
Evaluating the association between vascular remodeling and plaque calcification patterns of the carotid artery and its effects on ischemic symptoms using CT angiography
Highlight box
Key findings
• Arterial remodeling can be serves as a novel measure to help ascertain the risk stratification of ischemic events in carotid atherosclerotic disease.
What is known and what is new?
• Vascular remodeling is evident in atherosclerotic plaques of the internal carotid artery (ICA).
• Significant differences in the remodeling ratio (RR) existed between various plaque calcification types.
• There is a significant correlation between calcification thickness and positive remodeling.
What is the implication, and what should change now?
• Combining plaque calcium configurations (“qualitative”) with RR evaluations (“morpho-logical”) during computed tomography angiography plaque assessments offers greater accuracy for risk stratification of ischemic events in patients with ICA stenosis compared to evaluating the plaque component alone. The possibility will be explored in future prospective studies.
Introduction
Background
Carotid atherosclerotic plaque is the main cause of ischemic cerebrovascular events. The risk of future ischemic events is influenced not only by the degree of luminal narrowing but also by plaque morphology and its susceptibility to rupture (1). Identifying the high-risk features of carotid artery plaques could provide more accurate risk stratification for future cerebral ischemic events, especially to monitor the effects of medical therapeutic interventions on patients who are unsuitable for surgical procedures.
Rationale and knowledge gap
Arterial remodeling plays an important role in understanding the progression of atherosclerosis. Histopathological specimens and imaging studies of coronary arteries have shown that positive remodeling is related to plaque vulnerability and a high risk of ischemic events (2,3). Few authors have studied the association between vascular remodeling of the internal carotid artery (ICA) and ischemic events. Some studies have found that expansive arterial remodeling is related to plaque vulnerability (1,4,5), whereas others have suggested that there is no significant association between expansive remodeling observed in atherosclerotic plaques of the ICA and symptomatic arteries (6). It is still unclear whether the degree of carotid artery remodeling should be considered as an indicator of plaque stability or instability.
Calcium is a common component of atherosclerotic plaques. The size, shape, and position of calcification may influence plaque development (7). Plaque stability can be affected by not only the amount but also the subtype and chemical composition of calcium in atherosclerotic lesions (8). Calcification of atherosclerotic plaques can be detected using different imaging modalities, including MR imaging, computed tomography (CT), and ultrasound. Among these techniques, CT provides excellent visualization of calcifications owing to the marked X-ray attenuation properties of calcium (9). Therefore, computed tomography angiography (CTA) is considered an optimal technique to quantify and characterize carotid artery calcifications (10). Large calcifications have been more frequently associated with positive remodeling in coronary plaques (11). However, the potential association between this luminal morphological change and plaque calcifications in the ICA remains unclear.
Objective
In this retrospective study, we aimed to elucidate the relationship between plaque calcification, vascular remodeling, and ischemic events by analyzing clinical and imaging findings to understand the clinical implications of carotid artery calcification and changes in luminal morphology using CTA. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-428/rc).
Methods
Patient selection
Between September 2018 and March 2023, patients presenting with symptoms suggestive of carotid territory ischemia admitted to the Zhejiang Hospital were consecutively screened for inclusion in this retrospective, single-center study. The inclusion criteria were age ≥18 years and moderate-to-severe stenosis of the ICA. The exclusion criteria were as follows: (I) mild stenosis (<50%) or suspicion of near-occlusion; (II) repeat CTA examinations of the same patient. If multiple CTAs were available for analysis, only the earliest CTA indicating ICA stenosis; (III) other causes of stenosis, such as dissection, Moyamoya disease, or fibromuscular dysplasia; and (IV) poor-quality carotid CTA images degraded by artifacts (e.g., motion) or instances where the clinical data were incomplete. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Zhejiang Hospital (No. 2023-40K) and individual consent for this retrospective analysis was waived.
For eligible participants, baseline demographic information was collected from electronic medical records. It included general details such as age and sex; details of vascular risk factors, including hypertension, diabetes, dyslipidemia, coronary artery disease, smoking habits, statin use, and relevant laboratory data.
Definition of symptoms
Symptomatic ICA was characterized by ipsilateral ischemic events that occurred within a 6-month window. These included cerebral infarction, transient ischemic attack and amaurosis fugax. Diagnosis was either based on clear medical records or confirmed via MRI or CT imaging. According to our study protocol, the “symptomatic” artery was the one ipsilateral carotid to the symptoms, whereas its counterpart on the contralateral side was termed the “asymptomatic” artery when both carotid arteries of the patient were evaluated.
CT imaging protocol
All participants underwent a carotid artery CTA with either a 64-slice dual-source SOMATOM Force scanner (Siemens Medical Systems, Forchheim, Germany) or a 63-slice NeuViz scanner (Neusoft Medical Systems, Shenyang, China). Images were obtained from the aortic arch to the base of the skull to evaluate the carotid arteries. Intravenous access was established through the antecubital vein using an 18- or 20-gauge angiocatheter. A 100-mL contrast agent was administered at a rate of 4 mL/s. Bolus monitoring was conducted by placing a region of interest in the ascending aorta and triggering at 100 HU. Injections were performed with a 10-second delay. All data were reviewed on a picture archiving and communication system (PACS) workstation for image post-processing.
CTA imaging review
The reviewer was blinded to patient IDs and clinical information. All carotid imaging markers were determined by two experienced neuroradiologists (working 13 years and 15 years). Any disagreements were resolved through consensus. The severity of ICA stenosis was evaluated according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria (12). Maximum luminal narrowing was measured on contrast-enhanced axial images, using a width of 850 HU and a window level of 300 HU, to distinguish plaque from the vascular lumen (13). The remodeling ratio (RR) of the proximal ICA was calculated using the long-axis CTA image and the following formula: the maximum distance between the inner border of the arterial lumen at the plaque site and the outer borders of the plaque perpendicular to the axis of the ICA/the maximum luminal diameter of the distal ipsilateral ICA at a region (within 1 cm) unaffected by atherosclerosis (Figure 1) (6). The patterns of vascular remodeling were categorized based on the cutoff value for RR of ≥1.1 (positive remodeling) or <1.1 (negative remodeling) (14).
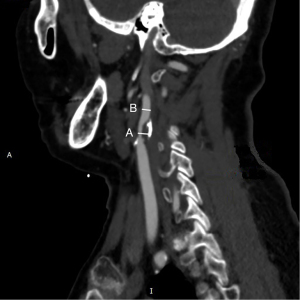
Plaque classification
In each artery, the characteristics of plaques at the proximal ICA responsible for the highest degree of stenosis were visually assessed. CTA markers evaluated included the presence of calcification, maximum calcification thickness and calcium score. Calcification thickness (expressed in mm) was measured on the largest longitudinal section of the responsible plaque, typically at a window setting of W850:L300 in a long-axis CTA image. This window level may show variable edge and halo blurring, which could change the size of the calcification (13,15). The calcium score was measured using semiautomated software (Syngo CaScoring; Siemens) on non-contrast CT, following the method described by Agatston et al. (16).
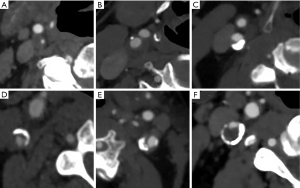
Based on the presence or absence of calcification, the plaque(s) was classified as non-calcified plaque and plaque containing calcified components. Plaques with calcified components were further classified into subgroups according to the presence of calcium configurations (15) (Figure 2), as follows:
- Type 1: complete absence of calcification within the plaque;
- Type 2: intimal or superficial calcifications;
- Type 3: deep or bulky calcifications;
- Type 4: adventitial calcifications with an internal soft plaque thickness of less than 2 mm; negative rim sign;
- Type 5: mixed patterns with both intimal and bulky calcifications;
- Type 6: adventitial calcifications (thickness <2 mm) with an internal soft plaque thickness greater than 2 mm; positive rim sign.
Statistical analysis
Categorical variables are presented as absolute numbers and relative frequencies. Numerical results are presented as mean ± standard deviation (SD) or medians with interquartile ranges. Shapiro-Wilk test was used to check continuous variables for normality. The t-test was used to compare continuous variables with normal distribution. The Mann-Whitney U test or Kruskal-Wallis test was used to compare non-normally distributed continuous variables. Categorical data were analyzed using the χ2 test or Fisher’s exact test to compare intergroup differences. Correlations were measured using Pearson’s or Spearman correlation coefficient. Predictors of ischemic symptoms were assessed using logistic regression analysis, with results expressed as odds ratio (ORs) with 95% confidence intervals (CIs). A two-sided P value <0.05 was considered to indicate statistical significance. All analyses were performed using SPSS version 27 (IBM, Armonk, NY, USA).
Results
Baseline participant characteristics
The study included 196 patients (men 81%, women 19%) with 242 arteries. The mean age of the patients was 71.5±9.9 (range, 37–91) years. Of these, 81 were symptomatic and 115 were asymptomatic (Figure 3). The clinical and demographic characteristics of the patients are summarized in Table 1. Of the 242 ICAs examined, 53.3% (129/242) exhibited moderate ICA stenosis arteries, while the remaining 46.7% (113/242) showed severe ICA stenosis.
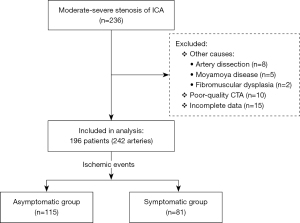
Table 1
| Characteristic | Asymptomatic patients (n=115) | Symptomatic patients (n=81) | P |
|---|---|---|---|
| Age, years | 72.05±8.90 | 70.80±11.24 | 0.38 |
| Male | 95 (82.6) | 63 (77.8) | 0.40 |
| Hypertension | 88 (76.5) | 64 (79.0) | 0.68 |
| Dyslipidemia | 32 (27.8) | 22 (40.7) | 0.91 |
| Diabetes | 32 (27.8) | 27 (27.2) | 0.31 |
| Coronary heart disease | 28 (24.3) | 19 (23.5) | 0.67 |
| Smoking habit | 46 (40.0) | 15(18.5) | 0.31 |
| Statin use | 30 (26.1) | 41 (50.6) | 0.14 |
| Total cholesterol, mg/dL | 3.78 (3.30–4.64) | 3.89 (3.31–4.81) | 0.70 |
| Blood glucose, mg/dL | 5.32 (4.69–6.41) | 4.93 (4.39–6.28) | 0.10 |
| Low-LDC-c, mg/dL | 2.15 (1.70–2.96) | 2.29 (1.58–2.80) | 0.87 |
| High-LDC-c, mg/dL | 1.07 (0.87–1.21) | 1.10 (0.87–1.28) | 0.64 |
| Glycosylated hemoglobin | 6.30 (5.80–7.20) | 6.30 (5.85–7.65) | 0.94 |
| Triglyceride, mg/dL | 1.22 (0.89–1.72) | 1.13 (0.86–1.69) | 0.79 |
Data are presented as mean ± standard deviation, n (%), and median (interquartile range). Low-LDC-c, low-density lipoprotein-cholesterol; High-LDC-c, high-density lipoprotein-cholesterol.
Symptomatic versus asymptomatic ICAs
As shown in Table 2, the RR in symptomatic arteries [median, 1.31 (interquartile range, 1.17–1.68)] was significantly greater than that in asymptomatic arteries [median, 1.20 (interquartile range, 1.05–1.45), P=0.006]. Notable differences in plaque calcification types between symptomatic and asymptomatic ICAs were observed (P=0.01). Type 6 calcification was more prevalent in symptomatic arteries (50.0% vs. 36.1%), whereas type 3 and type 4 plaques were more common in asymptomatic arteries (15.8% vs. 6.0% and 18.4% vs. 8.3%). No significant differences were observed in the percentage of stenosis, vascular risk factors, statin use, and calcification markers (calcification thickness and score) between the two groups. Logistic regression analysis showed no association between ischemic symptoms and clinical risk factors except for RR. RR was positively associated with ischemic symptoms (OR 3.22; 95% CI: 1.47–7.05; P=0.004; Table 3).
Table 2
| Variables | Asymptomatic ICA (n=158) | Symptomatic ICA (n=84) | P |
|---|---|---|---|
| Age, years | 71.73±8.68 | 70.79±11.31 | 0.50 |
| Male | 133 (84.2) | 65 (77.4) | 0.19 |
| RR | 1.20 [1.05–1.45] | 1.31 [1.17–1.68] | 0.006 |
| Carotid NASCET percentage stenosis | 65 [56–75] | 69 [59–75] | 0.28 |
| Intraplaque calcification present | 147 (93.0) | 72 (85.7) | 0.06 |
| Maximum calcification thickness, mm | 1.50 [1.08–2.33] | 1.45 [0.90–1.90] | 0.10 |
| Calcification score | 128.80 [34.90–338.98] | 86.85 [8.88–334.03] | 0.20 |
| Plaque calcification pattern | 0.01 | ||
| Type 1 | 11 (7.0) | 12 (14.3) | |
| Type 2 | 8 (5.1) | 4 (4.8) | |
| Type 3 | 25 (15.8) | 5 (6.0) | |
| Type 4 | 29 (18.4) | 7 (8.3) | |
| Type 5 | 28 (17.7) | 14 (16.7) | |
| Type 6 | 57 (36.1) | 42 (50.0) | |
| Hypertension | 123 (77.8) | 70 (83.3) | 0.31 |
| Dyslipidemia | 41 (25.9) | 25 (29.8) | 0.62 |
| Diabetes | 46 (29.1) | 30 (35.7) | 0.29 |
| Coronary heart disease | 37 (23.4) | 17 (20.2) | 0.57 |
| Smoking habit | 72 (45.6) | 44 (52.4) | 0.31 |
| Statin use | 42 (26.6) | 23 (27.4) | 0.89 |
Data are presented as mean ± standard deviation, n (%), and median (interquartile range). ICA, internal carotid artery; RR, remodeling ratio; NASCET, North American Symptomatic Carotid Endarterectomy Trial.
Table 3
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| RR | 3.16 | 1.46–6.83 | 0.003 | 3.22 | 1.47–7.05 | 0.004 | |
| % of stenosis | 3.17 | 0.25–40.48 | 0.37 | 3.41 | 0.24–48.91 | 0.36 | |
| Hypertension | 1.42 | 0.72–2.83 | 0.31 | 1.34 | 0.65–2.77 | 0.42 | |
| Dyslipidemia | 1.21 | 0.67–2.18 | 0.52 | 1.21 | 0.60–2.44 | 0.59 | |
| Diabetes | 1.35 | 0.77–2.38 | 0.29 | 1.42 | 0.78–2.57 | 0.25 | |
| Coronary heart disease | 0.83 | 0.43–1.59 | 0.57 | 0.67 | 0.31–1.46 | 0.31 | |
| Smoking habit | 1.31 | 0.77–2.23 | 0.31 | 1.30 | 0.75–2.24 | 0.35 | |
OR, odds ratio; CI, confidence interval; RR, remodeling ratio.
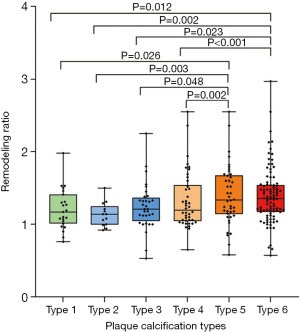
Analysis of vascular remodeling
In the analysis of the vascular remodeling associated with plaque calcification at the site of maximum ICA stenosis, the highest RR was observed for type 5 and 6 plaques compared to types 1 to 4 (Figure 4). No statistically significant difference in RR was observed between types 1 to 4 and type 5 and 6 (Table S1).
Of the 242 ICAs studied, 69 (28.5%) showed negative remodeling and 173 (71.5%) showed positive remodeling. Symptomatic ICAs were more common in the positive remodeling group compared to the negative remodeling one [38.7% (67/173) vs. 24.6% (17/69), P=0.03]. Table 4 compares these two remodeling types. The positive remodeling arteries showed a greater maximum calcification thickness (1.6 vs. 1.2 mm, P<0.001) and a higher calcification score (145.6 vs. 48.2, P<0.001). In terms of assessment of plaque calcification, a statistically significant significance was observed between the two groups (P<0.001). The type 6 classification, characterized by a positive rim sign, was significantly more common in the positive remodeling group compared to the negative remodeling group (46.2% vs. 27.5%). Conversely, the proportion of type 1 and type 4 plaques was higher in arteries with negative remodeling.
Table 4
| Variables | Negative remodeling (n=69) | Positive remodeling (n=173) | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||||
| Age, years | 70.06±10.37 | 71.94±9.34 | 1.02 (0.99–1.05) | 0.36 | |||
| Male | 60 (87.0) | 138 (79.8) | 0.59 (0.27–1.31) | 0.19 | |||
| Hypertension | 51 (73.9) | 142 (82.1) | 1.62 (0.83–3.14) | 0.15 | |||
| Diabetes | 22 (31.9) | 54 (31.2) | 0.97 (0.53–1.77) | 0.91 | |||
| Dyslipidemia | 16 (23.2) | 50 (28.9) | 1.35 (0.70–2.58) | 0.36 | |||
| Calcification thickness, mm | 1.20 (0.65–1.70) | 1.60 (1.20–2.30) | 1.82 (1.31–2.52) | <0.001 | 2.30 (1.06–5.01) | 0.03 | |
| Calcification score | 48.20 (4.60–192.90) | 145.60 (45.55–403.50) | 1.33 (1.13–1.57) | <0.001 | 1.00 (0.10–1.00) | 0.24 | |
| Plaque patterns | <0.001 | ||||||
| Type 1 | 11 (15.9) | 12 (6.9) | NA | ||||
| Type 2 | 6 (8.7) | 6 (3.5) | NA | 0.37 (0.08–1.82) | 0.22 | ||
| Type 3 | 8 (11.6) | 22 (12.7) | NA | 0.18 (0.02–1.61) | 0.12 | ||
| Type 4 | 18 (26.1) | 18 (10.4) | NA | 0.30 (0.08–1.17) | 0.08 | ||
| Type 5 | 7 (10.1) | 35 (20.2) | NA | 0.31 (0.04–2.51) | 0.27 | ||
| Type 6 | 19 (27.5) | 80 (46.2) | NA | 1.08 (0.29–3.98) | 0.91 | ||
Data are presented as mean ± standard deviation, n (%), and median (interquartile range). OR, odds ratio; CI, confidence interval; NA, not applicable.
On comparing negative and positive remodeling of carotid arteries, we found that calcification thickness (r=0.207; P=0.002), calcification score (r=0.218; P=0.001), and plaque calcification pattern (r=1.96; P=0.002) were significantly associated with the positive remodeling group based on bivariate analysis. On multivariable logistic regression analysis, calcification thickness remained significantly associated with positive remodeling of carotid arteries (OR 2.30; 95% CI: 1.06–5.01; P=0.036). The detailed results are summarized in Table 4.
Discussion
Key findings
This cross-sectional study demonstrated four main findings. Firstly, the RR in symptomatic arteries was significantly greater than that in asymptomatic ones, and the positive association between RR of ICA and ischemic symptoms was evident. Secondly, ischemic symptoms were more prevalent in carotid arteries showing positive remodeling compared to those with negative remodeling. Thirdly, there was statistical difference in RR between various plaque calcification types. Finally, we also observed a significant association existed between arterial positive remodeling and plaque calcification thickness.
Strengths and limitations
A strength of this study was the involvement of the association between vascular remodeling and plaque calcification patterns of the carotid artery and its effects on ischemic symptoms. Still, our study has several limitations that should be considered. First, the retrospective cross-sectional design meant that the evaluated parameters, such as plaque calcification types and vascular remodeling, might be resultant factors and may weaken the predictive power for future ischemic events. This implies that prospective studies are needed to further validate the predictive value of these parameters for future ischemic events. Second, there may have been a selection bias in patient inclusion. The findings of this study may be specific to patients with moderate-to-severe ICA stenosis, rendering them non-generalizable to those with mild stenosis. Third, compared to the automatic assessment of plaque calcification thickness and remodeling parameters using dedicated software, the manual measurement of these variables may be less precise and more susceptible to operator variability. Therefore, findings regarding the patterns of vascular remodeling and plaque calcification should be interpreted with caution, considering them exploratory in nature.
Comparison with similar researches
Arterial morphology can change in response to atherosclerotic progression through outward expansion of the vessel wall (positive remodeling) or vessel shrinkage (negative remodeling). In coronary events, positive remodeling may be associated with an unstable clinical presentation, which indicates plaque vulnerability to rupture (3,17).
With respect to the ICA, previous studies have reported a strong correlation between positive remodeling and ischemic complications (5,18). Our data suggest that ischemic symptoms are more common in arteries with positive remodeling than in those with negative remodeling. As regards the potential risk factors for ischemic events in our study, we found no association between ischemic symptoms and clinical risk factors, except for RR. The RR was significantly higher in symptomatic ICAs than in asymptomatic ones. Moreover, a positive association was observed between RR and ischemic symptoms, consistent with the findings of Yoshida et al. (5) and Miura et al. (19), both of whom reported a relationship between RR and symptoms in ICA atherosclerosis. This suggests that the extent of the RR value may be related to underlying atherosclerotic plaque vulnerability, and extensively remodeled plaques tend to be more clinically significant as markers of vulnerable plaques in patients with moderate-to-severe stenotic ICA.
Calcium is present in 50–60% of carotid plaques (20). Calcium deposition in vessels may occur either in the media or intima, with the adventitia also playing an important role in the pathogenesis of atherosclerosis. Adventitial inflammation in atherosclerosis may be linked to endothelial dysfunction triggered by lipoproteins and chronic inflammation (10). This alteration is characterized by adventitial vasa vasorum leakage and intraplaque hemorrhage (IPH) (10). An imaging study found that the CTA rim sign was significantly associated with IPH (12). Saba et al. (15) subsequently confirmed that the presence of a positive rim sign is significantly associated with cerebrovascular events. Our results suggest that type 6 calcification, i.e. the positive rim sign, is more common in symptomatic ICA than in asymptomatic one. Thus, adventitial calcifications with a positive rim sign may signify high-risk carotid plaques.
Explanations of the findings
The calcification subtypes have different roles in the progression of atherosclerotic lesions, with different implications on plaque biology and stability (8,10). Large calcifications, especially those ≥3 mm in size, have been frequently associated with positive remodeling in the coronary artery (11). Our findings show that the RR for type 5 and 6 plaques was higher compared to other types, and the proportion of type 6 calcifications was greater in the positive remodeling group. However, no significant differences were observed in the relationship between calcification phenotypes and positive remodeling. It is unclear whether there is a causative relationship between the two or if the remodeling and intraplaque calcification is a mere epiphenomenon of the concomitant atherosclerotic process.
We observed a significant correlation between calcification thickness and positive remodeling, which is consistent with a previous study (11). Although the precise mechanism underlying the relationship between calcifications and arterial remodeling remains unclear, it can be elucidated by a potential pipeline between plaque composition, biomechanics and hemodynamic changes. Plaque tissue components have different biomechanical properties. The morphometric features of calcifications (location and shape) in carotid plaques and their effect on the stress distribution in the fibrous plaque tissue at the calcification interface may cause elevated stresses in plaque tissue (21). Increased stress at sites of luminal narrowing can alter local hemodynamics and stimulate the production of metalloproteinases by endothelial macrophages. The hemodynamic stimuli and production of metalloproteinases may further increase the extraluminal area and remodeling index in lesions with stress (18,22).
Implications and actions needed
Combining plaque calcium configurations (“qualitative”) with RR evaluations (“morphological”) during CTA plaque assessments offers greater accuracy for risk stratification of ischemic events in patients with ICA stenosis compared to evaluating the plaque component alone.
Conclusions
In summary, vascular remodeling is evident in atherosclerotic plaques of the ICA, and the RR is significant in symptomatic arteries. In addition, a significant association exists between arterial positive remodeling and plaque calcification thickness. The measurement of RR is straightforward and can help ascertain the risk stratification for patients with carotid plaques. To evaluate the predictive value of RR for future stroke events, further prospective studies are warranted.
Acknowledgments
We would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for polishing our manuscript.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-428/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-428/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-428/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-428/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Zhejiang Hospital (No. 2023-40K) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen C, Tang W, Chen Y, et al. Computed tomography angiography-based radiomics model to identify high-risk carotid plaques. Quant Imaging Med Surg 2023;13:6089-104. [Crossref] [PubMed]
- Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316:1371-5. [Crossref] [PubMed]
- Kinoshita D, Suzuki K, Usui E, et al. High-Risk Plaques on Coronary Computed Tomography Angiography: Correlation With Optical Coherence Tomography. JACC Cardiovasc Imaging 2024;17:382-91. [Crossref] [PubMed]
- Yoshida K, Yang T, Yamamoto Y, et al. Expansive carotid artery remodeling: possible marker of vulnerable plaque. J Neurosurg 2019; Epub ahead of print. [Crossref] [PubMed]
- Yoshida K, Fukumitsu R, Kurosaki Y, et al. The association between expansive arterial remodeling detected by high-resolution MRI in carotid artery stenosis and clinical presentation. J Neurosurg 2015;123:434-40. [Crossref] [PubMed]
- Saam T, Habs M, Buchholz M, et al. Expansive arterial remodeling of the carotid arteries and its effect on atherosclerotic plaque composition and vulnerability: an in-vivo black-blood 3T CMR study in symptomatic stroke patients. J Cardiovasc Magn Reson 2016;18:11. [Crossref] [PubMed]
- Shi X, Gao J, Lv Q, et al. Calcification in Atherosclerotic Plaque Vulnerability: Friend or Foe? Front Physiol 2020;11:56. [Crossref] [PubMed]
- Bischetti S, Scimeca M, Bonanno E, et al. Carotid plaque instability is not related to quantity but to elemental composition of calcification. Nutr Metab Cardiovasc Dis 2017;27:768-74. [Crossref] [PubMed]
- McCollough CH, Boedeker K, Cody D, et al. Principles and applications of multienergy CT: Report of AAPM Task Group 291. Med Phys 2020;47:e881-912. [Crossref] [PubMed]
- Saba L, Nardi V, Cau R, et al. Carotid Artery Plaque Calcifications: Lessons From Histopathology to Diagnostic Imaging. Stroke 2022;53:290-7. [Crossref] [PubMed]
- Pugliese L, Spiritigliozzi L, Di Tosto F, et al. Association of plaque calcification pattern and attenuation with instability features and coronary stenosis and calcification grade. Atherosclerosis 2020;311:150-7. [Crossref] [PubMed]
- Eisenmenger LB, Aldred BW, Kim SE, et al. Prediction of Carotid Intraplaque Hemorrhage Using Adventitial Calcification and Plaque Thickness on CTA. AJNR Am J Neuroradiol 2016;37:1496-503. [Crossref] [PubMed]
- Saba L, Mallarin G. Window settings for the study of calcified carotid plaques with multidetector CT angiography. AJNR Am J Neuroradiol 2009;30:1445-50. [Crossref] [PubMed]
- Gauss S, Achenbach S, Pflederer T, et al. Assessment of coronary artery remodelling by dual-source CT: a head-to-head comparison with intravascular ultrasound. Heart 2011;97:991-7. [Crossref] [PubMed]
- Saba L, Chen H, Cau R, et al. Impact Analysis of Different CT Configurations of Carotid Artery Plaque Calcifications on Cerebrovascular Events. AJNR Am J Neuroradiol 2022;43:272-9. [Crossref] [PubMed]
- Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827-32. [Crossref] [PubMed]
- Cury RC, Abbara S, Achenbach S, et al. CAD-RADS™: Coronary Artery Disease - Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Am Coll Radiol 2016;13:1458-1466.e9. [Crossref] [PubMed]
- Kashiwazaki D, Kuwayama N, Akioka N, et al. Carotid plaque with expansive arterial remodeling is a risk factor for ischemic complication following carotid artery stenting. Acta Neurochir (Wien) 2017;159:1299-304. [Crossref] [PubMed]
- Miura T, Matsukawa N, Sakurai K, et al. Plaque vulnerability in internal carotid arteries with positive remodeling. Cerebrovasc Dis Extra 2011;1:54-65. [Crossref] [PubMed]
- Baradaran H, Al-Dasuqi K, Knight-Greenfield A, et al. Association between Carotid Plaque Features on CTA and Cerebrovascular Ischemia: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol 2017;38:2321-6. [Crossref] [PubMed]
- Gijsen FJH, Vis B, Barrett HE, et al. Morphometric and Mechanical Analyses of Calcifications and Fibrous Plaque Tissue in Carotid Arteries for Plaque Rupture Risk Assessment. IEEE Trans Biomed Eng 2021;68:1429-38. [Crossref] [PubMed]
- Han D, Starikov A, Ó Hartaigh B, et al. Relationship Between Endothelial Wall Shear Stress and High-Risk Atherosclerotic Plaque Characteristics for Identification of Coronary Lesions That Cause Ischemia: A Direct Comparison With Fractional Flow Reserve. J Am Heart Assoc 2016;5:e004186. [Crossref] [PubMed]


