) or mean ± standard deviation. IPTW, inverse probability weighting; DPP4i, dipeptidyl peptidase-4 inhibitors; SU, sulfonylureas; S.diff, standardized mean difference; LV, left ventricular; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non ST-segment elevation myocardial infarction; CAD, coronary artery disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; HbA1c, glycated hemoglobin.
Table 2
| Variables | Crude population | IPTW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Metformin and | P value | S.diff | Metformin and | P value | S.diff | ||||
| DPP4i (n=234) | SU (n=235) | DPP4i (n=468) | SU (n=468) | ||||||
| Angiographic and procedural characteristics | |||||||||
| Infarct-related artery | |||||||||
| LAD | 113 (48.3) | 98 (41.7) | 0.152 | −0.98 | 217 (46.4) | 211 (45.1) | 0.694 | −0.19 | |
| LCx | 37 (15.8) | 39 (16.6) | 0.818 | 0.19 | 73 (15.6) | 79 (16.9) | 0.585 | 0.33 | |
| RCA | 77 (32.9) | 93 (39.6) | 0.133 | 1.11 | 168 (35.8) | 169 (36.1) | 0.926 | 0.05 | |
| Left main | 7 (3.0) | 5 (2.1) | 0.554 | −0.54 | 11 (2.3) | 9 (1.9) | 0.655 | −0.29 | |
| Multi-vessel disease | 129 (55.1) | 143 (60.9) | 0.209 | 0.75 | 272 (58.0) | 262 (56.0) | 0.534 | −0.27 | |
| Drug-eluting stents | |||||||||
| Everolimus | 125 (53.4) | 131 (55.7) | 0.613 | 0.32 | 256 (54.6) | 252 (53.8) | 0.821 | −0.10 | |
| Zotarolimus | 2 (0.9) | 1 (0.4) | 0.623 | −0.54 | 2 (0.4) | 2 (0.4) | >0.999 | 0.00 | |
| Biolimus A9 | 47 (20.1) | 37 (15.7) | 0.220 | −1.03 | 88 (18.8) | 94 (20.1) | 0.609 | 0.30 | |
| Sirolimus | 7 (3.0) | 6 (2.6) | 0.773 | −0.26 | 13 (2.8) | 12 (2.6) | 0.844 | −0.13 | |
| Paclitaxel | 0 | 1 (0.4) | >0.999 | 0.92 | 0 | 1 (0.2) | >0.999 | 0.65 | |
| Number of stent | 1.20±0.43 | 1.21±0.45 | 0.935 | 0.01 | 1.22±0.47 | 1.21±0.45 | 0.918 | −0.01 | |
| Stent diameter, mm (max) | 3.12±0.44 | 3.09±0.42 | 0.375 | −0.08 | 3.08±0.44 | 3.08±0.42 | 0.869 | 0.01 | |
| Stent diameter, mm (mean) | 3.08±0.42 | 3.06±0.41 | 0.524 | −0.06 | 3.04±0.42 | 3.05±0.41 | 0.657 | 0.03 | |
| Total stent length, mm | 30.4±13.7 | 30.3±14.6 | 0.919 | −0.01 | 30.8±13.9 | 30.6±14.1 | 0.828 | −0.01 | |
| Discharge medication | |||||||||
| Aspirin | 232 (99.1) | 234 (99.6) | 0.623 | 0.04 | 466 (99.4) | 466 (99.6) | >0.999 | 0.02 | |
| Clopidogrel | 148 (63.2) | 175 (74.5) | 0.009 | 1.36 | 331 (70.7) | 345 (73.7) | 0.307 | 0.35 | |
| Prasugrel | 33 (14.1) | 20 (8.5) | 0.056 | −1.66 | 50 (10.7) | 42 (9.0) | 0.380 | −0.55 | |
| Cilostazol | 24 (10.3) | 38 (16.2) | 0.059 | 1.63 | 68 (14.5) | 60 (12.8) | 0.447 | −0.46 | |
| Ticagrelor | 51 (21.8) | 40 (17.0) | 0.191 | −1.08 | 85 (18.1) | 82 (17.5) | 0.810 | −0.14 | |
| Ca-channel blockers | 16 (6.8) | 13 (5.5) | 0.557 | −0.53 | 29 (6.2) | 37 (7.9) | 0.303 | 0.65 | |
| Beta-blockers | 206 (88.0) | 207 (88.1) | 0.986 | 0.01 | 419 (89.3) | 414 (88.5) | 0.669 | −0.09 | |
| RAAS inhibitors | 195 (83.3) | 204 (86.8) | 0.291 | 0.38 | 400 (85.5) | 408 (87.2) | 0.447 | 0.18 | |
| Statin | 218 (93.2) | 220 (93.6) | 0.843 | 0.05 | 439 (93.6) | 439 (93.8) | 0.900 | 0.02 | |
Data are presented as n (%) or mean ± standard deviation. IPTW, inverse probability weighting; DPP4i, dipeptidyl peptidase-4 inhibitors; SU, sulfonylureas; S.diff, standardized mean difference; LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery; RAAS, renin-angiotensin-aldosterone system.
Table 3 and Figure 2 show the cumulative incidences of major clinical outcomes during the 3-year follow-up. Before the adjustment, there was a trend toward lower cumulative incidence of MACE in the MET + DPP4 inhibitors group than in the MET + SU group (Figure 2A), but it did not show the significant difference between the two groups after IPTW adjustment (16.8% vs. 19.4%, P=0.302). There was no significant difference in all-cause death between the two groups, both before (Figure 2B) and after the adjustment. However, the cumulative incidences of recurrent MI (0.9% vs. 5.1%, P=0.007) (Figure 2C) and non-ST elevation MI (NSTEMI, 0.0 vs. 3.4%, P=0.007) were significantly lower in the MET + DPP4 inhibitors group before the adjustment. After IPTW adjustment, the incidences of recurrent MI [hazard ratio (HR): 0.228, 95% CI: 0.090–0.580, P=0.001] and NSTEMI (HR: 0.377, 95% CI: 0.094–1.504, P<0.001) in the MET + DPP4 inhibitors group were significantly lower than in the MET + SU group (Figure 3). Although the cumulative incidences of any repeat revascularization (7.3% vs. 12.8%, P=0.05) (Figure 2D), TLR (1.7% vs. 5.1%, P=0.04), and TVR (3.0% vs. 8.5%, P=0.01) in the MET + DPP4 inhibitors group were significantly lower than in the MET + SU group, these differences between the two groups were not significant after IPTW adjustment.
Table 3
| Variables | Crude population | IPTW | |||||
|---|---|---|---|---|---|---|---|
| Metformin and | P value | Metformin and | P value | ||||
| DPP4i (n=234) | SU (n=235) | DPP4i (n=468) | SU (n=468) | ||||
| MACE | 32 (13.7) | 48 (20.4) | 0.052 | 79 (16.8) | 91 (19.4) | 0.302 | |
| Target lesion failure | 13 (5.6) | 23 (9.8) | 0.085 | 38 (8.1) | 43 (9.2) | 0.554 | |
| Total death | 15 (6.4) | 18 (7.7) | 0.597 | 26 (5.6) | 35 (7.5) | 0.233 | |
| Cardiac death | 9 (3.8) | 9 (3.8) | 0.993 | 17 (3.6) | 18 (3.9) | 0.858 | |
| Non-cardiac death | 6 (2.6) | 9 (3.8) | 0.436 | 9 (1.9) | 16 (3.4) | 0.154 | |
| Myocardial infarction | 2 (0.9) | 12 (5.1) | 0.007 | 6 (1.3) | 23 (4.9) | 0.001 | |
| STEMI | 2 (0.9) | 4 (1.7) | 0.685 | 6 (1.3) | 8 (1.7) | 0.587 | |
| NSTEMI | 0 | 8 (3.4) | 0.007 | 0 | 15 (3.2) | <0.001 | |
| Revascularization | 17 (7.3) | 30 (12.8) | 0.047 | 51 (10.9) | 56 (12.0) | 0.608 | |
| CABG | 0 | 1 (0.4) | >0.999 | 0 | 2 (0.4) | 0.499 | |
| PCI | 17 (7.3) | 29 (12.3) | 0.065 | 51 (10.9) | 54 (11.5) | 0.756 | |
| TLR | 4 (1.7) | 12 (5.1) | 0.043 | 21 (4.5) | 21 (4.5) | 0.994 | |
| TVR | 7 (3.0) | 20 (8.5) | 0.010 | 27 (5.8) | 36 (7.7) | 0.237 | |
| Non-TVR | 10 (4.3) | 10 (4.3) | 0.992 | 24 (5.1) | 20 (4.3) | 0.537 | |
| Stent thrombosis | 1 (0.4) | 3 (1.3) | 0.623 | 2 (0.4) | 5 (1.1) | 0.451 | |
| Re-hospitalization due to HF | 8 (3.4) | 8 (3.4) | 0.993 | 15 (3.2) | 14 (3.0) | 0.855 | |
Data are presented as incidence (%). IPTW, inverse probability weighting; DPP4i, dipeptidyl peptidase-4 inhibitors; SU, sulfonylureas; MACE, major adverse cardiac events; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non ST-segment elevation myocardial infarction; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; TLR, target lesion revascularization; TVR, target vessel revascularization; HF, heart failure.
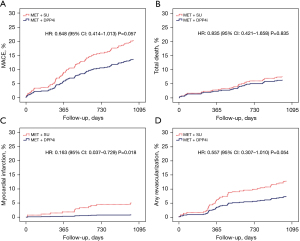
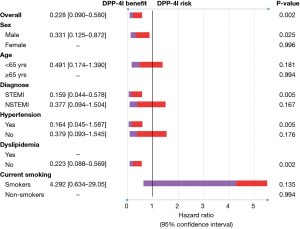
Figure 3 shows that in cases of male, initial diagnosis of ST-segment elevation MI (STEMI), history of hypertension, or no history of dyslipidemia, the use of MET + DPP4 inhibitors over MET + SU may have benefits to reduce recurrent MI in patients with AMI and type 2 DM.
Discussion
The main findings of this study are: (I) the cumulative incidence of MACE was similar between the two groups (MET + DPP4 inhibitors group vs. MET + SU group); (II) the cumulative incidence of recurrent MI was significantly lower in the MET + DPP4 inhibitors group than in the MET + SU group in patients with AMI and type 2 DM during 3-year follow-up. This analysis of Korean national registry data demonstrated that the use of MET combined with DPP4 inhibitors in patients with AMI and type 2 DM resulted in lower incidence of 3-year recurrent MI rates compared to MET combined with SU such as glimepiride.
SU have been used the common add-on second-line agents combined with MET, mainly because of their relatively lower cost and strong hypoglycemic effect. Several studies have reported that they have been associated with an increased risk of hypoglycemia, weight gain, and CV risks compared with other glucose-lowering agents (20-22). On the contrary, previous meta-analyses evaluating the safety of SU as a group or in combination with MET did not show higher risk of mortality or CV events (23,24). However, in direct comparisons with DPP4 inhibitors showed by other meta-analyses, SU were associated with significant increase in the incidence of CV events (25,26). Zhang et al. showed that DPP4 inhibitors were associated with 47% less CV events compared with SU (25). Morgan et al. demonstrated that MET-SU combination therapy was associated with an increased risk for MACE and all-cause mortality (HR: 1.71, 95% CI: 1.28–2.29) compared to the MET-DPP4 inhibitors combination (27). Furthermore, the combination of MET and SU was associated with increased mortality in comparison with SU alone (28).
DPP4 inhibitors inhibit the enzyme that degrades 2 gut-derived incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), and stimulate insulin secretion and reduce glucagon secretion (29). DPP4 inhibitors have progressively replaced SU in many countries because they are not associated with hypoglycemia or weight gain, and have a relatively good safety profile (30,31). Hypoglycemia and weight gain among SU users may be associated with an increased CV risk (32). In animal experimental model, DPP4 inhibitors were shown to reduce infarct size and attenuate left ventricular dysfunction and remodeling via the GLP-1 receptor-protein kinase A pathway in the post-MI settings (33-35).
Recently, the major prospective clinical trials have investigated the various uses and CV outcomes of DPP4 inhibitors in diabetic patients. In EXAMINE (the CV outcomes study of alogliptin in patients with acute coronary syndrome and type 2 DM), SAVOR-TIMI 53 (the saxagliptin assessment of vascular outcomes in patients with DM-thrombolysis in MI), and TECOS (the sitagliptin CV outcome study), no significant differences were observed about the incidence of MACE, MI, stroke, CV mortality, and all-cause mortality between DPP4 inhibitors or matched placebo (13,36,37). Therefore, the favorable cardiac and vascular impacts observed in animal models using DPP4 inhibitors did not find validation in clinical investigations, including CV outcome trials. These trials only demonstrated non-inferiority when compared to a placebo.
However, the results of present study were that the use of MET combined with DPP4 inhibitors in patients with AMI and type 2 DM resulted in lower incidence of 3-year recurrent MI rates and did not show higher risk of MACE, mortality or other clinical events compared to MET combined with SU. Our results also showed that the incidences of any repeat revascularization (7.3% vs. 12.8%, P=0.05), TLR (1.7% vs. 5.1%, P=0.04), and TVR (3.0% vs. 8.5%, P=0.01) in the MET + DPP4 inhibitors group were significantly lower, although they had no statistical significance after IPTW adjustment. The major differences of our present study comparing other published CV outcome trials of DPP4 inhibitors were that the duration of studies (3-year follow-up) and head-to-head comparison of MET combined therapy of DPP4 inhibitors and SU. Given the relatively short durations of other trials (1–2 years), the variance in hyperglycemia exposure between the groups was insufficient to detect any notable differences in CV outcomes, particularly among diabetic patients with pre-existing advanced CV disease (3). Cowley et al. showed a trend toward reduced incidence of CV outcomes in MET-treated patients contrasting with a trend for an increase in MACE in patients not receiving MET (38). It is crucial to examine the impact of MET on endothelial dysfunction in diabetic patients, and we have to consider its anti-inflammatory and anti-apoptotic effects on pericoronary fat from pre-diabetic AMI patients, achieved by modulating sodium-glucose cotransporter 2, leptin, and sirt 6 levels (39,40). DPP4 inhibitors and MET have both demonstrated efficacy in enhancing insulin resistance and mitigating myocardial injury resulting from ischemia-reperfusion injury. Some studies propose that the combined therapy yields superior outcomes compared to monotherapy, resulting in decreased CV events and all-cause mortality rates (41-44). Thus, it was considered that MET may act as a moderator or facilitator of DPP4 inhibitors for improving CV outcomes. This suggests that the decreased CV risks of MET combined with DPP4 inhibitors observed in this study may possibly be the results of the MET-SU combination reference group. Furthermore, it can be assumed that a negative interaction with MET could be partly responsible for the increase of CV risk with SU.
Finally, the beneficial effects of DPP4 inhibitors in patients with AMI pose several potential explanations, despite the absence of a definitive mechanism outlining the CV benefits of DPP4 inhibitors. One possible mechanism involves the potential of DPP4 inhibitors to diminish reperfusion injury by protecting mitochondrial function (45). They have demonstrated an ability to enhance the activity of reperfusion injury salvage kinase, thereby reducing reperfusion injury originating from cardiac tissue damage and associated arrhythmias (46,47). Furthermore, in patients with DM experiencing ischemia/reperfusion injury, DPP4 inhibitors exhibit the capacity to rescue cardiac mitochondrial dysfunction, diminish reactive oxygen species production, and alleviate oxidative stress (45,48). Lastly, DPP4 inhibitors may exert an inhibitory effect on atherosclerosis and proliferation of vascular smooth muscle cell. Previous studies have shown that GLP-1 based therapies, including both endogenous (DPP4 inhibitors) and exogenous (incretin: GLP-1 agonist) treatments, have anti-inflammatory effects that may reduce the progression of atherosclerosis. It was reported that they were associated with reduced MACE as well as improved clinical outcomes in patients with NSTEMI and STEMI (49-51). In addition, GLP-1 based therapies have a positive impact on reducing MACE and hospitalizations, even in advanced heart failure, and current guidelines recommend them as the preferred choice for patients with cardiovascular disease (52).
The strengths of this study include that the duration of studies (3-year follow-up) and head-to-head comparison of MET combined therapy of DPP4 inhibitors and SU compared to other studies. Since the duration of other studies was relatively too short and the designs of those were not direct comparison of medications, the results of other studies showed less reliability and validity than our study. This analysis of Korean national registry data demonstrated that the use of MET combined with DPP4 inhibitors in AMI patients with type 2 DM was associated with lower incidence of 3-year recurrent MI rates compared to MET combined with SU such as glimepiride.
The present study has some limitations. First, because this study was multicenter national prospective observational registry and was focused on assessing, whether there were differences in effectiveness between the two most commonly used combination therapies (MET-DPP4 inhibitors and MET-SU) in patients with AMI, these results can be applied to patients who have same characteristics as inclusion criteria. Second, the clinical impact of MET-DPP4 inhibitors and MET-SU combination therapy were compared based on medications at discharge. Thus, the dose of medications, long-term adherence, discontinuation, and incidence of adverse events were not available in this study. Third, because this study population was composed of a single race of Korean, our findings should be confirmed in different races and ethnic groups. Finally, while it is a significant limitation that this study enrolled patients prior to the recent guidelines, it is believed to hold valuable implications, particularly in the context of the Asian populations in low and middle-income countries.
Conclusions
The use of MET combined with DPP4 inhibitors in AMI patients with type 2 DM was associated with significantly reduced incidence of recurrent MI than that of MET combined with SU such as glimepiride during 3-year follow-up.
Acknowledgments
This study was done with the support of Korean Circulation Society (KCS) to commemorate the 50th Anniversary of KCS. The KAMIR study group of the KSC was as follows: Gachon University Gil Medical Center, Incheon, South Korea (Tae Hoon Ahn), Wonju Severance Christian Hospital, Wonju, South Korea (Junghan Yoon), Seoul National University Hospital, Seoul, South Korea (Hyo-Soo Kim), Seoul St. Mary’s Hospital, Seoul, South Korea (Ki-Bae Seung), Samsung Medical Center, Seoul, South Korea (Hyeon-Cheol Gwon), Kyung-pook National University Hospital, Daegu, South Korea (Shung Chull Chae), Kyunghee University Hospital At Gangdong, Seoul, South Korea (Chong-Jin Kim), Pusan National University Hospital, Busan, South Korea (Kwang Soo Cha), Yeungnam University Medical Center, Daegu, South Korea (Jung-Hee Lee), Chonbuk National University Hospital, Jeongju, South Korea (Jei Keon Chae), Jeju National University Hospital, Jeju, South Korea (Seung-Jae Joo), Seoul National University Bundang Hospital, Bundang, South Korea (Chang-Hwan Yoon), Keimyung University Dongsan Medical Center, Daegu, South Korea (Seung-Ho Hur), Chungnam National University Hospital, Daejeon, South Korea (In-Whan Seong), Chungbuk National University Hospital, Cheongju, South Korea (Kyung-Kuk Hwang), Inje University Haeundae Paik Hospital, Busan, South Korea (Doo-Il Kim), Wonkwang University Hospital, Iksan, South Korea (Seok Kyu Oh), Gyeongsang National University Hospital, Jinju, South Korea (Jin-Yong Hwang).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-349/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-349/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-349/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-349/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study protocol was approved by the Korea University Guro Hospital Institutional Review Board (IRB) (#2016GR0740) and was conducted according to the ethical guidelines of the Declaration of Helsinki (as revised in 2013). The other hospitals were informed and agreed with the study. Prior to enrollment, the written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013;34:3035-87. [Crossref] [PubMed]
- Gholap NN, Achana FA, Davies MJ, et al. Long-term mortality after acute myocardial infarction among individuals with and without diabetes: A systematic review and meta-analysis of studies in the post-reperfusion era. Diabetes Obes Metab 2017;19:364-74. [Crossref] [PubMed]
- Roussel R, Steg PG, Mohammedi K, et al. Prevention of cardiovascular disease through reduction of glycaemic exposure in type 2 diabetes: A perspective on glucose-lowering interventions. Diabetes Obes Metab 2018;20:238-44. [Crossref] [PubMed]
- Caturano A, Galiero R, Pafundi PC, et al. Does a strict glycemic control during acute coronary syndrome play a cardioprotective effect? Pathophysiology and clinical evidence. Diabetes Res Clin Pract 2021;178:108959. [Crossref] [PubMed]
- Marfella R, Rizzo MR, Siniscalchi M, et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention up-regulates endothelial progenitor cell level and differentiation during acute ST-elevation myocardial infarction: effects on myocardial salvage. Int J Cardiol 2013;168:3954-62. [Crossref] [PubMed]
- Scheen AJ, Charbonnel B. Effects of glucose-lowering agents on vascular outcomes in type 2 diabetes: a critical reappraisal. Diabetes Metab 2014;40:176-85. [Crossref] [PubMed]
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022;65:1925-66. [Crossref] [PubMed]
- Thein D, Christiansen MN, Mogensen UM, et al. Add-on therapy in metformin-treated patients with type 2 diabetes at moderate cardiovascular risk: a nationwide study. Cardiovasc Diabetol 2020;19:107. [Crossref] [PubMed]
- Engler C, Leo M, Pfeifer B, et al. Long-term trends in the prescription of antidiabetic drugs: real-world evidence from the Diabetes Registry Tyrol 2012-2018. BMJ Open Diabetes Res Care 2020;8:e001279. [Crossref] [PubMed]
- Morgan CL, Poole CD, Evans M, et al. What next after metformin? A retrospective evaluation of the outcome of second-line, glucose-lowering therapies in people with type 2 diabetes. J Clin Endocrinol Metab 2012;97:4605-12. [Crossref] [PubMed]
- Forst T, Hanefeld M, Jacob S, et al. Association of sulphonylurea treatment with all-cause and cardiovascular mortality: a systematic review and meta-analysis of observational studies. Diab Vasc Dis Res 2013;10:302-14. [Crossref] [PubMed]
- Leibovitz E, Gottlieb S, Goldenberg I, et al. Sitagliptin pretreatment in diabetes patients presenting with acute coronary syndrome: results from the Acute Coronary Syndrome Israeli Survey (ACSIS). Cardiovasc Diabetol 2013;12:53. [Crossref] [PubMed]
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26. [Crossref] [PubMed]
- GRADE Study Research Group. Glycemia Reduction in Type 2 Diabetes - Microvascular and Cardiovascular Outcomes. N Engl J Med 2022;387:1075-88. [Crossref] [PubMed]
- Choi SY, Choi BG, Rha SW, et al. Angiotensin-converting enzyme inhibitors versus angiotensin II receptor blockers in acute ST-segment elevation myocardial infarction patients with diabetes mellitus undergoing percutaneous coronary intervention. Int J Cardiol 2017;249:48-54. [Crossref] [PubMed]
- Byun JK, Choi BG, Rha SW, et al. Comparison of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with diabetes mellitus and non-ST-segment elevation myocardial infarction who underwent successful percutaneous coronary intervention. Atherosclerosis 2018;277:130-5. [Crossref] [PubMed]
- Grech ED. ABC of interventional cardiology: percutaneous coronary intervention. II: the procedure. BMJ 2003;326:1137-40. [Crossref] [PubMed]
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016;134:e123-55. [PubMed]
- Standards of medical care in diabetes--2010. Diabetes Care 2010;33:S11-61. [Crossref] [PubMed]
- Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 2009;339:b4731. [Crossref] [PubMed]
- Schramm TK, Gislason GH, Vaag A, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J 2011;32:1900-8. [Crossref] [PubMed]
- Lee TTL, Hui JMH, Lee YHA, et al. Sulfonylurea Is Associated With Higher Risks of Ventricular Arrhythmia or Sudden Cardiac Death Compared With Metformin: A Population-Based Cohort Study. J Am Heart Assoc 2022;11:e026289. [Crossref] [PubMed]
- Varvaki Rados D, Catani Pinto L, Reck Remonti L, et al. The Association between Sulfonylurea Use and All-Cause and Cardiovascular Mortality: A Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials. PLoS Med 2016;13:e1001992. [Crossref] [PubMed]
- Simpson SH, Lee J, Choi S, et al. Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol 2015;3:43-51. [Crossref] [PubMed]
- Zhang Y, Hong J, Chi J, et al. Head-to-head comparison of dipeptidyl peptidase-IV inhibitors and sulfonylureas - a meta-analysis from randomized clinical trials. Diabetes Metab Res Rev 2014;30:241-56. [Crossref] [PubMed]
- Monami M, Ahrén B, Dicembrini I, et al. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2013;15:112-20. [Crossref] [PubMed]
- Morgan CL, Mukherjee J, Jenkins-Jones S, et al. Combination therapy with metformin plus sulphonylureas versus metformin plus DPP-4 inhibitors: association with major adverse cardiovascular events and all-cause mortality. Diabetes Obes Metab 2014;16:977-83. [Crossref] [PubMed]
- Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008;299:1561-73. [Crossref] [PubMed]
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696-705. [Crossref] [PubMed]
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. CONSENSUS STATEMENT BY THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY ON THE COMPREHENSIVE TYPE 2 DIABETES MANAGEMENT ALGORITHM - 2018 EXECUTIVE SUMMARY. Endocr Pract 2018;24:91-120. [Crossref] [PubMed]
- American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S73-85. [Crossref] [PubMed]
- Scheen AJ. Safety of dipeptidyl peptidase-4 inhibitors for treating type 2 diabetes. Expert Opin Drug Saf 2015;14:505-24. [Crossref] [PubMed]
- Hausenloy DJ, Whittington HJ, Wynne AM, et al. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucose-dependent manner. Cardiovasc Diabetol 2013;12:154. [Crossref] [PubMed]
- Hocher B, Sharkovska Y, Mark M, et al. The novel DPP-4 inhibitors linagliptin and BI 14361 reduce infarct size after myocardial ischemia/reperfusion in rats. Int J Cardiol 2013;167:87-93. [Crossref] [PubMed]
- Connelly KA, Zhang Y, Advani A, et al. DPP-4 inhibition attenuates cardiac dysfunction and adverse remodeling following myocardial infarction in rats with experimental diabetes. Cardiovasc Ther 2013;31:259-67. [Crossref] [PubMed]
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327-35. [Crossref] [PubMed]
- Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2015;373:232-42. [Crossref] [PubMed]
- Crowley MJ, Williams JW Jr, Kosinski AS, et al. Metformin Use May Moderate the Effect of DPP-4 Inhibitors on Cardiovascular Outcomes. Diabetes Care 2017;40:1787-9. [Crossref] [PubMed]
- Sardu C, Paolisso P, Sacra C, et al. Effects of Metformin Therapy on Coronary Endothelial Dysfunction in Patients With Prediabetes With Stable Angina and Nonobstructive Coronary Artery Stenosis: The CODYCE Multicenter Prospective Study. Diabetes Care 2019;42:1946-55. [Crossref] [PubMed]
- Sardu C, D'Onofrio N, Torella M, et al. Metformin Therapy Effects on the Expression of Sodium-Glucose Cotransporter 2, Leptin, and SIRT6 Levels in Pericoronary Fat Excised from Pre-Diabetic Patients with Acute Myocardial Infarction. Biomedicines 2021;9:904. [Crossref] [PubMed]
- Scheller NM, Mogensen UM, Andersson C, et al. All-cause mortality and cardiovascular effects associated with the DPP-IV inhibitor sitagliptin compared with metformin, a retrospective cohort study on the Danish population. Diabetes Obes Metab 2014;16:231-6. [Crossref] [PubMed]
- Apaijai N, Chinda K, Palee S, et al. Combined vildagliptin and metformin exert better cardioprotection than monotherapy against ischemia-reperfusion injury in obese-insulin resistant rats. PLoS One 2014;9:e102374. [Crossref] [PubMed]
- Eriksson JW, Bodegard J, Nathanson D, et al. Sulphonylurea compared to DPP-4 inhibitors in combination with metformin carries increased risk of severe hypoglycemia, cardiovascular events, and all-cause mortality. Diabetes Res Clin Pract 2016;117:39-47. [Crossref] [PubMed]
- Yu OH, Yin H, Azoulay L. The combination of DPP-4 inhibitors versus sulfonylureas with metformin after failure of first-line treatment in the risk for major cardiovascular events and death. Can J Diabetes 2015;39:383-9. [Crossref] [PubMed]
- Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2014;2:702-14. [Crossref] [PubMed]
- Chinda K, Palee S, Surinkaew S, et al. Cardioprotective effect of dipeptidyl peptidase-4 inhibitor during ischemia-reperfusion injury. Int J Cardiol 2013;167:451-7. [Crossref] [PubMed]
- Yang CJ, Yang J, Yang J, et al. DPP-4 inhibitors: A promising feasible therapeutic approach for myocardial ischemia-reperfusion injury. Int J Cardiol 2015;201:253-4. [Crossref] [PubMed]
- Liu L, Liu J, Tian XY, et al. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal 2014;21:1571-81. [Crossref] [PubMed]
- Chen WR, Hu SY, Chen YD, et al. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J 2015;170:845-54. [Crossref] [PubMed]
- Marfella R, Sardu C, Calabrò P, et al. Non-ST-elevation myocardial infarction outcomes in patients with type 2 diabetes with non-obstructive coronary artery stenosis: Effects of incretin treatment. Diabetes Obes Metab 2018;20:723-9. [Crossref] [PubMed]
- Marfella R, Sardu C, Balestrieri ML, et al. Effects of incretin treatment on cardiovascular outcomes in diabetic STEMI-patients with culprit obstructive and multivessel non obstructive-coronary-stenosis. Diabetol Metab Syndr 2018;10:1. [Crossref] [PubMed]
- Sardu C, Paolisso P, Sacra C, et al. Cardiac resynchronization therapy with a defibrillator (CRTd) in failing heart patients with type 2 diabetes mellitus and treated by glucagon-like peptide 1 receptor agonists (GLP-1 RA) therapy vs. conventional hypoglycemic drugs: arrhythmic burden, hospitalizations for heart failure, and CRTd responders rate. Cardiovasc Diabetol 2018;17:137. [Crossref] [PubMed]


