
A proposal for a revision of the phlebographic classification of congenital venous malformations
Highlight box
Key findings
• Phlebographic differentiation into non-lacunar and lacunar structure of venous malformations (VM) is feasible and reliable.
What is known and what is new?
• For treatment planning, correct estimation and prevention of complications, an exact anatomical classification of the VM is crucial.
• Not only the drainage, as assessed in the established classification, but also the phlebographic aspect of the VM itself plays a decisive role.
What is the implication, and what should change now?
• The internal structure of the VM distinguishing a non-lacunar type (a) and lacunar type (b) should be integrated into the existing classification.
Introduction
Venous malformation (VM) is the most frequent type of congenital vascular malformation with an incidence of 1 to 2 in 5,000 and a prevalence of 1.5% in the Western population (1,2). They often manifest as a blue skin discoloration or as a soft subcutaneous mass when superficial in cutaneous, subcutaneous, or mucosal tissues, but may affect any tissue or organ. Although they are congenital in nature, they frequently become symptomatic later in life as they grow with the child and do not spontaneously regress (3). While their natural course is in general benign, they can cause local and systemic complications, leading to significant morbidity, pain and disability in affected patients. Most of the VM are isolated, but they can occur multifocal or as part of complex combined congenital vascular malformations or in combination with other anomalies. Conservative treatment options comprise symptomatic therapy with compression garments for extensive VM of the extremities, anticoagulation for patients with evidence of localized intravascular coagulation and adequate pain management. If these measures fail, a treatment aiming for complete occlusion or resection of VM should be planned by an interdisciplinary board of specialists (4). Not only symptoms that limit the patient’s quality of life, but also the VM threatening vital organs or causing complications such as bleeding or thromboembolism warrant invasive treatment. In terms of functional outcome local sclerotherapy remains the most important therapeutic tool (5,6). For planning and correct estimation and prevention of complications, an exact anatomical classification of the VM is crucial (7). In VM with enlarged drainage veins the sclerosant can have a reduced local effect and there is a risk of a non-target embolization due to flushing out of the sclerosis agent. Not only the drainage, as assessed in the established classification, but also the phlebographic aspect of the VM itself plays a decisive role. In our experience, the effect of a sclerosant is different in a non-lacunar, small channel VM as compared to ectatic, lacunar VM, in which the effectiveness can be significantly reduced due to lower wall contact and as emptying out of these lacunar veins often is impossible. The combination of coils with sclerotherapy has helped us reduce the need for high amounts of the sclerosing agents and increase the treatment effect in large lacunar lesions and ectatic venous channels. More recently molecularly targeted therapeutic options have begun to play a role in VM, as most VMs are found to be positive for somatic gain-of-function mutations in the angiopoietin receptor gene TEK and genes of the PIK3CA-AKT-mTOR signaling pathway (8-13).
However, due to the varying phenotypic penetration and extreme clinical heterogeneity, the association between phenotype and genotype remains poor and slows the implementation of molecularly targeted therapy (14). To what extent an exact phlebographic classification could allow a better understanding of genotype differences has not yet been investigated.
Biological and clinical classifications such as the Hamburg classification or the International Society for the Study of Vascular Anomalies (ISSVA) classification do not dedicatedly reflect the central angioarchitecture of dysplastic venous channels of VM. The first phlebographic classification by venous drainage (type I no visible drainage, type II drainage into a normal venous system, type III drainage into dysplastic veins) was proposed in 1991 by Dubois et al. (15). In 2001 the same group described three simplified categories (type I cavitary, type II spongy, type III dysmorphic veins) (16). In 2003, Puig et al. finally proposed a classification system combining the direct drainage of the VM with its connection to the normal venous system by defining four different types (17). Type I was defined as an almost isolated VM without visible drainage, type II as VM with drainage into normal veins, type III as VM draining into dysplastic veins and type IV as venous ectasia. This is the only classification system established and widely used for VM up to now.
The aim of this study is to validate a revised phlebographic VM classification system by distinguishing a non-lacunar (a) and a lacunar (b) type of internal VM structure as an additional element to take into count the flow patterns, not only of the draining veins, but also of the VM morphology itself. A more precise subgroup differentiation of patients with VM might help to improve individual treatment planning. We present this article in accordance with the STARD reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-378/rc).
Methods
Study participants
We retrospectively analyzed patients with clinically confirmed diagnoses of VM seen in the division of Angiology between 2009 and 2018. In 2008, a malformation-counseling program, including standardized diagnostic and therapeutic algorithms, was established at the University Hospital of Bern, Switzerland. Since then, consecutive patients with congenital vascular malformations have been enrolled in an independently monitored, prospective registry, the Bernese Congenital Vascular Malformation Registry. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted at the University Hospital of Bern, but the study was approved by the ethics committee in charge, which is in this case the Cantonal Ethics Committee of Bern, Switzerland (No. 2019-01321).
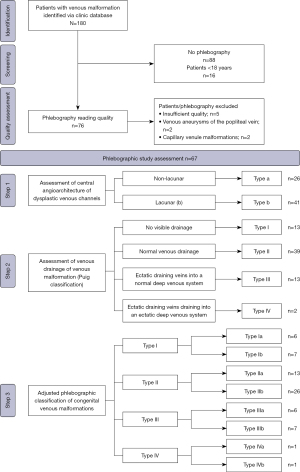
Inclusion criteria were patients >18 years of age with a diagnosis of VM defined by clinical characteristics, duplex ultrasound and magnetic resonance imaging plus direct puncture phlebography to assess morphology and drainage of the VM performed before first embolo-sclerotherapy. Exclusion criteria were intracranial VM of the spinal cord, other types of congenital vascular malformations or rejected informed consent. For the present study there were 506 patients with congenital vascular malformations at all sites, apart from intracranial and spinal cord, enrolled between 2009 and 2018. Patients were retrospectively reviewed to identify those, who have been treated by embolo-sclerotherapy with documented direct puncture phlebography. We identified 180 patients with VM, of whom 92 had had direct puncture phlebography. After exclusion of patients under the age of 18 at the time of plebography and those with documentation of denial to further use of patient’s data 76 patients were included into the quality assessment of imaging. After the exclusion of those with insufficient phlebographic quality (n=5) or because of the phlebographic identification of other subtypes of congenital VMs (n=4), for which the classification as proposed by Puig et al. is not applicable, 67 phlebograms of 67 patients were included in the reading process. All patients signed a general informed consent for anonymized data analysis.
Data collection
Baseline demographic data, including gender and age in years were collected at first diagnosis. Localization, size and affected tissue (skin, subcutaneous tissue, muscle, bone, parenchymatous organ, gastrointestinal tract), as well as infiltration of the malformation across tissue planes (i.e., affection of more than one tissue compartment), was assessed based on the obtained cross-sectional imaging (magnetic resonance imaging or computed tomography). D-dimer levels were routinely measured in venous blood samples. D-dimers were determined using an immunoturbidimetric method with a pathologic result defined as D-dimer >500 µg/L (18,19).
In all patients, which have been treated by embolo-sclerotherapy, a direct puncture phlebogram was routinely obtained by ultrasound-guided and fluoroscopy controlled percutaneous puncture and direct administration of nonionic contrast medium (iopamiro 300 mg/mL, Bracco Suisse SA, Cadempino, Switzerland) using 21–23 G needles.
Phlebograms were analyzed in a random order by two independent readers, which were blinded to clinical information, in a stepwise reading protocol, performed independently from one another. The readers had 10 and 5 years experiences, respectively, in the treatment of VMs. The first basic level of analysis (quality assessment) was to verify whether contrast phlebograms show sufficient image quality and evaluability to characterize phlebographic morphology and drainage of the VM. Criteria regarding morphological characteristics of the VM and flow characteristics of the draining venous system were predefined in order to guarantee a systematic analysis. Phlebographic study assessment included:
- Central angioarchitecture of dysplastic venous channels was assessed by visual estimation concerning the presence or absence of lacunar spaces. More than 80% of reticular dysplastic venous channels by visual estimation were defined as non-lacunar type “a” and dysplastic venous channels showing >80% lacunar spaces by visual estimation as lacunar type “b” (Figure 1).
- Drainage of VM and its relation to the adjacent deep venous system according to the classification proposed by Puig et al.: (I) no visible drainage; (II) normal venous drainage; (III) ectatic draining veins into a normal deep venous system; (IV) ectatic draining veins draining into an ectatic deep venous system.
- Adjusted phlebographic classification, combining assessment of drainage type (I–IV) with additional judgement of the central angioarchitecture of dysplastic venous channels (a or b) in a third and separate reading process (Figure 1).
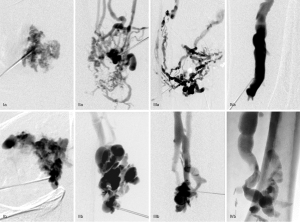
In case of disagreement, an agreement was reached by consent between the two readers.
Statistical analysis
Data analysis was conducted on the 67 patients for whom phlebography quality was judged sufficient by the two independent readers. Baseline characteristics of patients with non-lacunar and lacunar VM types were compared by the non-parametric Wilcoxon test for continuous data and by the Fisher’s exact test for categorical data. For each of the steps of the reading protocol, the agreement between the two readers in charge of interpreting the phlebograms was measured by the percentage of agreement, as well as by the Cohen’s kappa coefficient (κ). All analyses were performed in R version 3.6.0.
Results
The baseline data of the patients are shown in Table 1. The majority of the VM was localized at the extremities (78%) and was confined to a single tissue compartment (57%). Overall the median value of D-dimer levels was found to be 337 µg/L [interquartile range (IQR), 208 to 870 µg/L]. Values tended to be higher in patients with lacunar type lesions, but the difference was not significant [297 µg/L (IQR, 215 to 583 µg/L) in the non-lacunar group versus 423 µg/L (IQR, 206 to 1,149 µg/L) in the lacunar group, P=0.33].
Table 1
| Characteristics | Total (n=67) | Type a (non-lacunar) (n=26) | Type b (lacunar) (n=41) | P value |
|---|---|---|---|---|
| Sex | 0.31 | |||
| Female | 40 (60%) | 18 (69%) | 22 (54%) | |
| Male | 27 (40%) | 8 (31%) | 19 (46%) | |
| Age at diagnosis, years, median [IQR] | 24 [19, 37] | 28 [21, 42] | 22 [19, 34] | 0.11 |
| Localization | 0.47 | |||
| Extremities | 52 (78%) | 23 (88%) | 29 (71%) | |
| Trunk | 8 (12%) | 2 (8%) | 6 (15%) | |
| Head | 5 (7%) | 1 (4%) | 4 (10%) | |
| Other | 2 (3%) | 0 (0%) | 2 (5%) | |
| Extension | 0.62 | |||
| Localized | 38 (57%) | 16 (62%) | 22 (54%) | |
| Infiltrative | 29 (43%) | 10 (38%) | 19 (46%) | |
| Lesion size, cm | 0.67 | |||
| <8 | 32 (48%) | 14 (54%) | 18 (44%) | |
| 8–10 | 7 (10%) | 3 (12%) | 4 (10%) | |
| >10 | 28 (42%) | 9 (35%) | 19 (46%) | |
| Compartments | 0.70 | |||
| S | 10 (15%) | 4 (15%) | 6 (15%) | |
| M | 26 (39%) | 12 (46%) | 14 (34%) | |
| S, M | 18 (27%) | 7 (27%) | 11 (27%) | |
| S, M, B | 7 (10%) | 1 (4%) | 6 (15%) | |
| Other | 6 (9%) | 2 (8%) | 4 (10%) | |
| D-dimer, µg/L | n=63 | n=24 | n=39 | |
| Median [IQR] | 337 [208, 870] | 297 [215, 583] | 423 [206, 1,149] | 0.33 |
| ≤500 | 38 (60%) | 16 (67%) | 22 (56%) | 0.44 |
| >500 | 25 (40%) | 8 (33%) | 17 (44%) | |
| Puig classification | 0.74 | |||
| Type I | 13 (19%) | 6 (23%) | 7 (17%) | |
| Type II | 39 (58%) | 13 (50%) | 26 (63%) | |
| Type III | 13 (19%) | 6 (23%) | 7 (17%) | |
| Type IV | 2 (3%) | 1 (4%) | 1 (2%) |
Categorical data are compared using Fisher exact test. Continuous data are compared using Mann-Whitney test. IQR, interquartile range; S, subcutis; M, muscle; B, bone.
The distinction of the central angioarchitecture of dysplastic venous channels into non-lacunar and lacunar types was reliable using a binary visual cut off of more than 80% of the VM angioarchitecture showing lacunar formation or not. There were 26 (39%) patients with non-lacunar and 41 (61%) patients with lacunar type, respectively.
The drainage classification according to Puig et al. showed type I in 13 (19%) patients, type II in 39 (58%) patients, type III in 13 (19%) patients and type IV in 2 (3%) patients (Figure 2).
There was an almost perfect inter-reader reliability for the individual characteristic of angioarchitecture (κ=0.91). Concerning the drainage types I to IV an agreement of 87% (κ=0.78) was reached, that did not significantly change for the combined classification of angioarchitecture and drainage with 82% agreement and a substantial inter-reader reliability (κ=0.77) compared to reading the classification as proposed by Puig et al. alone (Table 2).
Table 2
| Phlebographic study assessment | Inter-reader agreement (%) | κ |
|---|---|---|
| 1. Central angioarchitecture of dysplastic venous channels: non-lacunar vs. lacunar | 96 | 0.91 |
| 2. Venous drainage of venous malformation: Puig classification (type I–IV) | 87 | 0.78 |
| Combination of assessment 1 and 2 | 82 | 0.77 |
Discussion
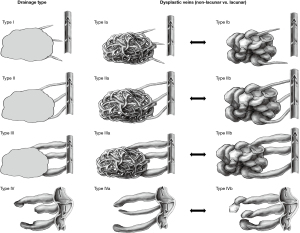
The analysis shows, that a reliable differentiation of the central angioarchitecture of dysplastic venous channels in patients with VMs by direct puncture phlebography is possible. Adjusting the venous drainage classification as suggested by Puig et al. by adding a binary criterion of lacunar or non-lacunar characteristics of the central angioarchitecture of dysplastic venous channels (Figure 3) does not change the inter-reader reliability.
Our patient population showed a comparable distribution of VM phenotypes defined by phlebography as described by Puig et al. In their series of 43 patients, used to establish the current classification, types I and II were most common and type IV the least common type (17). In the literature VM, outside of the central nervous system, is described in about 40% on the extremities, 20% on the trunk and 40% in the cervico-facial area (5). In our series we found slightly more lesions on the extremities and less on the trunk and the cervico-facial region. This might be due to the fact, that we analyzed symptomatic patients in whom an interventional treatment was indicated, as we otherwise do not routinely obtain direct puncture phlebograms. Lesions on the extremities might be more likely to cause symptoms than those of the trunk (20). Concerning cervicocranial VMs, they are mainly managed by neuroradiology in our hospital and these patients do not show within our database.
Phlebographic classification of VMs is mainly dedicated to allowing precise treatment planning for embolo-sclerotherapy and better estimation of possible complications due to the need of high volume of sclerosant. We therefore identified the need to assess and integrate the morphologic pattern of the central angioarchitecture of the dysplastic venous channels themselves in addition to the drainage pattern (Videos 1,2). With regard to embolo-sclerotherapy this goes along with dosage adjustments with probably more toxic effect on endothelial cells in non-lacunar type a and more volume needs in lacunar type b (Figure 1). Although this is done by experience, this might help to interpret treatment effects. Non-lacunar VM is likely to be more easily completely occluded by sclerotherapy than lacunar VM. On the other hand, non-lacunar VM might be more difficult to treat with new approaches such as electro-sclerotherapy, as the electrode positioning might be more challenging (21-23). However, a dedicated analysis of treatment outcomes in larger, probably multi-centric cohorts is necessary to clarify these possible implications.
The trend to show higher D-dimers in patients with lacunar VM morphology was interesting, but did not reach statistical significance. Other groups already described the association of large venous lacunae within VM in association with higher D-dimer levels (18,19,24,25). The importance of the internal flow patterns concerning the development of localized intravascular coagulopathy is known and needs to be taken into count if interventional procedures are planned, as they also impact toxicity and efficacy of sclerosants in terms of the endothelial contact surface and the duration and volume needed to fill the VM (26).
Moreover, with recent research identifying genetic alterations, which occur during embryogenesis and result in somatic mosaic pathogenic hotspot mutations as underlying causes for VM, further investigation of genotype-phenotype associations is essential to define meaningful molecularly targeted therapy opportunities. This task is demanding as identical genotypes go along with different phenotypes and different genotypes can lead to identical phenotypes. Further clinical differentiation of subgroups of VM patients therefore might help to improve the understanding the complex genetic interaction of known and unknown genetic oncogene variants in subgroups of patients with VM.
We propose a schematic and simple adjustment of the current classification by Puig et al. by adding a non-lacunar internal VM structure (type Ia, IIa, IIIa, IVa) or a lacunar internal VM structure (type Ib, IIb, IIIb, IVb) to the established classification (Figure 3).
The main limitation of our analysis is the small size of study population designed as single center project and dedicated standards for direct puncture phlebography, which might not coincide with other centers and therefore differences in interpretation. With the results of this validation study, we hope to have laid a foundation for better comparability of data in the field of VM research and reach wider acceptance and dissemination by expanding our knowledge through joint efforts to further validate our proposed revised VM classification.
We excluded intracranial and spinal VM due to logistic reasons, a major limitation when proposing a new classification system that should be universally applicable. However, based on the literature the angioarchitecture should not be significantly affected by the localization of extra-cranial VM (27,28). No difference was observed in terms of inter-reader reliability between the three size groups we defined and even small VMs can be visualized correctly by using a sufficient magnification during image acquisition. We therefore think, that the non-differentiation of VM <8 cm, should not impact our results.
A validation of this revised classification in a prospective context, as well as proof of the impact on treatment outcomes is needed and should ideally be conducted by different centers.
Conclusions
The internal structure of the VM itself is an important element for interventional embolo-sclerotherapy treatment planning and the differentiation of the central angioarchitecture of dysplastic venous channels of VM should be integrated into the existing classification in future studies in order to validate this approach.
Acknowledgments
We thank Armando Lenz for his statistical support and Lisa Cuthbertson for her support in drawing the schematic images of the different VM types in Figure 3. We also thank IDSC for their support with data delivery and the team of our clinical investigation unit for the administrative support.
Funding: The study was financially supported by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-378/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-378/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-378/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-378/coif). M.C.R. is affiliated with CTU Bern, University of Bern, which has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in design, conduct, or analysis of clinical studies funded by not-for-profit and for-profit organizations. In particular, pharmaceutical and medical device companies provide direct funding to some of these studies. For an up-to-date list of CTU Bern’s conflicts of interest, see http://www.ctu.unibe.ch/research/declaration_of_interest/index_eng.html. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted at the University Hospital of Bern, but the study was approved by the ethics committee in charge, which is in this case the Cantonal Ethics Committee of Bern, Switzerland (No. 2019-01321). All patients signed a general informed consent for anonymized data analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eifert S, Villavicencio JL, Kao TC, et al. Prevalence of deep venous anomalies in congenital vascular malformations of venous predominance. J Vasc Surg 2000;31:462-71. [Crossref] [PubMed]
- Wassef M, Blei F, Adams D, et al. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 2015;136:e203-14. [Crossref] [PubMed]
- Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr 2012;2012:645678. [Crossref] [PubMed]
- Dompmartin A, Vikkula M, Boon LM. Venous malformation: update on aetiopathogenesis, diagnosis and management. Phlebology 2010;25:224-35. [Crossref] [PubMed]
- Behravesh S, Yakes W, Gupta N, et al. Venous malformations: clinical diagnosis and treatment. Cardiovasc Diagn Ther 2016;6:557-69. [Crossref] [PubMed]
- Cao J, Liu J, Zhang X, et al. A systematic review and network meta-analysis of the effectiveness of sclerotherapy for venous malformation. J Vasc Surg Venous Lymphat Disord 2023;11:210-218.e3. [Crossref] [PubMed]
- Lee BB, Baumgartner I, Berlien P, et al. Diagnosis and Treatment of Venous Malformations. Consensus Document of the International Union of Phlebology (IUP): updated 2013. Int Angiol 2015;34:97-149. [PubMed]
- Adams DM, Trenor CC 3rd, Hammill AM, et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics 2016;137:e20153257. [Crossref] [PubMed]
- Castel P, Carmona FJ, Grego-Bessa J, et al. Somatic PIK3CA mutations as a driver of sporadic venous malformations. Sci Transl Med 2016;8:332ra42. [Crossref] [PubMed]
- Kangas J, Nätynki M, Eklund L. Development of Molecular Therapies for Venous Malformations. Basic Clin Pharmacol Toxicol 2018;123:6-19. [Crossref] [PubMed]
- Limaye N, Kangas J, Mendola A, et al. Somatic Activating PIK3CA Mutations Cause Venous Malformation. Am J Hum Genet 2015;97:914-21. [Crossref] [PubMed]
- Limaye N, Wouters V, Uebelhoer M, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet 2009;41:118-24. [Crossref] [PubMed]
- Van Damme A, Seront E, Dekeuleneer V, et al. New and Emerging Targeted Therapies for Vascular Malformations. Am J Clin Dermatol 2020;21:657-68. [Crossref] [PubMed]
- Wetzel-Strong SE, Detter MR, Marchuk DA. The pathobiology of vascular malformations: insights from human and model organism genetics. J Pathol 2017;241:281-93. [Crossref] [PubMed]
- Dubois JM, Sebag GH, De Prost Y, et al. Soft-tissue venous malformations in children: percutaneous sclerotherapy with Ethibloc. Radiology 1991;180:195-8. [Crossref] [PubMed]
- Dubois J, Soulez G, Oliva VL, et al. Soft-tissue venous malformations in adult patients: imaging and therapeutic issues. Radiographics 2001;21:1519-31. [Crossref] [PubMed]
- Puig S, Aref H, Chigot V, et al. Classification of venous malformations in children and implications for sclerotherapy. Pediatr Radiol 2003;33:99-103. [Crossref] [PubMed]
- Dompmartin A, Ballieux F, Thibon P, et al. Elevated D-dimer level in the differential diagnosis of venous malformations. Arch Dermatol 2009;145:1239-44. [Crossref] [PubMed]
- Mazoyer E, Enjolras O, Bisdorff A, et al. Coagulation disorders in patients with venous malformation of the limbs and trunk: a case series of 118 patients. Arch Dermatol 2008;144:861-7. [Crossref] [PubMed]
- Casanova D, Boon LM, Vikkula M. Venous malformations: clinical characteristics and differential diagnosis. Ann Chir Plast Esthet 2006;51:373-87. [Crossref] [PubMed]
- Liu JW, Ni B, Gao XX, et al. Comparison of bleomycin polidocanol foam vs electrochemotherapy combined with polidocanol foam for treatment of venous malformations. J Vasc Surg Venous Lymphat Disord 2024;12:101697. [Crossref] [PubMed]
- Wohlgemuth WA, Müller-Wille R, Meyer L, et al. Bleomycin electrosclerotherapy in therapy-resistant venous malformations of the body. J Vasc Surg Venous Lymphat Disord 2021;9:731-9. [Crossref] [PubMed]
- Muir T, Bertino G, Groselj A, et al. Bleomycin electrosclerotherapy (BEST) for the treatment of vascular malformations. An International Network for Sharing Practices on Electrochemotherapy (InspECT) study group report. Radiol Oncol 2023;57:141-9. [Crossref] [PubMed]
- Dompmartin A, Acher A, Thibon P, et al. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol 2008;144:873-7. [Crossref] [PubMed]
- Razek AA, Ashmalla GA. Prediction of venous malformations with localized intravascular coagulopathy with diffusion-weighted magnetic resonance imaging. Phlebology 2019;34:156-61. [Crossref] [PubMed]
- Han YY, Sun LM, Yuan SM. Localized intravascular coagulation in venous malformations: A system review. Phlebology 2021;36:38-42. [Crossref] [PubMed]
- Dasgupta R, Patel M. Venous malformations. Semin Pediatr Surg 2014;23:198-202. [Crossref] [PubMed]
- van der Vleuten CJ, Kater A, Wijnen MH, et al. Effectiveness of sclerotherapy, surgery, and laser therapy in patients with venous malformations: a systematic review. Cardiovasc Intervent Radiol 2014;37:977-89. [PubMed]


