
The relationship between epicardial adipose tissue volume on coronary computed tomography angiography and idiopathic ventricular tachycardia: a propensity score matching case-control study in Chinese population
Highlight box
Key findings
• The large epicardial adipose tissue (EAT) volume is associated with an extended repolarization process in idiopathic ventricular tachycardia (IVT) patients.
What is known and what is new?
• Large EAT volume is associated with the incidence of premature ventricular beats, but the relationship between EAT volume and IVT is not yet clear.
• In this case-control study, we first revealed that EAT volume, by coronary computed tomography angiography measurement, was increased in IVT patients compared with control subjects. This association was independent of other risk factors.
What is the implication, and what should change now?
• EAT volume might be a useful marker to identify the risk of IVT. EAT may be considered a promising therapeutic target.
Introduction
Idiopathic ventricular tachycardia (IVT) occurs in structurally normal heart patients and accounts for approximately 10% of all ventricular tachycardia (VT) patients (1). Severe levels of IVT may experience syncope, shock, and heart failure (2). An in-depth understanding of the relevant anatomy, underlying pathophysiology and electrocardiographic features of IVT is essential for effective management (3).
Epicardial adipose tissue (EAT) is a unique fatty deposit of the cardiac, which plays an important role in promoting arrhythmogenesis (4,5). Accumulation of EAT is associated with increased cardiometabolic risk (6). Under physiological conditions, EAT is 20% of heart mass. EAT can be considered as an endocrine organ, which promotes the occurrence and development of arrhythmia through the vasocrine or paracrine secretion of pro-inflammatory and pro-fibrotic factors (7). One study found a correlation between pericardial adipose tissue and ventricular fibrillation/tachycardia in patients with systolic heart failure (8). Another study revealed a larger EAT volume may be associated with IVT recurrence after catheter ablation in IVT patients (9). At present, the role of EAT in IVT remains unclear. Therefore, we hypothesize that increased EAT volume might be associated with the presence of IVT in this retrospective study, we enrolled a cohort to prove our hypothesis. We aimed to assess the relationship between EAT volume on coronary computed tomography angiography (CCTA) and the risk of IVT. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-345/rc).
Methods
Study population
IVT patients hospitalized undergoing CCTA from January 2020 to September 2022 at the First Affiliated Hospital of Zhengzhou University were consecutive retrospectively recruited. The inclusion criteria: (I) IVT patients underwent CCTA; (II) absence of coronary heart disease. The patients with age ≤18 years, a history of prior radiofrequency catheter ablation (RFCA), and poor/insufficient CCTA images were excluded. We performed a control group of hospitalized patients without IVT undergoing CCTA during the same time period. The patients in the control group were all hospitalized patients for different reasons, such as chest tightness, shortness of breath, chest pain, and so on. In our institution, CCTA was performed for hospitalized patients who screened for coronary heart disease. Patients diagnosed with IVT were identified using the International Classification of Disease (ICD) code in our hospital’s electronic health record systems. IVT diagnosis in patients was defined as an absence of structural heart disease and ventricular tachycardia lasting ≥30 s. (III) Smokers and drinkers were either former or active smokers and drinkers (10). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was authorized by the Research and Clinical Trial Ethics Committee of the First Affiliated Hospital of Zhengzhou University (No. 2022-KY-0288). Individual consent for this retrospective analysis was waived.
Clinical and laboratory data
Demographic parameters and clinical characteristics of each individual were collected from the patient’s medical records. The following data were collected for all patients: demographic parameters, comorbidities, electrocardiogram (ECG) parameters (QTc, Tp-e, and Tp-e/QTc), echocardiography parameters [left ventricular ejection fraction (LVEF), early peak/artial peak (E/A) ratios], CCTA parameters (EAT volume and attenuation), and medications on admission.
CCTA acquisition
All CCTA scanning was accomplished using dual-source computed tomography (CT) of a third-generation scanner (Somatom Force, Siemens Heathineers, Forchheim, Germany). CCTA images were acquired with the following scan protocol: Tube voltage was 120 kV. If the patient’s heart rate was >65 beats/min, an oral or intravenous β-blocker was administered to reduce the heart rate before scanning. Sublingual nitroglycerine (0.5 mg) was also administered immediately prior to CCTA scanning. Images were acquired after a bolus injection of 30 to 60 mL of contrast media (400 mg iodine per mL, Iomeprol Injection). Using prospective ECG-triggering with automatic tube current modulation, field matrix of 512×512, and scan slice thickness of 0.5 mm. Each reconstructed image was transferred to the reconstructed workstation for postprocessing.
EAT quantification
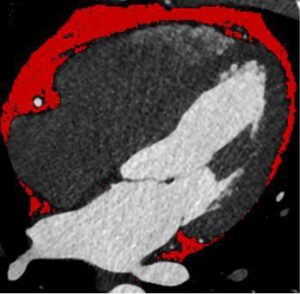
EAT is defined between the visceral epicardium and the myocardium (9). EAT was defined as CCTA density ranging from −190 to −30 Hounsfield units (HU) (11,12). EAT volume was quantified using dedicated semiautomatic software (syngo via Frontier Cardiac Risk Assessment, version 1.2.3, Siemens Healthineers, Erlangen, Germany), as described in Figure 1. The software automatically delineates and identifies EAT and manually adjusts the contour of EAT volume if necessary. We used the pulmonary artery bifurcation as the superior limit and the end of the left ventricular apex as the inferior limit of the heart. EAT volume measurements were performed by two radiologists who were unaware of the patient’s clinical data.
Statistical analysis
The values of the continuous variables were described as the mean ± standard deviation or median (Q1, Q3 quartiles) and compared among groups using the student’s t-test or Mann-Whitney U test, depending on whether data were normally distributed. The values of categorical variables are presented as numbers (percentages) and compared between two groups by Pearson Chi-squared test or Fisher exact test. The threshold value of EAT volume was determined by the median. The 1:1 propensity score (PS)-adjusted method was applied in the observational case-control study to reduce the bias in selecting the case controls, using a greedy and nearest neighbor matching algorithm with a caliper distance equal to 0.2. This process was done with the R package “MatchIt” (R Project for Statistical Computing). Matching of the two groups was performed for variables that univariate analysis showed P<0.10 (BMI ≥24 kg/m2, E/A ratios <1, and LVEF). The receiver operating curve (ROC) was used to evaluate the predictive value of EAT volume for the risk of IVT in the PS adjusted cohort.
The associations between EAT volume and continuous variables were investigated using Pearson’s correlation coefficient. Variables that univariate analysis showed P value <0.10 and important factors were included in the multivariate logistic analysis. Multivariable logistic analysis was performed to investigate the risk factors for IVT. Furthermore, we also assess the effect of EAT volume on ECG indexes. The sample size of this study was calculated in PASS software, based on an alpha of 0.05 and a beta of 0.1 (type I error), with a ratio of 1:1 between the experimental and control groups on the basis of previous literature (13), the sample size of the study was following the requirements. Statistical analysis was performed using R language version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value <0.05 was considered statistically significant.
Results
Baseline clinical features
A total of 104 IVT patients with CCTA were screened for eligibility, 81 IVT patients were included in the final analysis, as shown in Figure 2. The time from the IVT to CCTA was a median of 2 [1, 4] days in all patients. The mean age was 44.0±11.9 years old in IVT patients in IVT patients of the unadjusted cohort. Patients with IVT had significantly higher proportions of E/A ratios <1 (P=0.017). IVT patients had significantly larger BMI (P<0.001) and lower LVEF (P<0.001), compared with control patients in the unadjusted cohort.

We used PS-adjusted to reduce selection bias and potential clinical confounders. Following the 1:1 matching protocol, the differences in clinical characteristics between the two groups were eliminated. Compared to the patients with control, the IVT patients’ BMI, E/A ratios <1, and LVEF were not significantly different between the two groups in the PS-adjusted cohort, as described in Table 1. Based on the PS, PS-adjusted is an optimal balance between the two groups. The standardized mean difference value was all under 0.1, indicating that confounding factors bias was attenuated (Figure S1).
Table 1
| Variables | Unadjusted cohort | PS-adjusted cohort | |||||
|---|---|---|---|---|---|---|---|
| IVT (n=81) | Control (n=162) | P value | IVT (n=57) | Control (n=57) | P value | ||
| Age, years | 44.0±11.9 | 44.3±14.2 | 0.383 | 42.9±12.0 | 44.5±13.6 | 0.503 | |
| Female | 32 (39.5) | 70 (43.2) | 0.581 | 20 (35.1) | 20 (35.1) | >0.99 | |
| BMI ≥24 kg/m2 | 62 (76.5) | 60 (37.0) | <0.001 | 45 (78.9) | 47 (82.5) | 0.635 | |
| BMI, kg/m2 | 25.9±3.8 | 23.4±3.8 | <0.001 | 26.2±4.6 | 26.2±3.6 | 0.963 | |
| Smoker | 24 (24.0) | 37 (18.5) | 0.354 | 14 (24.6) | 12 (21.1) | 0.655 | |
| Drinker | 18 (22.2) | 33 (20.4) | 0.738 | 15 (26.3) | 16 (28.1) | 0.833 | |
| Hypertension | 19 (23.5) | 33(20.4) | 0.580 | 13 (22.8) | 10 (17.5) | 0.484 | |
| Diabetes mellitus | 5 (6.2) | 14 (8.6) | 0.499 | 4 (7.0) | 7 (12.5) | 0.361 | |
| Statins | 10 (12.3) | 11 (6.8) | 0.146 | 3 (5.3) | 7 (12.3) | 0.321 | |
| WBC, 109/L | 6.6±1.9 | 6.4±1.7 | 0.353 | 6.6±1.6 | 6.4±1.9 | 0.642 | |
| FPG, mmol/L | 5.0±1.3 | 4.7±1.5 | 0.104 | 4.8±0.7 | 4.8±1.4 | 0.920 | |
| TC, mmol/L | 4.1±1.0 | 4.2±0.7 | 0.312 | 4.3±0.7 | 4.2±1.1 | 0.748 | |
| TG, mmol/L | 1.3 (1.0–1.7) | 1.1 (0.8–1.5) | 0.692 | 1.4 (1.1–2.0) | 1.4 (0.9–2.0) | 0.615 | |
| HS-CRP (>2 mg/L) | 22 (27.2) | 37 (22.8) | 0.459 | 16 (28.1) | 20 (35.1) | 0.420 | |
| LVEF, % | 60.0±5.2 | 63.0±1.5 | <0.001 | 63.0±2.0 | 63.0±1.4 | 0.353 | |
| E/A ratios <1 | 43 (53.1) | 60 (37.0) | 0.017 | 21 (36.8) | 19 (33.3) | 0.695 | |
| EAT volume, mL | 169.2±48.2 | 92.7±35.8 | <0.001 | 171.1±50.0 | 109.6±37.1 | <0.001 | |
| EAT attenuation, HU | −81.0±4.6 | −80.7±4.9 | 0.701 | −79.5±4.5 | −81.4±4.6 | 0.026 | |
Data are presented as the mean ± SD, median (IQR) or n (%). IVT, idiopathic ventricular tachycardia; PS, propensity score; BMI, body mass index; WBC, white blood cell; FPG, fasting blood glucose; TC, total cholesterol; TG, triglycerides; HS-CRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction; EAT, epicardial adipose tissue; HU, Hounsfield units; SD, standard deviation.
EAT volume among IVT patients
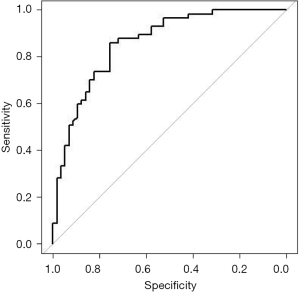
The volume of EAT distribution is described in Figure 3. Patients with IVT had significantly larger EAT volume compared with control patients in the unadjusted cohort (169.2±48.2 vs. 92.7±35.8 mL, P<0.001). IVT patients had also significantly larger EAT volumes compared with control patients in the PS-adjusted cohort (171.1±50.0 vs. 109.6±37.1 mL, P<0.001). The area under the curve of EAT volume to predict the risk of IVT patients in the PS-adjusted cohort was 0.859 [95% confidence interval (CI): 0.791–0.926]. The sensitivity was 86.0%. The specificity was 75.4%, as shown in Figure 4.

Variables with P<0.10 in the univariable analysis and important factors were included in the multivariable analysis model, including BMI, LVEF, E/A ratios <1, EAT attenuation, and EAT volume (per increase 10 mL). The multivariable logistic analysis found that EAT volume [per increase 10 mL, odds ratio (OR): 1.29, 95% CI: 1.17–1.41, P<0.001], BMI (OR: 1.19, 95% CI: 1.08–1.32, P=0.001) and LVEF (OR: 0.74, 95% CI: 0.64–0.85, P<0.001) were independent risk factors for IVT patients in the unadjusted cohort, as shown in Table 2. In the PS-adjusted cohort, EAT volume (per increase 10 mL, OR: 1.43, 95% CI: 1.25–1.64, P<0.001) and EAT attenuation (OR: 1.13, 95% CI: 1.01–1.27, P=0.035) were independently related to IVT, as depicted in Table 3.
Table 2
| Variables | OR (95% CI) | P value |
|---|---|---|
| BMI, kg/m2 | 1.19 (1.08–1.32) | 0.001 |
| E/A ratios <1 | 1.61 (0.80–3.22) | 0.180 |
| LVEF, % | 0.74 (0.64–0.85) | <0.001 |
| EAT attenuation, HU | 1.06 (0.98–1.14) | 0.135 |
| EAT volume (per increase 10 mL) | 1.29 (1.17–1.41) | <0.001 |
Variables that univariate analysis of the unadjusted cohort showed P value <0.10 were included in the multivariate analysis model, which included BMI, LVEF, E/A ratios <1, EAT attenuation, and EAT volume (per increase 10 mL). IVT, idiopathic ventricular tachycardia; OR, odds ratio; CI, confidence interval; BMI, body mass index; LVEF, left ventricular ejection fraction; EAT, epicardial adipose tissue; HU, Hounsfield units.
Table 3
| Variables | OR (95% CI) | P value |
|---|---|---|
| BMI, kg/m2 | 1.03 (0.90–1.18) | 0.660 |
| E/A ratios <1 | 1.43 (0.51–3.98) | 0.493 |
| LVEF, % | 0.85 (0.65–1.10) | 0.217 |
| EAT attenuation, HU | 1.13 (1.01–1.27) | 0.035 |
| EAT volume (per increase 10 mL) | 1.43 (1.25–1.64) | <0.001 |
Variables that univariate analysis of the unadjusted cohort showed P value <0.10 were included in the multivariate analysis model, which included BMI, LVEF, E/A ratios <1, EAT attenuation, and EAT volume (per increase 10 mL). IVT, idiopathic ventricular tachycardia; OR, odds ratio; CI, confidence interval; BMI, body mass index; LVEF, left ventricular ejection fraction; EAT, epicardial adipose tissue; HU, Hounsfield units.
Association between EAT volume and clinical characteristics
For IVT patients, the associations between EAT volume and clinical characteristics were evaluated. EAT volume had a positive correlation with age (r=0.321, P=0.003), BMI (r=0.376, P=0.001), white blood cell (WBC) (r=0.018, P=0.041), and fasting blood glucose (FBG) (r=0.158, P=0.027). Additionally, EAT volume had a negative correlation with LVEF (r=−0.120, P=0.013) (Table S1).
Further analysis of EAT volume in IVT patients
The median EAT volume was 147.3 mL in patients with IVT. There was no statistically significant increase of QTc in IVT patients with large EAT volume (≥147.3 mL) compared to low EAT volume (<147.3 mL) in IVT patients (437.7±26.8 vs. 425.7±29.0 ms, P=0.058). The large EAT volume (≥147.3 mL) patients had significantly longer Tp-e, and Tp-e/QTc, compared with low EAT volume (<147.3 mL) in IVT patients (114.1±12.8 vs. 108.0±13.0 ms, P=0.034; 0.26±0.03 vs. 0.24±0.04, P=0.020; respectively), as described in Figure 5.

Discussion
In this case-control study, we first revealed that EAT volume, by CCTA measurement, was increased in IVT patients compared with control subjects. This association was independent of other risk factors. The Tp-e, and Tp-e/QTc duration were long in IVT patients with large EAT volume. These insights are essential for understanding the mechanisms linking EAT with IVT.
The uniqueness of EAT is not only in its peculiar anatomy and unobstructed proximity to the heart but also in its specific transcriptome, which is different from that of other visceral fat depots (14). We performed a robust methodology CT acquisition and assessment following standard methods and used specific cardiac software to quantify EAT automatically. EAT volume by CCTA measurement is convenient, which is feasible and reliable.
To date, some studies have attempted to evaluate the relationship between EAT and arrhythmias (15,16). A meta-analysis study of 63 observational studies found that EAT volume is larger in patients with atrial fibrillation compared to sinus rhythm patients (17). Patients with frequent ventricular premature beats had increased EAT thickness compared to control subjects. EAT thickness was independently associated with frequent ventricular premature beats (18). Fat deposition in patients with post-myocardial infarction ventricular tachycardia undergoing catheter ablation is associated with scar size and may be a marker for poor outcomes, including all-cause mortality and VT recurrence (19). The EAT component changes the local electrophysiological properties and may provide new ideas for arrhythmias risk assessment and prevention (16). Our study first revealed that patients with IVT had a larger EAT volume than control patients in our study. EAT may be involved in the formation of IVT substrates (20).
Tp-e and Tp-e/QTc, as indicators of cardiac repolarization, could predict the occurrence of arrhythmias. Tp-e as a stable indicator could avoid the effect of heart rate (21). Prolonged Tp-e intervals are associated with the development of VT and sudden cardiac death (22). The Tp-e interval and Tp-e/QTc ratio were elevated in patients with higher EAT thickness (23). These repolarization indicators were increased in patients with a large EAT volume in our study, which may suggest that a large EAT volume is associated with increases cardiac repolarization in IVT patients. Hence, we revealed that EAT volume may potentially assess this risk of IVT.
The exact mechanism linking EAT and IVT has not been fully established. Several critical mechanisms can be used to illustrate this regulation. First, the accumulation of EAT could cause lipotoxicity, and secretion of some adipokines could cause electrical remodeling of cardiomyocytes and stimulate myocardial fibrosis (24). Second, EAT can secrete some inflammatory factors, such as tumor necrosis factor-alpha, plasminogen activator inhibitor-1, and interleukine-6. A high inflammatory state can lead to cardiac remodeling (25). Furthermore, EAT can increase myocardial repolarization through paracrine effects on the myocardium, leading to heterogeneity of myocardial action potentials, and inducing arrhythmias (16,20). Further studies are needed to explore the pathophysiological of EAT in the prognosis of IVT.
Given the important role of EAT in the development of arrhythmias, it is considered a promising therapeutic target. New evidence suggests that physical activity and low-calorie diets may be effective non-pharmacological strategies for reducing EAT (26). EAT may guide treatment decisions in patients with diabetes because drugs such as metformin, sodium glucose cotransporter 2 inhibitors, and glucagon-like peptide-1 receptor agonists are associated with reduced EAT (27). Some anti-inflammatory drugs such as colchicine and methotrexate may have indirect effects on reduce EAT (28). However, we should note that whether reduction of EAT can confer benefits for patients’ clinical outcomes requires further demonstration. When patients have a large EAT volume, whether drug treatment is needed should also be further confirmed.
Limitations
Our study had several limitations. First, this was a single-center study with a small cohort, which might bias the results. Second, patients enrolled in our study rarely underwent cardiac magnetic resonance imaging, which may have resulted in patients with cardiomyopathy being missed. Although structural heart disease is an exclusion criterion, some patients show low left ventricular ejection fraction. We cannot assume that these patients do not have non-ischemic cardiomyopathy, and ventricular tachycardia may be secondary, thus affecting the results. Third, our study did not include drugs such as hypoglycemia, which may have biased the results. Furthermore, some patients did not undergo radiofrequency ablation in our study, and we could not analysis the EAT volume effect on the locations of IVT origin, which may limit the generalizability of the results. Finally, the EAT volume quantitative software we used was unable to quantify the periventricular EAT volume. The effect of periventricular EAT volume on IVT needs to be further study. Further prospective studies are needed to confirm the findings.
Conclusions
EAT volume was larger in patients with IVT patients compared with control patients. A large EAT volume was associated with prolonged cardiac repolarization. EAT volume might be a useful marker to identify the risk of IVT.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-345/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-345/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-345/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-345/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was authorized by the Research and Clinical Trial Ethics Committee of the First Affiliated Hospital of Zhengzhou University (No. 2022-KY-0288). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stevenson WG, Tandri H, Roden DM. The Shape of Ventricular Tachycardia. Circulation 2023;148:1368-70. [Crossref] [PubMed]
- Della Bella P, Baratto F, Vergara P, et al. Does Timing of Ventricular Tachycardia Ablation Affect Prognosis in Patients With an Implantable Cardioverter Defibrillator? Results From the Multicenter Randomized PARTITA Trial. Circulation 2022;145:1829-38. [Crossref] [PubMed]
- Ward RC, van Zyl M, DeSimone CV. Idiopathic Ventricular Tachycardia. J Clin Med 2023;12:930. [Crossref] [PubMed]
- Zhao J, Zhang Y, Yin Z, et al. Impact of proinflammatory epicardial adipose tissue and differentially enhanced autonomic remodeling on human atrial fibrillation. J Thorac Cardiovasc Surg 2023;165:e158-74. [Crossref] [PubMed]
- Yuvaraj J, Cameron W, Andrews J, et al. Coronary computed tomography angiography-based assessment of vascular inflammation in patients with obstructive sleep apnoea and coronary artery disease. Cardiovasc Diagn Ther 2022;12:123-34. [Crossref] [PubMed]
- Sang C, Hu X, Zhang D, et al. The predictive value of left atrium epicardial adipose tissue on recurrence after catheter ablation in patients with different types of atrial fibrillation. Int J Cardiol 2023;379:33-9. [Crossref] [PubMed]
- Wang Z, Jiao S, Chen J, et al. The relationship between frequent premature ventricular complexes and epicardial adipose tissue volume. Front Endocrinol (Lausanne) 2023;14:1219890. [Crossref] [PubMed]
- Wu CK, Tsai HY, Su MY, et al. Pericardial fat is associated with ventricular tachyarrhythmia and mortality in patients with systolic heart failure. Atherosclerosis 2015;241:607-14. [Crossref] [PubMed]
- Sepehri Shamloo A, Schoene K, Stauber A, et al. Epicardial adipose tissue thickness as an independent predictor of ventricular tachycardia recurrence following ablation. Heart Rhythm 2019;16:1492-8. [Crossref] [PubMed]
- Lin A, Nerlekar N, Yuvaraj J, et al. Pericoronary adipose tissue computed tomography attenuation distinguishes different stages of coronary artery disease: a cross-sectional study. Eur Heart J Cardiovasc Imaging 2021;22:298-306. [Crossref] [PubMed]
- Liu J, Yu Q, Li Z, et al. Epicardial adipose tissue density is a better predictor of cardiometabolic risk in HFpEF patients: a prospective cohort study. Cardiovasc Diabetol 2023;22:45. [Crossref] [PubMed]
- Takahashi D, Fujimoto S, Nozaki YO, et al. Validation and clinical impact of novel pericoronary adipose tissue measurement on ECG-gated non-contrast chest CT. Atherosclerosis 2023;370:18-24. [Crossref] [PubMed]
- Cosson E, Nguyen MT, Rezgani I, et al. Epicardial adipose tissue volume and myocardial ischemia in asymptomatic people living with diabetes: a cross-sectional study. Cardiovasc Diabetol 2021;20:224. [Crossref] [PubMed]
- Sato Y, Kawai H, Hoshino M, et al. Relationship between epicardial adipose tissue and coronary artery stenoses on computed tomography in patients scheduled for carotid artery revascularization. J Cardiol 2022;79:588-95. [Crossref] [PubMed]
- Sani MM, Sung E, Engels M, et al. Association of epicardial and intramyocardial fat with ventricular arrhythmias. Heart Rhythm 2023;20:1699-705. [Crossref] [PubMed]
- Patel KHK, Hwang T, Se Liebers C, et al. Epicardial adipose tissue as a mediator of cardiac arrhythmias. Am J Physiol Heart Circ Physiol 2022;322:H129-44. [Crossref] [PubMed]
- Wong CX, Sun MT, Odutayo A, et al. Associations of Epicardial, Abdominal, and Overall Adiposity With Atrial Fibrillation. Circ Arrhythm Electrophysiol 2016;9:e004378. [Crossref] [PubMed]
- Kırış A, Turan OE, Kırış G, et al. The relationship between epicardial fat tissue thickness and frequent ventricular premature beats. Kardiol Pol 2015;73:527-32. [Crossref] [PubMed]
- Cheniti G, Sridi S, Sacher F, et al. Post-Myocardial Infarction Scar With Fat Deposition Shows Specific Electrophysiological Properties and Worse Outcome After Ventricular Tachycardia Ablation. J Am Heart Assoc 2019;8:e012482. [Crossref] [PubMed]
- Ernault AC, Meijborg VMF, Coronel R. Modulation of Cardiac Arrhythmogenesis by Epicardial Adipose Tissue: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;78:1730-45. [Crossref] [PubMed]
- Tse G, Yan BP. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace 2017;19:712-21. [Crossref] [PubMed]
- Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, et al. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol 2006;47:1828-34. [Crossref] [PubMed]
- Kaplan O, Kurtoglu E, Nar G, et al. Evaluation of Electrocardiographic T-peak to T-end Interval in Subjects with Increased Epicardial Fat Tissue Thickness. Arq Bras Cardiol 2015;105:566-72. [Crossref] [PubMed]
- Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol 2022;19:593-606. [Crossref] [PubMed]
- McLaughlin T, Schnittger I, Nagy A, et al. Relationship Between Coronary Atheroma, Epicardial Adipose Tissue Inflammation, and Adipocyte Differentiation Across the Human Myocardial Bridge. J Am Heart Assoc 2021;10:e021003. [Crossref] [PubMed]
- Tian S. The effects of physical exercise on epicardial adipose tissue: Some methodological issues could be considered. Obes Rev 2021;22:e13233. [Crossref] [PubMed]
- Myasoedova VA, Parisi V, Moschetta D, et al. Efficacy of cardiometabolic drugs in reduction of epicardial adipose tissue: a systematic review and meta-analysis. Cardiovasc Diabetol 2023;22:23. [Crossref] [PubMed]
- Opstad TB, Papotti B, Åkra S, et al. Sirtuin1, not NAMPT, possesses anti-inflammatory effects in epicardial, pericardial and subcutaneous adipose tissue in patients with CHD. J Transl Med 2023;21:644. [Crossref] [PubMed]


