
Thrombus discrimination with electron density: potential implications for non-contrast computed tomography imaging
Background
A few studies have shown the potential of non-contrast computed tomography (CT) to detect thrombus. This has been attributed to the high protein and more concentrated hemoglobin content, and to the significant decrease in the water content after thrombus retraction (1-3). Nonetheless, a substantial fraction of thrombus is undetected using non-contrast CT, therefore contrast-enhanced CT remains the reference standard. Dual-layer spectral CT enables the direct estimation of tissue electron density (ED), without the need to modify acquisition protocols. This can be achieved since the detector technology and design (top-layer: yttrium-based low-density scintillator for detection of lower energies, bottom-layer: gadolinium oxysulphide high density scintillator for detection of higher energies) has shown to improve energy separation and signal-to-noise ratio (SNR). ED estimation using this technology is based on a linear combination of Compton-scatter and photoelectric effect component, being the former the dominant component (4).
Few reports suggested a better visualization of thrombus and hematoma using ED imaging, although the potential clinical usefulness of ED, other than for radiotherapy planning, remains unknown (5-7). We therefore sought to explore in vitro whether ED imaging could improve thrombus identification compared to conventional imaging among material aspirated during mechanical thrombectomy for acute ischemic stroke.
Setting and in-vitro study
This study was performed in accordance with the Declaration of Helsinki and later amendments, and was approved by the institutional ethics review board of Instituto Medico ENERI, Clinica La Sagrada Familia. Written authorization for anonymized use of data (habeas data) was obtained from all patients.
We evaluated mechanical thrombectomy material obtained from patients with acute ischemic stroke treated in a tertiary level stroke center (Instituto Medico ENERI, Clinica La Sagrada Familia) and immediately fixed in 10% neutral buffered formalin and stored in polystyrene test tubes. The test tubes were immersed in a bucket of water for evaluation by spectral CT, along with scattered control tubes without thrombus material, only filled with formalin. All images were obtained using a dual-layer detector CT scanner (Spectral IQon, Philips Healthcare, Best, The Netherlands) using the following parameters: collimation 64×0.625 mm; tube voltage 120 kV; current 104 mA; thickness 0.67 mm (0.34 mm increment), field of view 237 mm, 512×512 matrix size, rotation time 0.40 s, YC reconstruction filter. Using specific software (Spectral Viewer, Philips Healthcare), images were assessed using average multiplanar reconstructions in search of thrombus material within the tubes. Each tube was assessed using multiparametric side-by-side view of conventional CT (120 kVp), low monoenergetic imaging (40 keV), and ED images. A region of interest (ROI) was placed at the site of maximum density of the identified material, commonly using the ED view since it usually displayed the material more clearly (Figures 1,2). Areas immediately adjacent to the polystyrene tube were avoided. This ROI was automatically colocalized and identical in size and location in the other (conventional CT and 40 keV) displays, thus the mean Hounsfield units (HU) and mean percent ED relative to water (%EDW) were automatically calculated for the same ROI. Additional ROIs were placed within an area including only formalin within each tube, and a larger ROI at the surrounding water. Contrast-to-noise ratio (CNR) was calculated as the difference between thrombus and formalin, divided by the background noise [standard deviation (SD) of water], whereas SNR was defined as the ratio between the mean density and the mean SD. Finally, a neuroradiologist (J.S.) evaluated independently the conventional images, adjusting the window level and width at his best discretion, and determined the presence of thrombi, without availability of spectral analysis, with the premise that the tubes could or could not have thrombotic material inside. Subsequently, 6 months after the original analysis, the same qualitative analysis was performed by the first cardiac imaging observer (G.A.R.G.) only using ED reconstructions. Histopathological analyses were performed by an experienced cardiovascular pathologist (C.M.) blinded to the clinical characteristics and to the CT results. For this purpose, sections were stained with hematoxylin and eosin for light microscopy. Thrombectomy material was considered suitable for further analysis if larger than 1 mm2 and, if thrombus was detected, thrombus age was discriminated according to predefined histopathology reports as fresh thrombus (<1 day), completely composed of layered patterns of platelets, fibrin, erythrocytes and intact granulocytes; lytic thrombus (1–5 days), with areas of necrosis and granulocyte karyorrhexis; and organized thrombus (>5 days) characterized by areas of smooth muscle cells, with or without depositions of connective tissue and capillary vessels. Cases with heterogeneous composition were defined as the oldest component. We finally evaluated the differences between CT density and thrombus age, although it should be noted that, aside from the fact that thrombus can comprise diverse components, material was not colocalized for these comparisons in order to establish the accurate CT density corresponding to each thrombus age.
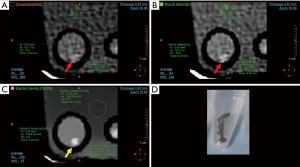
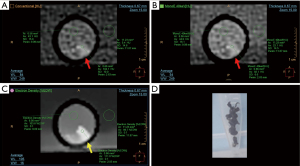
Discrete variables were reported as counts and percentages and continuous variables are presented as means ± SDs. Comparisons between groups of continuous variables were performed using independent samples t-tests and one-way analysis of variance. All statistical analyses were performed using SPSS software, version 22.0 (Armonk, NY, USA). A two-sided P value of less than 0.05 indicated statistical significance.
Findings
Material was obtained from 54 thrombectomy procedures. Two tubes were excluded since material obtained was less than 1 mm2. Six control tubes filled with formalin were included in the water bucket, thus leading to 58 tubes for the qualitative analysis. ED imaging identified accurately the presence of material in all tubes, whereas 2 (3%) of the tubes containing thrombus were not identified by conventional CT, leading to a very good agreement between observers for the presence of material using conventional CT and ED imaging (kappa =0.84, P<0.001). Using ED imaging, thrombus material showed a mean density of 108.8±2.9 %EDW, water had a mean density of 100.0±0.3 %EDW, and formalin a mean density of 103.5±1.2 %EDW (Table 1). Compared to conventional imaging and 40 keV monoenergetic, ED imaging had a significantly higher SNR (conventional 10.4±7.0, vs. 40 keV 11.5±8.4, vs. ED 490.0±304.5, P<0.001) and CNR (conventional 4.3±4.3, vs. 40 keV 5.7±11.2, vs. ED 37.8±29.1, P<0.001). According to histopathology, thrombus material was of heterogeneous age in 7 (13%) cases and defined as fresh in 28 (54%) cases, as lytic in 21 (40%) cases, and as organized in 2 (4%) cases. Fresh thrombus (<1 day) showed higher density than non-fresh (>1 day) thrombus, although only conventional imaging (<1 day 102.0±18.9 HU vs. >1 day 87.5±25.0 HU, P=0.02) and 40 keV monoenergetic (<1 day 109.7±30.5 HU, vs. >1 day 85.4±29.2 HU, P=0.006) were able to detect significant differences whereas ED were similar (<1 day 109.0±2.1 %EDW, vs. >1 day 108.1±2.9 %EDW, P=0.26). For this analysis, one calcified thrombus was excluded since it provided outlying results.
Table 1
| Variables | Conventional (HU) | 40 keV (HU) | Electron density (%EDW) | P value |
|---|---|---|---|---|
| Thrombus | 102.2±53.8 | 115.2±121.6 | 108.8±2.9 | – |
| Formalin | 36.6±15.0 | 41.9±20.3 | 103.5±1.2 | – |
| Water | 3.5±3.1 | 10.1±6.7 | 100.0±0.3 | – |
| Signal-to-noise ratio | 10.4±7.0 | 11.5±8.4 | 490.0±304.5 | <0.001 |
| Contrast-to-noise ratio | 4.3±4.4 | 5.7±11.2 | 37.8±29.1 | <0.001 |
Data are presented as mean ± standard deviation. HU, Hounsfield units; %EDW, percent electron density relative to water.
Interpretation
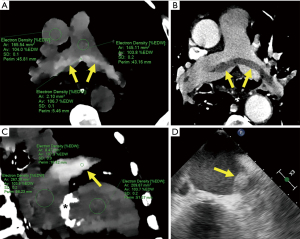
In this study, ED imaging enabled an improved visualization of thrombus compared to conventional imaging and low monoenergetic imaging in vitro, with a significantly higher CNR. Our findings, motivated and built upon promising early clinical experience (Figure 3), might provide further emerging evidence towards the potential application of ED imaging as an alternative for patients ineligible for contrast administration (6-8).
Dual-energy CT has demonstrated an incremental value over conventional CT in diverse cardiovascular scenarios, although most of such benefits have been documented with the use of contrast-enhanced examinations (9-11). Previous reports have suggested the potential role of non-contrast dual-energy CT for thrombus characterization (12-14). In a recent in-vitro study, Ding et al. demonstrated that low monoenergetic imaging may improve clot characterization, particularly of red blood cell rich thrombi (13). Furthermore, using Gammex phantoms with tissue-equivalent and iodine inserts, Hua et al. demonstrated a high accuracy of ED measurements compared to the expected values, with a median deviation between 0.1% and 1.1% for tissue inserts, with all soft tissues (adipose, brain, breast, and liver) being well separated, and results not affected by acquisition or reconstruction parameters (4).
To date, ED imaging is mostly used for radiotherapy planning. The approximate estimation of ED provided by conventional CT is based on the simple correlation with the HU. This is a suboptimal approach since HU values also depend on the effective atomic number and the X-ray spectrum. Alternatively, dual energy CT has shown to provide an accurate ED calculation across different tissues (4-8).
Nonetheless, although we have also documented early promising findings in-vivo (Figure 3), very few reports have explored the potential incremental value of ED imaging for in-vivo tissue characterization among diverse clinical scenarios (6,15-17). Among these, in a recent study ED imaging showed an area under the curve of 0.915 for the detection of bone metastasis (15). In keeping with such reports, we found improved visualization of thrombus compared to conventional imaging, with high CNR. Detection of thrombus without the requirement of intravenous contrast might become valuable particularly for patients with impaired renal function or for those at risk for contrast-induced acute kidney injury.
It should be acknowledged that although the mean ED of formalin was 103.5±1.2 %EDW, which seemed comparable to blood in the in-vivo examples provided (Figure 3), extrapolation of our results to in-vivo clinical imaging, influenced by surrounding tissues, should be cautious. Therefore, prospective clinical investigations are warranted involving thrombus of different sources and locations. In addition, whether the improved thrombus discrimination using ED might be influenced by differences in reconstruction methods for ED images affecting noise reduction deserves further research.
In conclusion, we demonstrated improved visualization of thrombus with ED imaging compared to conventional imaging and low monoenergetic imaging, with a significant increase in CNR. Our findings warrant further investigations exploring the potential role of non-contrast CT detection of thrombus.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-28/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-28/coif). G.A.R.G. serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2023 to August 2025, and is a consultant of MultipleAI Health and Caristo Diagnostics. P.L. is a consultant of Philips Healthcare. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki and later amendments, and was approved by the institutional ethics review board of Instituto Medico ENERI, Clinica La Sagrada Familia. Written authorization for anonymized use of data (habeas data) was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ehsanbakhsh A, Hatami F, Valizadeh N, et al. Evaluating the Performance of Unenhanced Computed Tomography in the Diagnosis of Pulmonary Embolism. J Tehran Heart Cent 2021;16:156-61. [PubMed]
- Weyland CS, Papanagiotou P, Schmitt N, et al. Hyperdense Artery Sign in Patients With Acute Ischemic Stroke-Automated Detection With Artificial Intelligence-Driven Software. Front Neurol 2022;13:807145. [Crossref] [PubMed]
- Velasco Gonzalez A, Buerke B, Görlich D, et al. Clot Analog Attenuation in Non-contrast CT Predicts Histology: an Experimental Study Using Machine Learning. Transl Stroke Res 2020;11:940-9. [Crossref] [PubMed]
- Hua CH, Shapira N, Merchant TE, et al. Accuracy of electron density, effective atomic number, and iodine concentration determination with a dual-layer dual-energy computed tomography system. Med Phys 2018;45:2486-97. [Crossref] [PubMed]
- Sedaghat S, Langguth P, Larsen N, et al. Diagnostic Accuracy of Dual-Layer Spectral CT Using Electron Density Images to Detect Post-Traumatic Prevertebral Hematoma of the Cervical Spine. Rofo 2021;193:1445-50. [Crossref] [PubMed]
- Bae K, Jeon KN. Diagnosis of Pulmonary Embolism in Unenhanced Dual Energy CT Using an Electron Density Image. Diagnostics (Basel) 2021;11:1841. [Crossref] [PubMed]
- Mochizuki J, Nakaura T, Hashimoto K, et al. Detection of ventricular thrombi via electron density imaging in non-contrast spectral computed tomography performed to exclude pneumonia: a case report. Eur Heart J Case Rep 2022;6:ytac148. [Crossref] [PubMed]
- Mei K, Ehn S, Oechsner M, et al. Dual-layer spectral computed tomography: measuring relative electron density. Eur Radiol Exp 2018;2:20. [Crossref] [PubMed]
- Rodriguez-Granillo GA. Delayed enhancement cardiac computed tomography for the assessment of myocardial infarction: from bench to bedside. Cardiovasc Diagn Ther 2017;7:159-70. [Crossref] [PubMed]
- Carrascosa PM, Deviggiano A, Capunay C, et al. Incremental value of myocardial perfusion over coronary angiography by spectral computed tomography in patients with intermediate to high likelihood of coronary artery disease. Eur J Radiol 2015;84:637-42. [Crossref] [PubMed]
- Li W, Yu F, Zhu W, et al. Detection of left atrial appendage thrombi by third-generation dual-source dual-energy CT: Iodine concentration versus conventional enhancement measurements. Int J Cardiol 2019;292:265-70. [Crossref] [PubMed]
- Brinjikji W, Michalak G, Kadirvel R, et al. Utility of single-energy and dual-energy computed tomography in clot characterization: An in-vitro study. Interv Neuroradiol 2017;23:279-84. [Crossref] [PubMed]
- Ding YH, Abbasi M, Michalak G, et al. Characterization of thrombus composition with multimodality CT-based imaging: an in-vitro study. J Neurointerv Surg 2021;13:738-40. [Crossref] [PubMed]
- Mochizuki J, Nakaura T, Matsumi H, et al. Evaluation of coronavirus-2019-related arterial thrombosis in noncontrast spectral computed tomography with electron density imaging. Radiol Case Rep 2022;18:49-52. [Crossref] [PubMed]
- Cheng CT, Gwini S, Craig G. A quantitative analysis of spectral computed tomography characteristics of osseous metastases. J Med Imaging Radiat Oncol 2023;67:595-601. [Crossref] [PubMed]
- Daoud B, Cazejust J, Tavolaro S, et al. Could Spectral CT Have a Potential Benefit in Coronavirus Disease (COVID-19)? AJR Am J Roentgenol 2021;216:349-54. [Crossref] [PubMed]
- Rodriguez-Granillo GA, Cirio J, Vila JF, et al. Noncontrast Myocardial Characterization in Acute Myocardial Infarction Using Electron Density Imaging. J Thorac Imaging 2023; Epub ahead of print. [Crossref] [PubMed]


