The Cardio-PadTM project: progress and remaining challenges
Low-income countries are facing a surge in the burden of cardiovascular diseases (CVDs) which, along with other non-communicable diseases, are overtaking infectious diseases as top killers in these settings (1,2). Rural populations are particularly vulnerable owing to their very limited access to either qualified health professionals or effective interventions for CVDs, notably diagnostic tools and essential medicines (3,4). Effective delivery of adequate cardiovascular care to rural populations in sub-Saharan Africa, South and East Asia should therefore be a priority for global health programs.
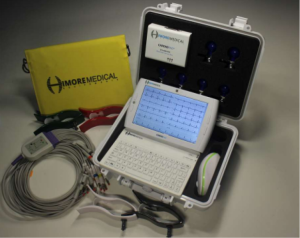
As acknowledged by the World Health Organization and increasingly by national health authorities, telemedicine is an important strategy to overcome the shortage of health professionals and improve delivery of health care to rural populations in low-to-middle-income countries (5). It has the potential to reduce socioeconomic disparities in the management of CVDs. Two years ago, we presented the Cardio-PadTM (Figure 1), a tele-cardiology device which enables to perform electrocardiograms (ECG) in remote underserved areas and transfer the test results wirelessly to specialist physicians who can interpret them and provide assistance for cases management (6). The Cardio-PadTM was designed and developed by Arthur Zang, a young Cameroon-based engineer, who received several awards including the 2014 Rolex Award for Enterprise (7) and the 2016 Africa Prize for engineering innovation from the Royal Academy of Engineering (8) for this invention recognized as the first medical computer tablet originating from Africa. In this paper, we present updates on the Cardio-PadTM initiative and some of its limitations and remaining challenges.
The technical features of the Cardio-PadTM have been previously described (6). In brief, the Cardio-PadTM is a touchscreen tablet whose main internal components include a Dual Core processor, a Double Data Rate Random Access Memory (DDR RAM), an internal memory for data storage, a Global System for Mobile/General Packet Radio Services (GSM/GPRS) for data transmission, a SIM card connector, GPS, Bluetooth and Wi-Fi modules, a lithium battery and two USB ports. External components include ECG electrodes and cables, and a Bluetooth sensor box which detects and digitalizes the heart signals from the electrodes connected to the patient and afterwards transfers them to the Cardio-PadTM via Bluetooth. The Cardio-PadTM is a multifunction cardiograph with two main functionalities: first, through the CardioPadgraphTM program, it performs a 12-lead ECG displayed on the pad; secondly, it allows transfer of patients’ data via the mobile phone network using a SIM card, to a National Data Centre and then to a specialist who can interpret the data and assist in the diagnosis and management of patients. The Cardio-PadTM can also function as a scope as it is able to perform real-time monitoring and recording of the heart’s electrical activity using the CardioPadScopeTM program. In the Cardio-PadTM user guide V.1, it is claimed that the device can also perform real-time monitoring of vital parameters such as respiratory rate, blood pressure, temperature and oximetry. However, no accessories are provided in the Cardio-Pad kit for the recording of these parameters, and the procedure to use the scope function is not described.
Some improvements have been made on the prototype available in 2014. First, although the autonomy of the built-in battery has not been improved, a solar panel is now provided to charge the battery using solar energy, making it more suitable for use in remote villages where there is limited electricity supply. Second, the ECG displayed on the Cardio-PadTM can now be printed on an A4 paper compatible with all printers, and stored in patients’ folders. This is an interesting feature, especially for hospitals where patients’ medical information are not archived electronically. Third, according to the developer, the information recorded by a Cardio-PadTM can now be transferred not only to another Cardio-PadTM but also to any smartphone. Through a software that can be freely downloaded on the Cardio-PadTM website, the smartphone can receive signals from a Cardio-PadTM, treat them and generate ECG images in various formats including JPEG. Fourth, a wireless mouse and a keyboard are now provided as backup solutions in case of damaged touchscreen or for users less comfortable with it.
The commercialization of the Cardio-Pad started in early 2016. According to Himore Medical Equipments, the company which produces and commercializes the Cardio-PadTM, 300 pads have been produced and about 50 have been sold to date. Most of the buyers were Non-Governmental Organizations in Nepal, India, Comores Island and Gabon. The manufacturer assures that there is a close follow-up of the products delivered. Local partnerships to ensure maintenance are being put in place in high sales countries and there is even a possibility to replace dysfunctional pads.
Despite the rapid commercialization of the Cardio-PadTM driven by the clear potential to help improve the care of patients with CVDs in remote underserved areas in low-to-middle-income countries, several questions remain unanswered, pertaining to its reliability and cost-effectiveness as a public health intervention. Moreover, there is virtually no peer-reviewed publication to support the Cardio-PadTM in the competition with similar technologies and devices being developed worldwide. Below are some recommendations to be considered by Himore Medical Equipments in order to ensure the success of the Cardio-PadTM.
First, there is a need to validate the Cardio-PadTM against standard ECG devices. This should ideally be done by assessing the performance of the device in a group of healthy volunteers and publishing the results in an academic peer-reviewed biomedical journal. The results should include measures of inter-observer and intra-observer agreement (9-11); error margins for each of the multiple ECG parameters; optimal operating conditions; and quantification of the effects of some ecological or patient-related factors on the performance of the device, notably age, gender, body mass index, patient’s or examiner’s mobility during the recording, exercise, skin moisture, tobacco or caffeine consumption.
Second, the performance of the Cardio-PadTM for the diagnosis of cardiac diseases should be rigorously demonstrated in the pre-hospital and intra-hospital emergency settings where such device would be the most useful especially because it could facilitate the coordination of medical decisions and interventions by allowing simultaneous real-time visualization of patients’ ECGs by several teams. The priority should be given to some specific conditions such as myocardial infarction, conduction blocks, atrial fibrillation, dyskalemia, dyscalcemias, prolongation of the QT interval, and ventricular tachycardia. It should be noted that the Cardio-PadTM is actually lagging far behind its competitors. Indeed, several other portable electronic ECG devices are being developed all around the world with promising results (12-17). Therefore, the designers of the Cardio-PadTM should manage to catch up quickly in order to safeguard its competitiveness on the market.
Third, it is advisable that medico-economic studies are conducted to quantify the clinical and economic benefits of using the Cardio-PadTM rather than current standard ECG devices even in rural settings with limited resources. Important items to consider include the average reduction of time to access meaningful information, the average gain of space allowed by cloud-based archiving, the average gain of time to diagnosis and treatment which converts into reduction of disability- and quality-adjusted life years (18-20), and the average reduction of the ECG cost due to savings on printing charges.
Fourth, while implementing technical and clinical validation studies, the staff at Himore Medical Equipments should also try to enhance the safety of patients’ data transfer and storage and incorporate new algorithms to assist users in the diagnostic process. Such advanced algorithms should be able to perform real-time analysis of the ECG and comparison with previous traces stored on the device or in the cloud. The safety of data transfer and storage is critical and should be taken seriously when contemplating the potential consequences of either the falsification or the deletion of patients’ medical information (21,22).
As a conclusion, we would like to congratulate and encourage Arthur Zang and his team for their invention and encourage them to keep aiming high in their quest of excellence. It is indeed a great achievement to have designed the Cardio-PadTM and constantly improved its functionalities over the years. We hope that the recommendations in this paper will help them take the necessary steps to ensure the worldwide success of the Cardio-PadTM especially in western countries were regulations on drugs and medical devices are more coercive and companies developing rival products are thriving. They should also realize that the implementation of these recommendations will request a diversification of the staff to include experts from various domains (cardiology, public health, biostatistics, data management, cybersecurity, economics, ethics, and law) and the initiation of international collaborations with various biomedical engineering institutes around the world. We would also like to take advantage of this tribune to raise the awareness of governments in low-to-middle income countries on the need to create regulatory boards that will closely monitor and guide the progressive introduction of new medical devices in their countries so as to minimize the health risks for their populations.
Acknowledgements
The authors are grateful to Arthur Zang for providing information of the Cardio-PadTM. All the information on the Cardio-PadTM presented in this paper were obtained from the inventor of the Cardio-PadTM and CEO of Himore Medical Equipements, Arthur Zang, or from the users documentation provided with the commercialized Cardio-PadTM kit.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245-54. [Crossref] [PubMed]
- WHO. Global status report on noncommunicable diseases 2014. Geneva: World Health Organization, 2014.
- Jingi AM, Noubiap JJ, Ewane Onana A, et al. Access to diagnostic tests and essential medicines for cardiovascular diseases and diabetes care: cost, availability and affordability in the West Region of Cameroon. PLoS One 2014;9:e111812. [Crossref] [PubMed]
- Khatib R, McKee M, Shannon H, et al. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet 2016;387:61-9. [Crossref] [PubMed]
- WHO. Telemedicine: opportunities and developments in Member States: report on the second global survey on eHealth 2009 (Global Observatory for eHealth Series, 2). Geneva: World Health Organization, 2010.
- Noubiap JJ, Jingi AM, Kengne AP. Local innovation for improving primary care cardiology in resource-limited African settings: an insight on the Cardio Pad(®) project in Cameroon. Cardiovasc Diagn Ther 2014;4:397-400. [PubMed]
- Zang A. Rolex Young Laureate 2014: reinventing cardiological care in Cameroon: Rolex; 2014 [Accessed July 29, 2016]. Available online: http://magazine.rolexawards.com/laureate/arthur-zang
- Africa Prize for Engineering Innovation: current and recent awards London: Royal Academy of Engineering; 2016 [Accessed July 29, 2016]. Available online: http://www.raeng.org.uk/grants-and-prizes/international-research-and-collaborations/africa-prize/current-and-recent-awards
- Liehr P, Dedo YL, Torres S, et al. Assessing agreement between clinical measurement methods. Heart Lung 1995;24:240-5. [Crossref] [PubMed]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. [Crossref] [PubMed]
- Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med 2005;37:360-3. [PubMed]
- Baquero GA, Banchs JE, Ahmed S, et al. Surface 12 lead electrocardiogram recordings using smart phone technology. J Electrocardiol 2015;48:1-7. [Crossref] [PubMed]
- Desteghe L, Raymaekers Z, Lutin M, et al. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2016. [Epub ahead of print].
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT Interval Readings in Normal Sinus Rhythm Between a Smartphone Heart Monitor and a 12-Lead ECG for Healthy Volunteers and Inpatients Receiving Sotalol or Dofetilide. J Cardiovasc Electrophysiol 2016;27:827-32. [Crossref] [PubMed]
- Haberman ZC, Jahn RT, Bose R, et al. Wireless Smartphone ECG Enables Large-Scale Screening in Diverse Populations. J Cardiovasc Electrophysiol 2015;26:520-6. [Crossref] [PubMed]
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS Pilot Study. J Electrocardiol 2015;48:249-59. [Crossref] [PubMed]
- Nguyen HH, Van Hare GF, Rudokas M, et al. SPEAR Trial: Smartphone Pediatric ElectrocARdiogram Trial. PLoS One 2015;10:e0136256. [Crossref] [PubMed]
- Bravo Vergel Y, Sculpher M. Quality-adjusted life years. Pract Neurol 2008;8:175-82. [Crossref] [PubMed]
- Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ 1994;72:429-45. [PubMed]
- Schoenhagen P, Mehta N. Big data, smart computer systems, and doctor-patient relationship. Eur Heart J 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Callahan ME. Cybersecurity and Hospitals: four questions every hospital leader should ask in order to prepare for and manage cybersecurity risks. Washington: American Hospital Association, 2013.
- Schoenhagen P, Roselli EE, Harris CM, et al. Online network of subspecialty aortic disease experts: Impact of "cloud" technology on management of acute aortic emergencies. J Thorac Cardiovasc Surg 2016;152:39-42. [Crossref] [PubMed]