
Persistence of SARS-CoV-2 colonization and high expression of inflammatory factors in cardiac tissue 6 months after COVID-19 recovery: a prospective cohort study
Highlight box
Key findings
• Certain coronavirus disease 2019 (COVID-19) recovered patients have persistent colonization of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in their cardiac tissue 6-month post-recovery, accompanied by a local increase in inflammatory factors.
What is known and what is new?
• The presence of SARS-CoV-2 in myocardial autopsy tissues has been observed in certain individuals with COVID-19.
• Among the patients undergoing cardiac surgery from May 25 to June 10, 2023 in our hospital, SARS-CoV-2 RNA was detected in left atrial tissue of four of nine patients, indicating viral colonization in cardiac tissue.
• Compared to cardiac tissue SARS-CoV-2 (−) patients, the expression of interleukin (IL)-6 and IL-1β in the cardiac tissue were significantly increased in two of four cardiac tissue SARS-CoV-2 (+) patients.
What is the implication, and what should change now?
• Our study indicates persistent viral presence in cardiac tissue, highlighting the importance of prioritizing long COVID in most patients with chronic conditions, including heart disease.
Introduction
Background
Although the World Health Organization (WHO) announced on May 5, 2023, that the coronavirus disease 2019 (COVID-19) global pandemic had ended, the impact of COVID-19 persists. For example, long COVID is a multisystem disease that occurs in at least 10% of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. More than 200 symptoms affecting multiple organ systems, including the heart, have been identified. At least 65 million people worldwide suffer from chronic viral infection (1-3). An analysis of the US Department of Veterans Affairs database showed that over 150,000 people infected with SARS-CoV-2 had a significantly increased risk of various cardiovascular diseases within one year, including heart failure, arrhythmia, and stroke, as well as postoperative respiratory syndrome, all related to the severity of the initial COVID-19 presentation (4). In addition, a study found that at an average of 71 days after COVID-19, cardiac magnetic resonance imaging (MRI) revealed heart damage in 78% of 100 individuals (5), and 58% of patients with long COVID had heart damage 12 months after infection (6), reflecting the persistence of cardiac abnormalities.
Rationale and knowledge gap
Hypotheses have been proposed regarding the pathogenesis of the above phenomenon, including immune disorders (7-9), microbial disruption, autoimmunity, coagulation, endothelial abnormalities, and dysfunction of neural signals, as well as persistence of SARS-CoV-2 within tissues (10,11). SARS-CoV-2 is often found in myocardial autopsy tissues (12). Intriguingly, existing literature indicated that the presence of myocardial injury is not associated with myocardial inflammatory cell infiltrates (13). Additionally, a postmortem study documented a substantive SARS-CoV-2 viral load (exceeding 1,000 copies) in 16 of 39 (41.0%) COVID-19 cases, with increased proinflammatory genes expression within the myocardium, even in the absence of significant infiltration of inflammatory cells (14). In some case reports involving post-infection myocardial biopsy, the SARS-CoV-2 genome was detected in patients with heart failure symptoms, and an endocardial biopsy was performed four weeks after the onset of pulmonary symptoms. SARS-CoV-2 infection was accompanied by myocarditis, indicating myocardial infection (15). Thus, the timing of viral colonization or clearance in the cardiac tissues of patients recovering from COVID-19, as well as the relevant myocardial inflammatory infiltrates, remains uncertain at present.
Objective
Studies have confirmed a significant role of serum interleukin-6 (IL-6) levels in response to SARS-CoV-2 infection. IL-6 is not only a predictor of COVID-19 disease severity and adverse outcomes but is also a long-term prognostic factor in COVID-19 (16). Interleukin-1β (IL-1β) is also an inflammatory factor that has a significant impact on the heart (17). Therefore, in this study, we aimed to evaluate the long-term presence of SARS-CoV-2 within cardiac tissue. We then investigated the differences in myocardial expression of the inflammatory factors IL-6 and IL-1β induced by SARS-CoV-2 colonization. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-381/rc).
Methods
Study cohort and tissue sampling
According to our institutional protocol, all patients were tested for SARS-CoV-2 antigen and polymerase chain reaction (PCR) before admission, and they were re-tested for SARS-CoV-2 after admission. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Review Board at Southwest Hospital, Army Medical University (Third Military Medical University) (No. KY2023041). Informed consent was taken from all the patients. Clinical details of patients such as gender, age, body mass index (BMI), and medical history were documented upon admission. Patients with two negative test results and no other contraindications were prepared for cardiac surgery. In addition, all cardiac surgery patients underwent preoperative chest computed tomography (CT) to exclude lung diseases, such as COVID-19 or other interstitial pneumonia. Following a multidisciplinary consensus advising avoiding elective surgery within 2 weeks of SARS-CoV-2 infection (18), we enrolled patients 3 weeks after the WHO declared the end of the global COVID-19 pandemic (May 5, 2023). Additionally, patients were selected within a 15-day timeframe to minimize variations in viral clearance over an extended duration. All patients undergoing open-heart surgery between May 25 and June 10, 2023, were included in this study. Other inclusion criteria included (I) receiving mitral valve replacement (MVR) + left atrial (LA) volume reduction surgery; (II) age over 18 years; (III) negative SARS-CoV-2 PCR test and no infection symptoms for at least 6 months after recovery from COVID-19 during the Omicron wave (December 2022 in China); (IV) two negative SARS-CoV-2 antigen detection tests before surgery; and (V) patient consent to participate in the study. The patient inclusion flowchart is presented in Figure 1. Patients underwent the planned open-heart surgery. Two groups of expert-level surgeons completed all cardiac surgeries. During the study period, anesthesia and surgical techniques were performed in accordance with routine procedures. During MVR + LA procedures, LA tissues were prospectively collected and used as samples for testing (they are normally discarded). The surgical LA tissues were washed by saline and then snap-frozen in liquid nitrogen and subjected to viral detection or other detections. Thrice-repeated reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis of IL-6 and IL-1β mRNA levels in the same tissue samples was used to detect relative expression levels.
RNA extraction from cardiac tissues
The fresh LA tissue sample was immediately placed in a cry storage tube with RNAsolidTM tissue RNA preservation solution (G3019; Servicebio, China) after receipt, and then frozen in liquid nitrogen. The samples will be stored in a −80 ℃ ultra-low temperature refrigerator and processed at the same time after all samples are collected. All cardiac tissue was homogenized using a high-speed freezing homogenizer and RNA extraction was performed using the RNeasy Mini Kit (74104, Qiagen, Germany). RNA concentration was determined by NanoDrop2000 ultra microspectrophotometer. Purity is also inferred from the ratio A260 to A280. The purified RNA was stored in an ultra-low temperature refrigerator at −80 ℃.
SARS-CoV2 detection by Kit assays
Total RNA was extracted from cardiac tissue using the RNeasy Mini Kit (74104, Qiagen). The samples were treated with buffers ATL (Qiagen) and protease K (Qiagen) for inactivation and then incubated at 56 ℃ for 30 min. The novel coronavirus 2019-nCoV nucleic acid detection kit (fluorescent PCR method) was used for detection (Sansure, China). The kit detects the ORF1ab and N genes of the novel coronavirus. Kit assays included multiple assays of three targets: (I) viral N gene; (II) ORF1ab gene; and (III) synthetic internal controls. According to the number of samples tested, the number of positive controls and negative controls tested, the corresponding number of components was taken in proportion, fully mixed into PCR mixture, and 20 µL of RNA samples were added after inactivation, and then centrifuged instantaneously. Bio-Rad CFX96 real-time fluorescent quantitative PCR was used for amplification. Bio-Rad CFX Manager 3.0 software was used to set the threshold, and the negative and positive results of COVID-19 were interpreted according to the cyclic threshold. Each experiment was repeated three times.
RT-qPCR
The first strand of cDNA was synthesized with 1 µg RNA using SynScript®III RT SuperMix for qPCR (+gDNA Remover) kit (cat: TSK314S, Tsingke, China), and the cDNA was stored at −20 ℃ for later use. RT-qPCR amplification was performed using the dye QuantiNova SYBR Green PCR Kit (208054, Qiagen). Total volume of each PCR reaction was 20 µL:2 µL cDNA (50 ng), 0.3 µL forward and reverse primers (10 µM primer concentration), 7.4 µL double-steamed water, and 2× SYBR Green PCR Master MiX 10 µL were added, respectively. PCR amplification conditions: initial denaturation at 95 ℃ for 3 min for one cycle, followed by 42 cycles of denaturation at 95 ℃ for 20 s and annealing and extension at 60 ℃ for 30 s. Followed by 42 cycles of denaturation at 95 ℃ for 20 s and annealing and extension at 60 ℃ for 30 s. Bio-Rad CFX96 quantitative PCR instrument was used for computer detection and were calculated using the 2−ΔΔCq method. The primers used were as follows: IL-6, forward 5'-ACTCACCTCTTCAGAACGAATTG-3', reverse 5'-CCATCTTTGGAAGGTTCAGGTTG-3'; IL-1β, forward 5'-AGCTACGAATCTCCGACCAC-3', reverse 5'-CGTTATCCCATGTGTCGAAGAA-3'; GAPDH, forward 5'-CTGGGCTACACTGAGCACC-3', reverse 5'-AAGTGGTCGTTGAGGGCAATG-3'.
Measuring serum interleukins and biochemical factors
To determine the serum levels of interleukins and biochemical factors in SARS-CoV-2 (+) or (−) groups, fasting blood samples were collected from each enrolled patient at 24 h pre- and post-operation. The serum fractions were obtained by immediate centrifugation of the blood samples and stored at −80 ℃ until further use. IL-6 and IL-1β were used as markers of inflammation and N-terminal pro-brain natriuretic peptide (NT-proBNP) was employed as an indicator of cardiac function. Furthermore, various additional indicators including blood urea nitrogen (BUN), creatinine (Cr), estimated glomerular filtration rate (eGFR), alanine transaminase (ALT), total bilirubin (TBIL), direct bilirubin (DBIL), and activated partial thromboplastin time (APTT) were integrated to comprehensively evaluate the medical status and organ function of the patients. Serum IL-6 levels were determined using the Cobas e601 electro-chemiluminescence immunoassay (Roche Diagnostics, Germany), while serum IL-1β levels were measured using the Immulite 1000 Immunoassay Analyzer (Siemens, Germany). NT-proBNP levels were checked by the Cobas e601 electro-chemiluminescence immunoassay (Roche Diagnostics). The BUN, Cr, ALT, TBIL, and DBIL levels were obtained from the 7060 full-automatic chemical analyzer (HITACHI, Japan), and the eGFR results were collected from routine clinical biochemistry reports. APTT was analyzed using the CA-1500 automatic blood coagulation analyzer (Sysmex, Japan).
Immunofluorescence staining
The LA tissue used for immunofluorescence staining was obtained from the SARS-CoV-2 (+) patient, whose IL-6 levels and IL-1β levels were approximately 55- and 110-fold higher than those of SARS-CoV-2 (−) patients. The fixed cardiac tissue sections were deparaffinized by a series of solutions including xylene, gradient alcohol, and pure water. High-temperature antigen retrieval was performed. After blocking, the IL-6 antibody (Zen-bio, China) or IL-1β antibody (Zen-bio) was added onto the sections and incubated at 4 ℃ overnight. Subsequently, the sections were washed three times with phosphate buffered saline (PBS). The sections were then incubated with fluorescein isothiocyanate (FITC)-labeled or Cy3-labeled goat anti-rabbit secondary antibody (Beyotime, China) at 37 ℃ for 1 h. Three additional washes with PBS were performed. The sections were then incubated with 4',6-diamidino-2-phenylindole (DAPI) solution (Beyotime, China) at room temperature for 10 min. The fluorescence microscope (ZEISS, Germany) was used to detect the fluorescence for pathological evaluation.
Statistical analysis
All statistical analyses were performed using SPSS 25.0 software (IBM Corp., Armonk, NY, USA). The data were expressed as medians and interquartile range (p25, p75). Categorical variables were compared using the Chi-square test or Fisher’s exact test. In our study, continuous variables were compared using Mann-Whitney U test due to their non-normal distribution. P<0.05 was considered statistically significant.
Results
Baseline characteristics
During the study period, a total of 41 patients underwent preoperative CT examination, and none of them presented with unilateral or bilateral pneumonia, interstitial pneumonia, unilateral or bilateral pleural effusion, or enlarged mediastinal lymph nodes. Eight patients had scattered nodules in either one lung (two patients) or both lungs (six patients), all of which were considered to be inflammatory foci (diameter ranged from 2–5 mm × 3–7 mm). Twenty-eight of 41 patients underwent open-heart surgery in the cardiac surgery department of Southwest Hospital. Among them, nine patients met the inclusion criteria (Figure 1). Of these nine patients, SARS-CoV-2 RNA genomes were detected within surgical LA tissue by RT-qPCR in four cases, indicating infection (positive control Ct value =36.88±0.5) (Figure S1). In the remaining five patients, SARS-CoV-2 RNA genomes were not detected (Ct value >40). The patients were assigned to two groups based on their cardiac tissue SARS-CoV-2 (+) or (−) status. In the SARS-CoV-2 (−) group, the age of the patients was 49.00 (40.00, 55.00) years, with 80% being male (4/5). In the SARS-CoV-2 (+) group, the age of the patients was 51.00 (27.50, 64.75) years, with 25% being male (1/4). There were no significant differences in terms of gender, age, BMI, or any other demographic or clinical characteristics between the two groups (Table 1). Each group had two patients with a prior diagnosis of chronic obstructive pulmonary disease (COPD; Table 1). The patients’ conditions were well-managed with regular treatment prior to surgery.
Table 1
| Characteristics | SARS-CoV-2-RNA genomes (−) | SARS-CoV-2-RNA genomes (+) | P value |
|---|---|---|---|
| Male | 4 (80.0) | 1 (25.0) | 0.21 |
| Age (years) | 49.00 (40.00, 55.00) | 51.00 (27.50, 64.75) | 0.54 |
| BMI (kg/m2) | 27.10 (23.96, 27.74) | 21.74 (19.57, 25.15) | 0.050 |
| LVEF (%) | 59.00 (55.50, 72.00) | 58.00 (46.50, 65.00) | 0.39 |
| LVEDV (mm) | 38.00 (37.50, 51.50) | 42.00 (35.50, 62.00) | 0.81 |
| Medical history | |||
| Cerebral infarction | 1 (20.0) | 0 | >0.99 |
| COPD | 2 (40.0) | 2 (50.0) | >0.99 |
| Diabetes | 0 | 1 (25.0) | 0.44 |
| Major operation | |||
| MVR | 3 (60.0) | 2 (50.0) | >0.99 |
| MVR + AVR | 1 (20.0) | 1 (25.0) | >0.99 |
| MVR + CABG | 1 (20.0) | 1 (25.0) | >0.99 |
| CPB duration (min) | 114.00 (89.00, 176.00) | 118.50 (52.50, 146.25) | 0.81 |
| Cross-clamp time (min) | 59.00 (19.00, 125.00) | 78.50 (13.25, 110.00) | 0.90 |
| Peroperative bleeding (mL) | 300.00 (200.00, 400.00) | 250.00 (87.50, 300.00) | 0.31 |
| Mechanical ventilation time (h) | 6.00 (5.00, 71.50) | 30.00 (8.25, 58.50) | 0.62 |
| Length of ICU stay (days) | 5.00 (3.00, 5.50) | 5.00 (1.75, 6.75) | 0.71 |
| Length of postoperation hospital stays (days) | 10.00 (8.00, 12.50) | 9.50 (6.00, 12.25) | 0.53 |
| Tissue IL-6 relative transcript level | 1.00 (0.62, 2.88) | 4.43 (0.51, 44.33) | 0.46 |
| Tissue IL-1β relative transcript level | 1.00 (0.67, 1.58) | 4.60 (0.73, 89.97) | 0.33 |
Data are reported as n (%) or medians and interquartile range (p25, p75). SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; BMI, body mass index; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume abbreviation; COPD, chronic obstructive pulmonary disease; MVR, mitral valve replacement; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ICU, intensive care unit; IL-6, interleukin-6; IL-1β, interleukin-1β.
Operative data
All nine patients underwent MVR + LA procedures (Figure 1, Table 1). In both SARS-CoV-2 (−) group and SARS-CoV-2 (+) group, one patient underwent MVR combined with aortic valve replacement (AVR), and another patient underwent MVR combined with coronary artery bypass grafting (CABG) (Table 2). There were no significant differences observed between the two groups in terms of surgical procedures. Furthermore, no significant differences were found in the duration of cardiopulmonary bypass (CPB) time [114.00 (89.00, 176.00) vs. 118.50 (52.50, 146.25) min, P=0.81], cross-clamp time [59.00 (19.00, 125.00) vs. 78.50 (13.25, 110.00) min, P=0.90], or other intraoperative parameters between the SARS-CoV-2 (−) and SARS-CoV-2 (+) groups. Additionally, there were no significant differences between the two groups in terms of postoperative indicators, including mechanical ventilation time, length of stay in the intensive care unit (ICU), and duration of hospital stay after surgery.
Table 2
| Characteristics | SARS-CoV-2-RNA genomes (−) | SARS-CoV-2-RNA genomes (+) | P value |
|---|---|---|---|
| IL-6 (ng/L) | |||
| Pre-operation | 6.17 (2.95, 129.47) | 4.14 (3.31, 6.32) | 0.46 |
| Post-operation | 95.55 (88.97, 385.27) | 95.20 (79.19, 240.15) | 0.56 |
| IL-1β (pg/mL) | |||
| Pre-operation | 0.00 (0.00, 6.13) | 0.00 (0.00, 10.20) | 0.88 |
| Post-operation | 0.00 (0.00, 12.90) | 0.00 (0.00, 5.43) | 0.85 |
| NT-proBNP (pg/mL) | |||
| Pre-operation | 443.20 (154.87, 2,183.50) | 214.11 (5.83, 1,058.23) | 0.46 |
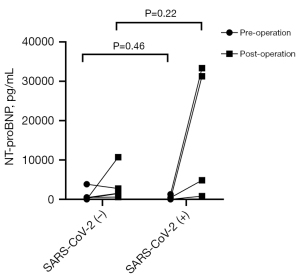
| Post-operation | 1,552.00 (1,046.00, 6,731.00) | 18,081.50 (1,816.73, 32,839.75) | 0.22 |
| BUN (mmol/L) | |||
| Pre-operation | 5.56 (4.36, 7.16) | 5.87 (4.26, 9.24) | 0.71 |
| Post-operation | 6.54 (1.13, 13.61) | 10.49 (6.92, 15.71) | 0.46 |
| Cr (μmol/L) | |||
| Pre-operation | 68.20 (65.29, 88.00) | 70.25 (55.23, 95.48) | 0.81 |
| Post-operation | 127.75 (83.96, 159.73) | 81.75 (58.60, 109.10) | 0.15 |
| eGFR (mL/min/L) | |||
| Pre-operation | 107.15 (69.89, 116.56) | 91.51 (59.54, 117.76) | 0.81 |
| Post-operation | 88.89 (67.96, 109.77) | 140.13 (92.67, 194.88) | 0.15 |
| ALT (IU/L) | |||
| Pre-operation | 35.90 (21.60, 46.85) | 24.60 (17.38, 36.93) | 0.46 |
| Post-operation | 66.70 (29.93, 927.65) | 45.60 (25.70, 515.73) | 0.15 |
| TBIL (μmol/L) | |||
| Pre-operation | 20.82 (13.30, 22.05) | 17.75 (10.20, 38.13) | >0.99 |
| Post-operation | 39.66 (14.36, 75.45) | 23.95 (16.00, 29.20) | 0.77 |
| DBIL (μmol/L) | |||
| Pre-operation | 2.80 (1.30, 4.55) | 3.75 (2.14, 7.78) | 0.54 |
| Post-operation | 14.55 (1.23, 37.48) | 6.75 (3.45, 9.17) | 0.77 |
| APTT (s) | |||
| Pre-operation | 27.50 (26.20, 30.00) | 25.50 (24.93, 26.38) | 0.050 |
| Post-operation | 33.10 (15.80, 44.15) | 38.90 (28.45, 98.70) | 0.62 |
The serum biochemical factors among cardiac tissue SARS-CoV-2 (+) or (−) groups were analyzed at 24 h pre- and post-operation. Data are reported as medians and interquartile range (p25, p75) for those with a skewed distribution. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IL-6, interleukin-6; IL-1β, interleukin-1β; NT-proBNP, N-terminal pro-brain natriuretic peptide; BUN, blood urea nitrogen; Cr, creatinine; eGFR, estimated glomerular filtration rate; ALT, alanine transaminase; TBIL, total bilirubin; DBIL, direct bilirubin; APTT, activated partial thromboplastin time.
Inflammatory factors in cardiac tissue and serum
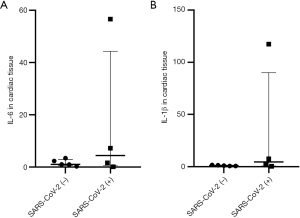
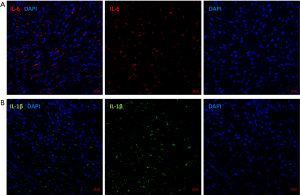
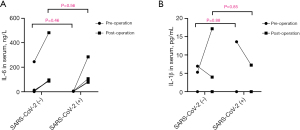
The RT-qPCR analysis revealed that the expression levels of IL-6 and IL-1β in cardiac tissues of all SARS-CoV-2 (−) patients were relatively low (Figure 2). Among the four SARS-CoV-2 (+) cases, the levels of IL-6 and IL-1β in two patients did not show significant differences compared to those in SARS-CoV-2 (−) patients (Figure 2). However, in the remaining two SARS-CoV-2 positive patients, there was a significant increase observed in the relative expression levels of IL-6 and IL-1β in the cardiac tissue of one patient [approximately 55- and 110-fold higher, respectively, than those of SARS-CoV-2 (−) patients, Figure 2]. Further immunofluorescence testing revealed the presence of inflammatory cytokines (IL-6 and IL-1β) in cardiac tissue from this SARS-CoV-2 (+) patient (Figure 3). Similarly, the other patient also exhibited significantly elevated levels of relative IL-6 and IL-1β expression (both 7-fold higher) compared to patients without viral colonization (Figure 2). Importantly, neither of these two patients exhibited any coexisting significant diseases or conditions, such as systemic lupus erythematosus or rheumatoid arthritis, which could account for the increased levels of inflammatory factors. Interestingly, the preoperative serum levels of inflammatory factors in these two patients did not exhibit abnormal increases compared to the other patients (Figure 4, Table 2). All the serum IL-6 levels of patients exhibited a significant increase on postoperative day one, while only one SARS-CoV-2 (−) patient displayed an abnormal elevation in IL-6 levels following surgery (245.1 ng/L pre-operation vs. 481.5 ng/L post-operation, Figure 4).
Perioperative outcomes
Table 2 presents the perioperative levels of serum biochemical factors of the SARS-CoV-2 (+) and SARS-CoV-2 (−) patients. There were no significant differences in the preoperative levels of NT-proBNP, BUN, Cr, ALT, TBIL, APTT and other serum biochemical factors between two groups. Similarly, there were no differences in these levels between the two groups postoperatively. Notably, SARS-CoV-2 (+) patients exhibited higher levels of NT-proBNP compared to SARS-CoV-2 (−) patients after surgery [1,552.00 (1,046.00, 6,731.00) vs. 18,081.50 (1,816.73, 32,839.75) pg/mL, P=0.22], although these differences did not reach statistical significance (Table 2, Figure 5). None of the patients required continuous renal replacement therapy (CRRT), extracorporeal membrane oxygenation (ECMO), intra-aortic balloon pumping (IABP), or other mechanical support throughout the perioperative period. All patients survived until hospital discharge and demonstrated a complete recovery after 3 months of follow-up.
Discussion
This study assessed the presence of SARS-CoV-2 within cardiac tissues collected during open-heart surgery. Notably, despite negative throat swab or antigen test results before surgery, some patients exhibited detectable SARS CoV-2 genomes in their cardiac tissues. Furthermore, among those with detectable SARS-CoV-2 (+) genomes in the cardiac tissues, some displayed elevated mRNA levels of local inflammatory factors IL-6 and IL-1β within the same tissues. Currently, it remains uncertain regarding the frequency of such occurrences, the timeline for patients returning to normalcy, and potential long-term impacts following cardiac surgery.
On May 5, 2023, The WHO officially announced the termination of the global COVID-19 pandemic. A multidisciplinary consensus recommended that elective surgery should be withheld within 2 weeks of SARS-CoV-2 infection (18). Therefore, in this study, we enrolled patients who were 3 weeks post the WHO’s declaration of the cessation of the global pandemic. To minimize potential variations in viral clearance over an extended duration, surgical patients within a concise timeframe of 15 days were selected. COVID-19 is defined as an infectious disease caused by the SARS-CoV-2 virus (19). Although most individuals achieve full recovery, 45% of COVID-19 survivors may experience diverse persistent symptoms even 4 months post-diagnosis, which is commonly referred to as long COVID (20). The patients included in this study had achieved a recovery period exceeding 4 months after SARS-CoV-2 infection. The symptoms experienced by cardiac patients, such as fatigue, shortness of breath, and palpitations, are often indistinguishable from those associated with long COVID or the underlying heart disease itself. Therefore, it is difficult to determine whether the patients in this study were experiencing long COVID.
Weckbach et al. reported that five COVID-19 patients with myocarditis diagnosed by cardiac magnetic resonance were SARS-CoV-2 RNA-negative within 7 to 43 days from COVID-19 symptoms to endomyocardial biopsy (EMB) (21). A previous study reported a case of SARS-CoV-2-positive myocarditis 4 weeks after COVID-19 (22). Another study reported the myocardium of one patient was found to have detectable SARS-CoV-2 RNA 5 months after COVID-19 (23). The timing and conditions of viral colonization in convalescent or asymptomatic patients infected with SARS-CoV-2 remains unclear. Our study revealed that even if 6 months after COVID-19, approximately 44% (4/9) of patients still exhibited presence of SARS-CoV-2 within their cardiac tissues. Besides, inflammatory cytokines (IL-6 and IL-1β) were found to be present in the cardiac tissue from SARS-CoV-2 (+) patients, indicating SARS-CoV-2 may induced inflammatory injury to the cardiac tissues even after the patient recovering from it. Furthermore, the SARS-CoV-2 (+) group exhibited an increase in NT-proBNP levels when compared to the SARS-CoV-2 (−) group, although this difference did not reach statistical significance. This observation may simply be coincidental, given the small size of the sample. Alternatively, it might potentially indicate the ongoing myocardial involvement in the SARS-CoV-2 (+) patients, since both groups underwent identical procedures. Further research is needed in this area.
A study has indicated that COVID-19 patients may experience persistent capillary paralysis for up to 18 months after infection. It is unclear whether, when, and to what extent the observed damage is reversible (24). Continuous circulation of the SARS-CoV-2 spike protein in patients with long COVID is associated with sequelae (11), and some patients also have elevated levels of cytokines in serum, especially IL-1, IL-6, tumor necrosis factor alpha (TNF-α), and interferon-γ-induced protein 10 (IP10) (25). Currently, the precise localization of virus-infected cardiac tissue and the induction mechanism of inflammatory factors in rehabilitated patients remain elusive (13,26). Among the four virus-positive patients included in this study, two exhibited abnormal elevations of IL-6 and IL-1β levels in local tissues. Additionally, our study revealed a lack of concordance between the expression patterns of inflammatory factors in both cardiac tissue and serum of these two patients. SARS-CoV-2 can be detected in the lung tissues of deceased patients with negative nasopharyngeal swabs (23). Other studies have reported similar phenomena (27,28). Our study also identified a similar phenomenon, despite these nine patients testing negative for SARS-CoV-2 in two pre-operative antigen tests. Therefore, the presence of residual virus in their cardiovascular tissues suggests that a negative viral swab does not necessarily indicate complete eradication of the virus from the body. Unfortunately, we were unable to obtain serial cardiac samples from the same patient. Serial samples would allow for analysis at different time points, such as 60 days, 90 days, and 1 year after SARS-CoV-2 infection. This would provide clarification regarding the clearance of the virus from cardiovascular tissue, normalization of local inflammatory factor levels, and synchronization between viral clearance and disappearance of inflammatory factors. The most crucial aspect pertains to the impact of this condition on long-term follow-up of patients after cardiac surgery and how to address various issues.
Limitation
There are several limitations in this study. Firstly, our study was conducted at a single center with a limited timeframe for patient selection, which might introduce a selection bias to the results. Additional studies involving multiple facilities would be beneficial for understanding prolonged viral colonization in cardiac tissue. Secondly, the number of enrolled patients was relatively small. Thirdly, the limited size of the collected LA tissue necessitated a narrow focus on the analysis of IL-6 and IL-1β levels, consequently precluding the assessment of additional interleukins such as interleukin-8. Lastly, we did not further investigate the underlying mechanism through which the SARS-CoV-2 virus contributes to the elevated inflammatory factors in myocardial tissue. These areas may need further exploration in future research.
Conclusions
In summary, this study reports the detection of SARS-CoV-2 within cardiac tissue acquired during cardiac surgery, thus confirming persistent colonization even 6 months after initial infection. The findings suggest that the presence of SARS-CoV-2 in cardiac tissues may induce a significant increase in local inflammatory mediators, highlighting the need for increased focus on long COVID in a large proportion of patients with chronic conditions, including heart disease.
Acknowledgments
We would like to thank all the patients participated in our study. We would like to thank Lingfeng Tang, Ya Chen, Rongshu Liao and Hong Tao for their help in collecting samples. We would like to thank Editage for the help in polishing our paper.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-381/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-381/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-381/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-381/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Review Board at Southwest Hospital, Army Medical University (Third Military Medical University) (No. KY2023041). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023;21:133-46. [Crossref] [PubMed]
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun 2022;101:93-135. [Crossref] [PubMed]
- Bull-Otterson L, Baca S, Saydah S, et al. Post–COVID Conditions Among Adult COVID-19 Survivors Aged 18–64 and ≥65 Years — United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep 2022;71:713-7. [Crossref]
- Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022;28:583-90. [Crossref] [PubMed]
- Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265-73. [Crossref] [PubMed]
- Roca-Fernandez A, Wamil M, Telford A, et al. Cardiac abnormalities in Long COVID 1-year post-SARS-CoV-2 infection. Open Heart 2023;10:e002241. [Crossref] [PubMed]
- Glynne P, Tahmasebi N, Gant V, et al. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med 2022;70:61-7. [Crossref] [PubMed]
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 2022;23:210-6. [Crossref] [PubMed]
- Klein J, Wood J, Jaycox JR, et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023;623:139-48. [Crossref] [PubMed]
- Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front Microbiol 2021;12:698169. [Crossref] [PubMed]
- Swank Z, Senussi Y, Manickas-Hill Z, et al. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae. Clin Infect Dis 2023;76:e487-90. [Crossref] [PubMed]
- Delorey TM, Ziegler CGK, Heimberg G, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021;595:107-13. [Crossref] [PubMed]
- Dal Ferro M, Bussani R, Paldino A, et al. SARS-CoV-2, myocardial injury and inflammation: insights from a large clinical and autopsy study. Clin Res Cardiol 2021;110:1822-31. [Crossref] [PubMed]
- Lindner D, Fitzek A, Bräuninger H, et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol 2020;5:1281-5. [Crossref] [PubMed]
- Nappi F, Avtaar Singh SS. SARS-CoV-2-Induced Myocarditis: A State-of-the-Art Review. Viruses 2023;15:916. [Crossref] [PubMed]
- Giannitrapani L, Mirarchi L, Amodeo S, et al. Can Baseline IL-6 Levels Predict Long COVID in Subjects Hospitalized for SARS-CoV-2 Disease? Int J Mol Sci 2023;24:1731. [Crossref] [PubMed]
- Schultheiß C, Willscher E, Paschold L, et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med 2022;3:100663. [Crossref] [PubMed]
- El-Boghdadly K, Cook TM, Goodacre T, et al. Timing of elective surgery and risk assessment after SARS-CoV-2 infection: 2023 update: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, Federation of Surgical Specialty Associations, Royal College of Anaesthetists and Royal College of Surgeons of England. Anaesthesia 2023;78:1147-52. [Crossref] [PubMed]
- Yuan Y, Jiao B, Qu L, et al. The development of COVID-19 treatment. Front Immunol 2023;14:1125246. [Crossref] [PubMed]
- O'Mahoney LL, Routen A, Gillies C, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine 2022;55:101762. [Crossref] [PubMed]
- Weckbach LT, Curta A, Bieber S, et al. Myocardial Inflammation and Dysfunction in COVID-19-Associated Myocardial Injury. Circ Cardiovasc Imaging 2021;14:e012220. [Crossref] [PubMed]
- Wenzel P, Kopp S, Göbel S, et al. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res 2020;116:1661-3. [Crossref] [PubMed]
- Blagova O, Ainetdinova D, Sedov A, et al. Progressive chronic SARS-CoV-2-positive giant cell myoendocarditis with atrial standstill and sudden cardiac death. ESC Heart Fail 2021;8:4296-300. [Crossref] [PubMed]
- Osiaevi I, Schulze A, Evers G, et al. Persistent capillary rarefication in long COVID syndrome. Angiogenesis 2023;26:53-61. [Crossref] [PubMed]
- Peluso MJ, Lu S, Tang AF, et al. Markers of Immune Activation and Inflammation in Individuals With Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Infect Dis 2021;224:1839-48. [Crossref] [PubMed]
- Sewanan LR, Clerkin KJ, Tucker NR, et al. How Does COVID-19 Affect the Heart? Curr Cardiol Rep 2023;25:171-84. [Crossref] [PubMed]
- Bussani R, Zentilin L, Correa R, et al. Persistent SARS-CoV-2 infection in patients seemingly recovered from COVID-19. J Pathol 2023;259:254-63. [Crossref] [PubMed]
- Cheung CCL, Goh D, Lim X, et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 2022;71:226-9. [Crossref] [PubMed]


