
Triglyceride levels and risk of cardiovascular disease and all-cause mortality in Chinese adults younger than 40 years old: a prospective cohort study
Highlight box
Key findings
• Among Chinese young adults, elevated fasting triglyceride (TG) levels were associated with increased risk of cardiovascular disease (CVD) and all-cause mortality.
What is known and what is new?
• TG levels were positively associated with CVD and all-cause mortality in the middle-aged or elderly population.
• TG levels were positively associated with CVD and all-cause mortality in Chinese young adults.
What is the implication, and what should change now?
• TG levels have the potential to serve as a reference biomarker for long-term incidents of CVD and all-cause mortality among young adults.
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity (1) and mortality (2). Globally, the incidence of CVD has risen rapidly in recent decades (1), especially among younger adults (3). Dyslipidemia is a key modifiable risk factor for CVD and can be modified through lifestyle changes and/or medications (4). As dyslipidemia has a cumulative effect on the risk of CVD, one of the most effective strategies to prevent CVD by slowing the development and progression of plaque is achieving a healthy lipid level as early as possible and maintaining this healthy lipid level throughout life (5).
Low-density lipoprotein cholesterol (LDL-C) is a well-established risk factor for CVD. In recent years, growing evidence suggests that elevated triglyceride (TG) levels may contribute to residual cardiovascular risk, even in patients with controlled LDL-C levels (6). The positive association between higher TG levels and CVD events has been reported in participants of different sexes (7,8) and ethnicities (9,10) as well as in certain high-risk individuals, such as those with abnormal glucose metabolism (11) and hypertension (12). Additionally, studies have reported a predictive role of TG levels for CVD mortality (13) and all-cause mortality (14).
However, prior studies regarding TG-associated risks of CVD, CVD subtypes, and mortality have mainly focused on the middle-aged or elderly population (7-14), with only three studies examining the younger population (15-17). In addition, all three studies focused on South Korean young adults (15-17), with no studies on Chinese young adults. Furthermore, the current guidelines mainly focus on the management of TG among adults aged 40 to 75 years (18). Therefore, it is imperative to carry out studies to determine whether more attention should be given to TG levels among adults younger than 40 years.
In the present study, Chinese adults younger than 40 years were recruited to examine the association of baseline fasting TG levels with CVD and all-cause mortality risks in later life. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-412/rc).
Methods
Study population
The Kailuan study, a prospective dynamic cohort study, was started in 2006 and conducted in Tangshan, China. The study design and procedures have been previously described in detail (19). In brief, participants from the Kailuan Group, which is a coal miner group, received a unified standard physical health examination and questionnaire survey at 11 affiliated hospitals of the Kailuan Group and were followed up every 2 years. Every participant was then followed until their death or until December 31, 2020, whichever came first.
In the current study, participants who attended health checkups for the first time between 2006 and 2016 and were <40 years old were selected as the research subjects (n=44,715). Participants without baseline TG values were excluded (n=698). Individuals with a history of CVD or cancer were also excluded (n=77). Additionally, participants using lipid-lowering medications at baseline were excluded (n=58). Ultimately, 43,882 participants were enrolled as the analytical sample (Figure S1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Kailuan General Hospital (No. 200605) and informed consent was obtained from all individual participants. All participating hospitals were informed and agreed on the study.
Data collection and definitions
Baseline data on demographic, behavioral, and clinical information, including age, sex, smoking status, alcohol consumption, physical exercise, education level, medical history (hypertension and diabetes), medication use (antihypertensive agents, hypoglycemic agents, and lipid-lowering agents), and family history of CVD, were collected via face-to-face questionnaires by trained interviewers. Smokers who smoked more than 1 cigarette per day on average over the past year were defined as current smokers. Drinkers who consumed more than 100 mL of alcohol per day on average with an alcohol concentration of ≥50% v/v in the past year were defined as current drinkers (20). Physical activity that exceeded 20 minutes per session and more than 3 times per week was classified as active (21). Education level was classified as high school or above and middle school or below. The height and weight of the participants were measured by trained nurses. Body mass index (BMI) was calculated by dividing the weight in kilograms by the height in meters squared. Obesity was defined as BMI ≥28 kg/m2 according to the criteria of the Chinese Working Group on Obesity (20). A mercury sphygmomanometer was used to measure blood pressure in the seated position after at least five minutes of rest, and an average value of at least three measurements of both systolic blood pressure (SBP) and diastolic blood pressure (DBP) was obtained. Mean arterial pressure (MAP) was calculated using the following formula: MAP = DBP + 1/3 (SBP − DBP). Hypertension was defined as an SBP over 140 mmHg or a DBP over 90 mmHg, any use of antihypertensive agents, or a history of hypertension.
After overnight fasting (8–12 h), blood samples were obtained via the participants’ elbow veins. At the Kailuan General Hospital, serum specimens were stored at −80 °C in the central laboratory. All serum samples were assessed using a Hitachi 747 autoanalyzer (Hitachi, Tokyo, Japan). The enzymatic colorimetric method was used to measure serum TG, LDL-C, high-density lipoprotein cholesterol (HDL-C), and total cholesterol (TC) (22). The hexokinase/glucose-6-phosphate dehydrogenase method was used for the measurement of fasting blood glucose (FBG). Diabetes was defined as self-reported physician-diagnosed type 2 diabetes, FBG levels ≥7.0 mmol/L, or any use of hypoglycemic agents. Lipid-lowering agents refer to self-reported lipid-lowering medication use, including statins, niacin, and fibrates.
Study outcomes and follow-up
The primary outcomes were incident CVD [a composite endpoint of myocardial infarction (MI) and ischemic stroke] and all-cause mortality. The secondary outcomes were individual CVD subtypes, including MI, and ischemic stroke. Assessment of CVD and all-cause mortality has been described previously (23,24). Briefly, during the follow-up period, the Municipal Social Insurance Institution of Tangshan and the Hospital Discharge Register of the Kailuan Group’s 11 affiliated hospitals provided information on CVD, which was updated annually. MI was diagnosed based on the 2007 universal definition (25) determined by clinical symptoms, dynamic changes in myocardial enzymes, and electrocardiogram results. Ischemic stroke was defined following the World Health Organization criteria according to symptoms, signs, and neuroimaging data (computed tomography or magnetic resonance imaging) (26). Data on death were collected from provincial vital statistics offices and confirmed by physicians. The study population was followed up from the baseline date to the death date or December 31, 2020, whichever came first.
Statistical analysis
In the present study, the dose-response relationship was explored by restricted cubic splines, and non-linear positive associations were found between TG levels and CVD, CVD subtypes, and all-cause mortality (Figures S2,S3). To better examine these associations, the study participants were subdivided into four groups based on the baseline TG levels: quartile 1 (Q1) <67 mg/dL, Q2 67 to 97 mg/dL, Q3 98 to 150 mg/dL, and Q4 ≥151 mg/dL. Continuous variables are described as the mean ± standard deviation (SD) or the median (interquartile range) and were compared using analysis of variance (ANOVA) or Kruskal-Wallis tests based on distribution. Categorical variables are described as numbers (percentages) and were compared using the chi-square test. To handle the missing values of the covariates (as shown in Table S1, all covariates with a missing rate of less than 15%), we applied multiple imputation with 10 rounds using chained equations as previously described (27,28).
The person-years were determined from the baseline date to either the date of outcomes (CVD or death) or December 31, 2020, whichever came first. The Kaplan-Meier curve and log-rank test were adopted for univariable survival analysis. Two Cox proportional hazard regression models were constructed to estimate the hazard ratios (HRs) of CVD, all-cause mortality, and CVD subtypes. Model 1 was adjusted for age and sex; Model 2 was further adjusted for obesity, HDL-C, LDL-C, smoking status, alcohol consumption, physical activity, education level, hypertension, diabetes, and family history of CVD. For each model, P values for the trend were determined using the quartiles of TG as an ordinal variable. All models were tested for proportional hazard assumption, and no appreciable violation was observed. Based on the variance inflation factor, multicollinearity among covariables in Model 2 was found to be acceptable (Table S2). To gain insight into the linearity of associations between baseline TG levels and the risk of CVD, CVD subtypes, and all-cause mortality, restricted cubic splines were constructed. The receiver operating characteristic (ROC) curve was plotted to identify the optimal cutoff value (the best Youden Index: sensitivity + specificity −1) for each primary outcome.
To assess the robustness of our results, we also performed the following sensitivity analyses excluding participants with factors that impact TG levels, CVD events, or all-cause mortality (29,30): (I) exclusion of participants with LDL-C ≥190 mg/dL at baseline due to the elevated risk of CVD and mortality (31); (II) exclusion of participants on antihypertensive or hypoglycemic agents at baseline; (III) exclusion of participants with new-onset cancer during follow-up; (IV) exclusion of patients who experienced outcomes during the first 2-year follow-up period; (V) exclusion of participants with missing covariable values and complete-case analysis; (VI) exclusion of current drinkers at baseline; (VII) exclusion of participants with obesity at baseline; (VIII) exclusion of participants with hypertension at baseline; (IX) exclusion of participants with diabetes at baseline; (X) exclusion of participants with metabolic syndrome at baseline.
All analyses were carried out using Stata (version 15.1, StataCorp, College Station, TX, USA). A two-sided P<0.05 was considered statistically significant.
Results
The mean age of the 43,882 participants was 30.6±5.56 years, and 35,173 (80.2%) were males. Overall, participants with higher TG levels tended to be older, male, obese, current smokers, and current drinkers. They also had higher levels of MAP, FBG, and LDL-C and had a higher prevalence of hypertension and diabetes mellitus. In addition, participants with higher TG levels had a lower education level and were more likely to use antihypertensive and glucose-lowering medications (Table 1).
Table 1
| Variables | TG (mg/dL) | P value | ||||
|---|---|---|---|---|---|---|
| Total | Q1 [<67] | Q2 [67–97] | Q3 [98–150] | Q4 [≥151] | ||
| Participants, n | 43,882 | 11,063 | 10,886 | 10,849 | 11,084 | |
| Age, years | 30.6±5.56 | 29.8±5.55 | 30.2±5.54 | 30.7±5.60 | 31.6±5.38 | <0.001 |
| Male | 35,173 (80.2) | 7,332 (66.3) | 8,390 (77.1) | 9,172 (84.5) | 10,279 (92.7) | <0.001 |
| Obesity | 7,125 (16.2) | 593 (5.4) | 1,127 (10.4) | 1,970 (18.2) | 3,435 (31.0) | <0.001 |
| MAP, mmHg | 92.3 (83.8, 97.8) | 88.3 (81.3, 94.0) | 90.4 (83.3, 96.7) | 93.3 (85.6, 98.7) | 94.7 (90.0, 103) | <0.001 |
| FBG, mg/dL | 91.9±24.9 | 89.3±27.5 | 90.2±21.3 | 92.1±22.3 | 95.8±27.2 | <0.001 |
| HDL-C, mg/dL | 53.4 (46.0, 61.9) | 56.1 (48.0, 65.0) | 53.8 (46.8, 61.5) | 53.0 (46.0, 61.1) | 50.7 (42.9, 59.9) | <0.001 |
| LDL-C, mg/dL | 92.8 (76.6, 112) | 83.9 (66.9, 102) | 92.8 (78.1, 109) | 96.7 (82.4, 115) | 98.2 (79.3, 118) | <0.001 |
| Current smoker | 15,863 (36.1) | 3,144 (28.4) | 3,583 (32.9) | 4,000 (36.9) | 5,136 (46.3) | <0.001 |
| Current drinker | 19,517 (44.5) | 4,213 (38.1) | 4,397 (40.4) | 4,866 (44.9) | 6,041 (54.5) | <0.001 |
| Active physical activity | 3,545 (8.1) | 911 (8.2) | 881 (8.1) | 817 (7.5) | 936 (8.4) | 0.08 |
| High school or above | 24,352 (55.5) | 6,697 (60.5) | 6,223 (57.2) | 5,859 (54.0) | 5,573 (50.3) | <0.001 |
| Hypertension | 3,617 (8.2) | 303 (2.7) | 605 (5.6) | 1,106 (10.2) | 1,603 (14.5) | <0.001 |
| Diabetes | 532 (1.2) | 54 (0.5) | 74 (0.7) | 132 (1.2) | 272 (2.5) | <0.001 |
| Antihypertensive agents | 591 (1.3) | 68 (0.6) | 95 (0.9) | 152 (1.4) | 276 (2.5) | <0.001 |
| Hypoglycemic agents | 135 (0.3) | 20 (0.2) | 26 (0.2) | 31 (0.3) | 58 (0.5) | <0.001 |
| Family history of CVD | 1,127 (2.6) | 244 (2.2) | 263 (2.4) | 277 (2.6) | 343 (3.1) | <0.001 |
a, continuous variables with normal distribution are described as mean ± standard deviation and were compared using ANOVA; continuous variables with skewed distribution are presented as median (interquartile range) and were compared using the Kruskal-Wallis test; categorical variables are described as number (percentage) and were compared using the chi-square test. TG, triglyceride; Q, quartile; MAP, mean arterial pressure, FBG, fasting blood glucose; HDL-C, high-density lipid cholesterol; LDL-C, low-density lipid cholesterol; CVD, cardiovascular disease; ANOVA, analysis of variance.
During a median follow-up of 11.2 (7.54 to 13.8) years, 298 incident CVD and 345 all-cause mortality cases were recorded. In terms of CVD subtypes, 74 cases of MI, 227 cases of ischemic stroke, and 3 cases of concurrent MI and stroke were documented.
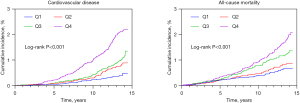
The incidence of CVD and all-cause mortality were 0.67 and 0.76 per 1,000 person-years, respectively. From the lowest TG quartile to the second, third, and highest quartiles, the incidence of CVD increased from 0.25 to 0.47, 0.63, and 1.25 per 1,000 person-years, respectively, and the incidence of all-cause mortality increased from 0.45 to 0.55, 0.85, and 1.17 per 1,000 person-years, respectively (Table 2). Participants with higher baseline TG levels had a higher risk of CVD events and all-cause mortality (Figure 1; all log-rank P<0.001). After adjusting for age, sex, obesity, high-density lipid cholesterol, low-density lipid cholesterol, smoking status, alcohol consumption, physical activity, education level, hypertension, diabetes, and family history of CVD, compared with those for participants in the lowest quartile, the adjusted HR of incident CVD for participants in the highest quartile was 2.26 [95% confidence interval (CI): 1.56 to 3.29; P=0.001]. The corresponding HR of all-cause mortality for participants in the highest quartile was 1.61 (95% CI: 1.14 to 2.28; P=0.007) (Table 2). Additionally, the results of the prespecified sensitivity analyses showed consistent positive associations between TG levels and CVD and all-cause mortality as did the primary analysis (Tables S3,S4).
Table 2
| Triglyceride, mg/dL | Events/total | Incidence, per 1,000 person-years | Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | P for trend | HR (95% CI) | P value | P for trend | ||||
| CVD | 298/43,882 | 0.67 | <0.001 | <0.001 | |||||
| Q1 [<67] | 28/11,063 | 0.25 | Reference | – | Reference | – | |||
| Q2 [67–97] | 52/10,886 | 0.47 | 1.57 (0.99, 2.48) | 0.05 | 1.36 (0.87, 2.19) | 0.17 | |||
| Q3 [98–150] | 72/10,849 | 0.63 | 1.77 (1.14, 2.75) | 0.01 | 1.38 (0.88, 2.14) | 0.16 | |||
| Q4 [≥151] | 146/11,084 | 1.25 | 2.96 (1.96, 4.46) | <0.001 | 2.26 (1.56, 3.29) | 0.001 | |||
| Per SD increase | 1.09 (1.05, 1.14) | <0.001 | 1.08 (1.02, 1.15) | 0.01 | |||||
| All-cause mortality | 345/43,882 | 0.76 | <0.001 | 0.001 | |||||
| Q1 [<67] | 50/11,063 | 0.45 | Reference | – | Reference | – | |||
| Q2 [67–97] | 61/10,886 | 0.55 | 1.08 (0.74, 1.57) | 0.70 | 1.05 (0.72, 1.54) | 0.78 | |||
| Q3 [98–150] | 97/10,849 | 0.85 | 1.47 (1.04, 2.07) | 0.02 | 1.40 (0.98, 1.99) | 0.06 | |||
| Q4 [≥151] | 137/11,084 | 1.17 | 1.75 (1.25, 2.45) | 0.001 | 1.61 (1.14, 2.28) | 0.007 | |||
| Per SD increase | 1.07 (1.02, 1.12) | 0.004 | 1.06 (1.01, 1.12) | 0.02 | |||||
Model 1 was adjusted for age and sex; Model 2 was adjusted for age, sex, obesity, high-density lipid cholesterol, low-density lipid cholesterol, smoking status, alcohol consumption, physical activity, education level, hypertension, diabetes, and family history of CVD. HR, hazard ratio; CI, confidence interval; CVD, cardiovascular disease; Q, quartile; SD, standard deviation.
The area under the curve (AUC) of the TG levels for CVD and all-cause mortality was 0.664 (95% CI: 0.634 to 0.695) and 0.616 (95% CI: 0.586 to 0.645), respectively. The cutoff points of TG levels for incident CVD were 110 mg/dL with 66.4% sensitivity and 57.3% specificity; the corresponding cutoff points for all-cause mortality were 102 mg/dL with 66.7% sensitivity and 52.0% specificity, respectively (Table S5). In multivariable Cox-regression models, TG ≥110 mg/dL was associated with a higher risk for CVD (adjusted HR: 1.46; 95% CI: 1.13 to 1.88; P=0.003), and TG ≥102 mg/dL was associated with a higher risk for all-cause mortality (adjusted HR: 1.50; 95% CI: 1.18 to 1.91; P=0.001) (Table 3).
Table 3
| Triglyceride, mg/dL | Events/total | Incidence, per 1,000 person-years | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||||
| CVD | 298/43,882 | 0.66 | |||||
| <110 | 98/25,072 | 0.38 | Reference | – | Reference | – | |
| ≥110 | 200/18,810 | 1.01 | 1.86 (1.45, 2.37) | <0.001 | 1.46 (1.13, 1.88) | 0.003 | |
| All-cause mortality | 345/43,882 | 0.76 | |||||
| <102 | 115/22,747 | 0.50 | Reference | – | Reference | – | |
| ≥102 | 230/21,135 | 1.03 | 1.59 (1.27, 2.00) | <0.001 | 1.50 (1.18, 1.91) | 0.001 | |
Model 1 was adjusted for age and sex; Model 2 was adjusted for age, sex, obesity, high-density lipid cholesterol, low-density lipid cholesterol, smoking status, alcohol consumption, physical activity, education level, hypertension, diabetes, and family history of CVD. HR, hazards ratio; CI, confidence interval; CVD, cardiovascular diseases.
In terms of CVD subtypes, the incidence of MI, and ischemic stroke were 0.16, and 0.50 per 1,000 person-years, respectively. Participants with higher baseline TG levels tended to have higher risks of MI and ischemic stroke (Figure 2; all log-rank P<0.001). Compared with those for participants in the lowest quartile, the adjusted HRs (95% CIs) for MI for participants in the highest quartile was 3.25 (95% CI: 1.33 to 7.97; P=0.01), respectively; for ischemic stroke, that was 1.88 (95% CI: 1.16 to 3.04; P=0.01), respectively (Table 4).
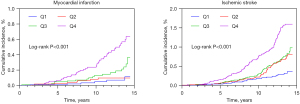
Table 4
| Triglyceride, mg/dL | Events/total | Incidence, per 1,000 person-years | Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | P for trend | HR (95% CI) | P value | P for trend | ||||
| Myocardial infarction | 74/43,882 | 0.16 | <0.001 | 0.001 | |||||
| Q1 [<67] | 6/11,063 | 0.05 | Reference | – | Reference | – | |||
| Q2 [67–97] | 8/10,886 | 0.07 | 1.26 (0.44, 3.63) | 0.67 | 1.07 (0.37, 3.08) | 0.90 | |||
| Q3 [98–150] | 18/10,849 | 0.16 | 2.55 (1.01, 6.42) | 0.04 | 1.83 (0.71, 4.70) | 0.20 | |||
| Q4 [≥151] | 42/11,084 | 0.36 | 5.35 (2.27, 12.6) | <0.001 | 3.25 (1.33, 7.97) | 0.01 | |||
| Per SD increase | 1.10 (1.04, 1.16) | 0.001 | 1.09 (1.02, 1.19) | 0.02 | |||||
| Ischemic stroke | 227/43,882 | 0.50 | <0.001 | 0.01 | |||||
| Q1 [<67] | 22/11,063 | 0.20 | Reference | – | Reference | – | |||
| Q2 [67–97] | 45/10,886 | 0.40 | 1.73 (1.05, 2.85) | 0.03 | 1.31 (0.79, 2.17) | 0.29 | |||
| Q3 [98–150] | 54/10,849 | 0.47 | 1.75 (1.06, 2.89) | 0.03 | 1.53 (0.91, 2.56) | 0.10 | |||
| Q4 [≥151] | 106/11,084 | 0.91 | 2.80 (1.76, 4.48) | <0.001 | 1.88 (1.16, 3.04) | 0.01 | |||
| Per SD increase | 1.09 (1.04, 1.14) | 0.001 | 1.08 (1.01, 1.15) | 0.01 | |||||
Model 1 was adjusted for age and sex; Model 2 was adjusted for age, sex, obesity, high-density lipid cholesterol, low-density lipid cholesterol, smoking status, alcohol consumption, physical activity, education level, hypertension, diabetes, and family history of cardiovascular disease. HR, hazard ratio; CI, confidence interval; Q, quartile; SD, standard deviation.
Discussion
In the present study, we assessed the association between fasting TG and the risk of incident CVD and all-cause mortality in adults younger than 40 years from the Kailuan cohort. We reported two main findings: (I) elevated TG levels were associated with higher risks of CVD and all-cause mortality, which were independent of potential confounders such as age, sex, obesity, high-density lipid cholesterol, low-density lipid cholesterol, smoking status, alcohol consumption, physical activity, education level, hypertension, diabetes, and family history of CVD; (II) this association still existed even in participants with “normal” TG levels (150 mg/dL) according to the current guidelines.
Until now, there has been no large prospective study reporting any association between baseline TG and long-term CVD among Chinese young adults. Although the association between TG levels and CVD risk has been extensively studied in observational studies (32,33), only three studies have focused on young adults and reported a positive association between baseline TG level and incident CVD (including MI and stroke) (15-17). However, all three studies were from the same database in South Korea (the claims database from the National Health Insurance Service), which limits the extrapolation of the results to other populations, so it makes sense to conduct studies among young people in other countries. Consistent with those studies, our study provided the first evidence that high levels of TG were independently related to increased future CVD risk in Chinese young adults. This finding highlights the necessity of early screening of TG levels.
The underlying mechanisms between TG and the risk of CVD are unclear. Evidence supports that TG may act as a biomarker of other risk factors rather than a direct driver for incident CVD. First, a high TG level has been regarded as an early marker of underlying metabolic dysfunction, leading to additional cardiovascular risk factor accumulation, such as elevated TC and LDL-C levels and obesity (34). Second, high TG levels are more closely related to unhealthy lifestyles such as heavy alcohol consumption and lack of exercise, which reflect different pathophysiological effects in the development of CVD (35). Third, the majority of TG molecules are carried in two different lipoproteins: chylomicrons and very low-density lipoproteins. It is well-known that patients with familial chylomicronemia syndrome (FCS) have a low risk of CVD despite extremely high TG levels (>1,000 mg/dL) since birth (36). Therefore, the type of lipoproteins, rather than TG itself, is an important factor. Fourth, the lipolysis of those lipoproteins results in the formation of remnant lipoproteins that can penetrate arterial walls and can be taken up by macrophages, which will further transform into foam cells (37,38). Last, the lipolysis of TG-rich lipoproteins produces a high amount of oxidized free fatty acids, monoacylglycerols, and other molecules, which can induce endothelial cell inflammation and promote atherogenesis (38-40).
Prior studies have reported conflicting findings regarding the association between TG levels and all-cause mortality risk among adults older than 40 years. Several studies have shown a significant rise in all-cause mortality risk among adults with a high level of TG (9,32). In contrast, a retrospective study of 373,389 American adults aged over 45 years receiving statin therapy found that elevated TG levels were associated with a reduced all-cause mortality risk (41). The possible reasons for this inconsistency may be population heterogeneity (i.e., age, ethnicity, and treatment), different follow-up durations, and various confounders. To date, limited studies have explored the association between TG levels and all-cause mortality risk among young adults. A nationwide cohort study included 5,688,055 Korean participants aged 20–39 years with a median follow-up of 7.1 years and found that elevated TG levels were independently associated with increased mortality (17). Our study with a much longer follow-up period (11.2 years) obtained a consistent result with this study. We provide the first epidemiological evidence in Chinese young adults that a higher TG level significantly contributes to an increased risk of long-term all-cause mortality.
According to current clinical practice guidelines, only individuals with TG >200 mg/dL were recommended to control hypertriglyceridemia to reduce CVD risk (42). However, several population-based studies consistently showed that elevated baseline TG levels even below 150 mg/dL, previously considered “optimal”, were associated with increased risk of CVD (43-45) and all-cause mortality (14). In agreement with those studies, our data provided the first evidence that participants with a cutoff value of 110 mg/dL had a 46% higher risk of CVD and a cutoff value of 102 mg/dL had a 50% higher risk of all-cause mortality in young adults. The results suggested that TG-lowering intervention may prove beneficial for even moderately elevated TG levels (110 to 150 mg/dL) in young adults.
Strengths and limitations
Several strengths are evident in our study. The associations between the fasting TG level and the risk of CVD, CVD subtypes, and all-cause mortality were examined for the first time among Chinese young adults comprising a prospective cohort. Our study involved only adults younger than 40 years, for whom few recommendations on TG management are provided in current guidelines (18). Accordingly, this study is poised to provide unprecedented insight into TG management among this age group.
However, there are some inherent limitations in this study. First, despite excluding participants with previous CVD at baseline and individuals who experienced outcomes during the first 2-year follow-up period to mitigate the potential concerns of reverse causality, we cannot prove causality in our study. Second, although we have made every effort to adjust for some important confounders, there is always a chance of unmeasured residual confounding (such as the level of lipoproteins (46) and hypertriglyceridemia-prone medications use). Third, since the study population was recruited from Tangshan City, which is located in northern China, the findings may not be generalizable to other parts of China, other countries, or ethnic populations. In addition, the majority of the participants are coal miners, which may limit the generalizability of the results as well. Last, due to the inherent limitations of our cohort, we were not able to provide detailed information on specific causes of mortality. As such, it may be worthwhile to investigate the effects of TG on specific causes of death in young adults in the future.
Conclusions
The present study shows that elevated fasting TG levels are significantly associated with increased long-term CVD and all-cause mortality risk among Chinese young adults. This study indicated that TG levels have the potential to serve as a reference biomarker for long-term prognosis among young adults.
Acknowledgments
We appreciate those who were involved in the Kailuan study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-412/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-412/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-412/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-412/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Kailuan General Hospital (No. 200605) and informed consent was obtained from all individual participants. All participating hospitals were informed and agreed on the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982-3021. [Crossref] [PubMed]
- Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145-58. [Crossref] [PubMed]
- Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol 2018;15:230-40. [Crossref] [PubMed]
- Gotto AM Jr. Evolving concepts of dyslipidemia, atherosclerosis, and cardiovascular disease: the Louis F. Bishop Lecture. J Am Coll Cardiol 2005;46:1219-24. [Crossref] [PubMed]
- Ference BA, Graham I, Tokgozoglu L, et al. Impact of Lipids on Cardiovascular Health: JACC Health Promotion Series. J Am Coll Cardiol 2018;72:1141-56. [Crossref] [PubMed]
- Chapman MJ, Zamorano JL, Parhofer KG. Reducing residual cardiovascular risk in Europe: Therapeutic implications of European medicines agency approval of icosapent ethyl/eicosapentaenoic acid. Pharmacol Ther 2022;237:108172. [Crossref] [PubMed]
- Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309-16. [Crossref] [PubMed]
- Jeppesen J, Hein HO, Suadicani P, et al. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation 1998;97:1029-36. [Crossref] [PubMed]
- Arca M, Veronesi C, D'Erasmo L, et al. Association of Hypertriglyceridemia with All-Cause Mortality and Atherosclerotic Cardiovascular Events in a Low-Risk Italian Population: The TG-REAL Retrospective Cohort Analysis. J Am Heart Assoc 2020;9:e015801. [Crossref] [PubMed]
- Lee JS, Chang PY, Zhang Y, et al. Triglyceride and HDL-C Dyslipidemia and Risks of Coronary Heart Disease and Ischemic Stroke by Glycemic Dysregulation Status: The Strong Heart Study. Diabetes Care 2017;40:529-37. [Crossref] [PubMed]
- Bos G, Dekker JM, Nijpels G, et al. A combination of high concentrations of serum triglyceride and non-high-density-lipoprotein-cholesterol is a risk factor for cardiovascular disease in subjects with abnormal glucose metabolism--The Hoorn Study. Diabetologia 2003;46:910-6. [Crossref] [PubMed]
- Huang YQ, Huang JY, Liu L, et al. Relationship between triglyceride levels and ischaemic stroke in elderly hypertensive patients. Postgrad Med J 2020;96:128-33. [Crossref] [PubMed]
- Chan WB, Tong PC, Chow CC, et al. Triglyceride predicts cardiovascular mortality and its relationship with glycaemia and obesity in Chinese type 2 diabetic patients. Diabetes Metab Res Rev 2005;21:183-8. [Crossref] [PubMed]
- Klempfner R, Erez A, Sagit BZ, et al. Elevated Triglyceride Level Is Independently Associated With Increased All-Cause Mortality in Patients With Established Coronary Heart Disease: Twenty-Two-Year Follow-Up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes 2016;9:100-8. [Crossref] [PubMed]
- Kim MK, Han K, Kim HS, et al. Lipid cutoffs for increased cardiovascular disease risk in non-diabetic young people. Eur J Prev Cardiol 2022;29:1866-77. [Crossref] [PubMed]
- Park JB, Kim DH, Lee H, et al. Mildly Abnormal Lipid Levels, but Not High Lipid Variability, Are Associated With Increased Risk of Myocardial Infarction and Stroke in "Statin-Naive" Young Population A Nationwide Cohort Study. Circ Res 2020;126:824-35. [Crossref] [PubMed]
- Lee H, Park JB, Hwang IC, et al. Association of four lipid components with mortality, myocardial infarction, and stroke in statin-naïve young adults: A nationwide cohort study. Eur J Prev Cardiol 2020;27:870-81. [Crossref] [PubMed]
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1046-81. [PubMed]
- Zhao M, Song L, Sun L, et al. Associations of Type 2 Diabetes Onset Age With Cardiovascular Disease and Mortality: The Kailuan Study. Diabetes Care 2021;44:1426-32. [Crossref] [PubMed]
- Liu P, Wang Y, Zhang X, et al. Obesity and Cardiac Conduction Block Disease in China. JAMA Netw Open 2023;6:e2342831. [Crossref] [PubMed]
- Wu Z, Chen S, Tao X, et al. Risk and effect modifiers for poor glycemic control among the chinese diabetic adults on statin therapy: the kailuan study. Clin Res Cardiol 2024; Epub ahead of print. [Crossref] [PubMed]
- Wang A, Wang G, Liu Q, et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc Diabetol 2021;20:46. [Crossref] [PubMed]
- Jin C, Chen S, Vaidya A, et al. Longitudinal Change in Fasting Blood Glucose and Myocardial Infarction Risk in a Population Without Diabetes. Diabetes Care 2017;40:1565-72. [Crossref] [PubMed]
- Li W, Jin C, Vaidya A, et al. Blood Pressure Trajectories and the Risk of Intracerebral Hemorrhage and Cerebral Infarction: A Prospective Study. Hypertension 2017;70:508-14. [Crossref] [PubMed]
- Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173-95. [Crossref] [PubMed]
- Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 1989;20:1407-31. [Crossref] [PubMed]
- Bartlett JW, Seaman SR, White IR, et al. Multiple imputation of covariates by fully conditional specification: Accommodating the substantive model. Stat Methods Med Res 2015;24:462-87. [Crossref] [PubMed]
- Liu H, Chen S, Li Z, et al. Long-term risks for cardiovascular disease and mortality across the glycaemic spectrum in a male-predominant Chinese cohort aged 75 years or older: the Kailuan study. Age Ageing 2022;51:afac109. [Crossref] [PubMed]
- Fuchs FD, Whelton PK. High Blood Pressure and Cardiovascular Disease. Hypertension 2020;75:285-92. [Crossref] [PubMed]
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:2969-89. [Crossref] [PubMed]
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082-143. [PubMed]
- Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299-308. [Crossref] [PubMed]
- Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007;115:450-8. [Crossref] [PubMed]
- Paynter NP, Kiefe CI, Lewis CE, et al. Accumulation of metabolic cardiovascular risk factors in black and white young adults over 20 years. J Am Heart Assoc 2015;4:e000940. [Crossref] [PubMed]
- Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993-2000. [Crossref] [PubMed]
- Paquette M, Bernard S, Hegele RA, et al. Chylomicronemia: Differences between familial chylomicronemia syndrome and multifactorial chylomicronemia. Atherosclerosis 2019;283:137-42. [Crossref] [PubMed]
- Goldberg IJ, Eckel RH, McPherson R. Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol 2011;31:1716-25. [Crossref] [PubMed]
- Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014;384:626-35. [Crossref] [PubMed]
- Wang L, Gill R, Pedersen TL, et al. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res 2009;50:204-13. [Crossref] [PubMed]
- Reiner Ž. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol 2017;14:401-11. [Crossref] [PubMed]
- Ambrosy AP, Yang J, Sung SH, et al. Triglyceride Levels and Residual Risk of Atherosclerotic Cardiovascular Disease Events and Death in Adults Receiving Statin Therapy for Primary or Secondary Prevention: Insights From the KP REACH Study. J Am Heart Assoc 2021;10:e020377. [Crossref] [PubMed]
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111-88. [Crossref] [PubMed]
- Aberra T, Peterson ED, Pagidipati NJ, et al. The association between triglycerides and incident cardiovascular disease: What is "optimal"? J Clin Lipidol 2020;14:438-447.e3. [Crossref] [PubMed]
- Lawler PR, Kotrri G, Koh M, et al. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur Heart J 2020;41:86-94. [Crossref] [PubMed]
- Kajikawa M, Maruhashi T, Kishimoto S, et al. Target of Triglycerides as Residual Risk for Cardiovascular Events in Patients With Coronary Artery Disease - Post Hoc Analysis of the FMD-J Study A. Circ J 2019;83:1064-71. [Crossref] [PubMed]
- Duarte Lau F, Giugliano RP. Lipoprotein(a) and its Significance in Cardiovascular Disease: A Review. JAMA Cardiol 2022;7:760-9. [Crossref] [PubMed]


