
Performance of artificial intelligence in detecting the chronic total occlusive lesions of coronary artery based on coronary computed tomographic angiography
Highlight box
Key findings
• Artificial intelligence (AI) can automatically identify coronary chronic total occlusion (CTO) lesions with high diagnostic accuracy and efficiency.
What is known and what is new?
• The pre-procedure assessment of the CTO is critical to improving the procedure’s success and the prognosis of the patients. The manual procedure of detecting CTO lesions using coronary computed tomography angiography (CCTA) is time-consuming and laborious. AI has been applied in detecting cardiovascular diseases, but in the detection of CTO lesions is difficult, and few studies have assessed its performance.
• We proposed a new AI algorithm to detect CTO lesions, which is with good diagnostic accuracy and efficiency.
What is the implication, and what should change now?
• AI is an automatic vascular segmentation and analysis tool with promising possibilities and applications. With the progress of the continuous evolution of algorithms, the accuracy and the clinical implementation will be further increased.
Introduction
Background
Coronary chronic total occlusion (CTO) refers to a total occlusion of a coronary artery over a period of 3 months, as evidenced by angiography or clinical examination (1). Around patients with coronary artery disease 30% of them will develop CTO, which increases the risk of developing major adverse cardiovascular events (MACE) and cardiogenic shock (2,3). To achieve success with percutaneous coronary intervention (PCI) for CTO lesions, a high level of technical skill is required as well as extensive planning (1). Performing a pre-procedure assessment of both the lesion characteristics and the anatomy of the CTO is critical to improving the procedure’s success and the prognosis of the patient (4).
Rationale and knowledge gap
Coronary computed tomography angiography (CCTA) is a safe, noninvasive method to diagnose CTO lesions (5,6). It enables visualization of fine morphological features and anatomical details of CTO lesions, including proximal stump morphology, the length of lesion, the extent of calcification, and the tortuosity of vessel (7) which are important for grading CTO before PCI (8). A preprocedural CCTA guided-CTO procedure resulted in a higher success rate with numerically fewer immediate periprocedural complications (6).
However, the manual procedure of detecting and evaluating CTO lesions using CCTA not only requires complicated three-dimensional post-processing, which is time-consuming and laborious but also highly relies on the experience of radiologists, which is prone to subjective variability errors. It is therefore important to develop a method for detecting and assessing CTO lesions that is more efficient and objective.
With the development of artificial intelligence (AI), great changes have occurred in cardiovascular imaging. Owing to its superior performance in medical image analysis, AI has been broadly applied in cardiovascular image quality optimization (9), structure segmentation (10,11), lesion feature extraction (12), risk stratification (13), aided diagnosis (14,15), guidance of treatment decision (16) and prognosis assessment (17). Moreover, AI has also been used to automatically assess collateral physiology in CTO using angiography and automatically segment and reconstruct for CT of CTO (18,19).
However, the reduction in the contrast medium at the occlusion site or the distal vessel is prone to cause vessel segmentation errors, following which, the vessel segments were disconnected, and those before and after occlusion were difficult to be demonstrated in reconstructed images. AI-based detection of CTO lesions from CCTA images is difficult, and few studies have assessed its performance in this area.
Objective
In this study, we aimed to develop a new AI model to automatically detect CTO lesions based on CCTA images, compared with the manual diagnosis of CTO lesions, with invasive coronary angiography (ICA) as the reference standard. We present this article in accordance with the STARD reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-407/rc).
Methods
Study population
To reduce patient selection bias, we retrospectively and consecutively reviewed patients with suspected coronary atherosclerosis disease (CAD) who underwent CCTA examinations from June 2021 and June 2022 in Beijing Anzhen Hospital. The patients were finally included according to the inclusion and exclusion criteria of our study. They were randomly assigned to the training dataset and the testing dataset at a rate of 4:1 (Figure 1). The explanation of allocation of the training set and the test set, and determination of the sample size was shown in Appendix 1.

The exclusion criteria are as follows: (I) without ICA examination or more than 3 months between ICA and CCTA; (II) history of revascularization; (III) history of acute myocardial infarction (AMI) before CCTA or ICA within 3 months; (IV) poor CCTA image quality.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Beijing Anzhen Hospital in China (No. 2021164X) and informed consent was obtained from all the patients.
CCTA scanning protocols and analysis
All patients were scanned using a dual-source computed tomography (CT) scanner (Somatom Definition Flash, Siemens Healthineers, Forchheim, Germany) and a 256-slice CT scanner (Revolution CT, GE Healthcare, Milwaukee, WI, USA) within 3 months before ICA. We used retrospectively echocardiographic gating for all scans. The acquisition was triggered by a bolus tracking technique and the region of interest was placed in the ascending aorta. During CT scan, 0.8 mL/kg of contrast (Iohexol 350, GE Ltd., Boston, MA, USA) was injected at a flow rate of 4.0–5.0 mL/s followed by a 30 mL saline flush. Details of the scan parameters are as follows: (I) dual-source CT scanner: rotation time 0.28 seconds, pixel matrix 512×512, collimation 2×64×0.6 mm, tube voltage 100 or 120 kV, with automatically selected tube current. The slice thickness was 0.6 mm with a reconstruction increment of 0.4 mm; (II) 256-slice CT scanner: rotation time 0.28 seconds, pixel matrix 512×512, collimation 256×0.625 mm, tube voltage 100 or 120 kV, and the Smart mA was applied. The slice thickness was 0.625 mm with a reconstruction increment of 0.4 mm. Radiation dose estimates for CCTA were calculated using recommended conversion factors, k =0.014 mSvd·mGy−1·cm−1 (20).
Two experienced radiologists (both with >5-year experience in cardiovascular image analysis) analyzed the CCTA images using a commercial workstation (Vitrea fx3.0, Canon Corporation, Tokyo, Japan). They independently reconstructed and detected the lesions with ≥50% stenosis (including CTO lesions) and were blinded to the results of the ICA. Quantitative assessment of coronary stenosis was performed according to the Society of Cardiovascular Computed Tomography (SCCT) guidance (21). This study included coronary arteries with diameter of 1.5 mm or more. The coronary lesions with ≥50% stenosis (including CTO lesions) was considered positive (22,23). Image analysis time was recorded from loading the images to the diagnosis of all target lesions.
Deep learning (DL) model for coronary CTO lesions segmentation and detection
To achieve myocardial and coronary artery segmentation and target lesion identification, convolutional neural network (CNN) was used, which include feed-forward neuronal networks and contain neurons with learnable weights and biases (24). The proposed DL framework consisted of three models: (I) a two-stage 3D U-Net-based myocardium segmentation network to determine the coordinates of the heart contour and segment the myocardium fine structure; (II) a modified 3D U-Net for coronary segmentation, which includes encoding and decoding parts, and a connected growth prediction model (CGPM) to eliminate vascular segmentation errors and then avoid partial or missing vascular segments of CTO lesions effectively; and (III) a vessel-connect algorithm to identify the missing segments of the vessels and connect them with main branches, which in turn localizes and displays the region of CTO lesions (Figure 2). Detailed steps regarding our AI model development are shown in Appendix 2.
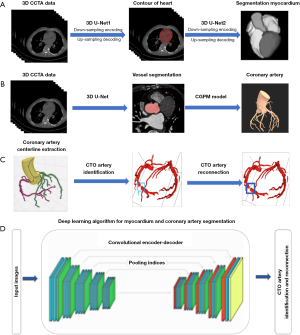
Statistical analysis
Statistical analysis was performed using SPSS version 23 (SPSS, Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation if they are normally distributed, or as median and IQR if they are not. Using the probability-probability plot, a normal distribution was assessed. Categorical variables are expressed as the number and percentage. Using independent t-tests and Mann-Whitney U tests, we compared differences in continuous and dichotomous demographic information between the training dataset and the testing dataset. Diagnostic performance of the AI was evaluated through sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy. McNemar’s test and receiver operating characteristic (ROC) analysis was also used to evaluate the accuracy of AI method and manual method with ICA as the reference in detecting coronary CTO lesions and lesions with 50–99% stenosis. The area under the curve (AUC) =0.50 was considered a valueless diagnostic indicator, 0.50< AUC ≤0.7 was low diagnostic accuracy, 0.7< AUC ≤0.9 was moderate, and 0.9< AUC ≤1.0 was good. Intra-class correlation coefficient (ICC) test was used to evaluate the intra-observer and inter-observer consistency, using a two-way random model and the absolute agreement definition. ICC value ≥0.75 indicates good reliability; 0.4≤ ICC <0.75 indicates medium reliability; <0.40 indicates poor reliability. Two-tailed P<0.05 was considered to have a significant difference.
Results
The demographics of the patients
Detailed demographics for the training and the testing dataset are shown in Table 1. A total of 537 patients with 1,569 ICA-confirmed atherosclerotic lesions (including 672 lesions with <50% stenosis, 493 lesions with 50–99% stenosis, and 404 CTO lesions) were finally included. In the training dataset, there were 429 patients with 1,255 atherosclerotic lesions (including 550 lesions with <50% stenosis, 396 lesions with 50–99% stenosis, and 309 CTO lesions). In the testing dataset, there were 108 patients with 314 atherosclerotic lesions (including 122 lesions with <50% stenosis, 97 lesions with 50–99% stenosis, and 95 CTO lesions). There was no notable difference in the demographic characteristics of the patients included in the training and testing datasets. The mean effective radiation dose of the CT exam was 3.2±2.2 mSv.
Table 1
| Characteristics | Training dataset (N=429) | Testing dataset (N=108) | P value |
|---|---|---|---|
| Male | 351 (81.8) | 93 (86.1) | 0.29 |
| Age (years) | 59.9±9.7 | 59.4±10.9 | 0.16 |
| BMI (kg/m2) | 26.1±3.6 | 26.0±3.5 | 0.59 |
| Coronary risk factors | |||
| Hypertension | 273 (63.6) | 61 (56.5) | 0.17 |
| Hyperlipidemia | 272 (63.4) | 68 (63.0) | 0.90 |
| Diabetes mellitus | 143 (33.3) | 37 (34.3) | 0.86 |
| Smoking | 227 (52.9) | 58 (53.7) | 0.90 |
| Drinking | 75 (17.5) | 24 (22.2) | 0.26 |
Values are mean ± standard deviation or n (%). BMI, body mass index.
Efficiency of AI in locating and detecting coronary CTO lesions and lesions with 50–99% stenosis
Compared to the traditional manual post-processing and diagnostic method (CCTA-manual, 472±45 seconds), the average time of our AI-assisted post-processing method in CCTA (CCTA-AI) for each patient was dramatically reduced to 116±15 seconds and reduced time by 75%, and the report was also automatically generated.
Accuracy of AI in locating and detecting coronary CTO lesions and lesions with 50–99% stenosis
Table 2 shows the diagnostic performance of CCTA-AI and CCTA-manual in identifying CTO lesions and lesions with 50–99% stenosis. The example of the lesion detection of the CCTA-AI method was shown in Figure 3.
Table 2
| Variables | Training dataset | Testing dataset | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CTO lesions | Lesions with 50–99% stenosis | CTO lesions | Lesions with 50–99% stenosis | ||||||||
| CCTA-AI | CCTA-manual | CCTA-AI | CCTA-manual | CCTA-AI | CCTA-manual | CCTA-AI | CCTA-manual | ||||
| Number of lesions | 309 | 309 | 396 | 396 | 95 | 95 | 97 | 97 | |||
| Sensitivity (95% CI) (%) | 90.3 (86.3, 93.3) | 86.7 (82.3, 90.2) | 92.7 (88.7, 95.5) | 92.4 (88.2, 95.2) | 80.0 (70.3, 87.2) | 83.2 (73.8, 89.9) | 87.7 (75.7, 94.5) | 91.2 (80.0, 96.7) | |||
| Specificity (95% CI) (%) | 98.1 (95.1, 99.2) | 97.6 (94.6, 99.0) | 97.7 (95.2, 99.0) | 98.0 (95.6, 99.2) | 96.4 (86.8, 99.3) | 96.5 (86.8, 99.4) | 97.9 (91.9, 99.6) | 96.7 (90.4, 99.2) | |||
| Positive predictive value (95% CI) (%) | 98.2 (95.2, 99.4) | 97.8 (95.1, 99.1) | 97.1 (93.8, 98.7) | 95.8 (93.3, 97.9) | 97.4 (90.2, 99.5) | 97.5 (90.5, 99.5) | 96.1 (85.7, 99.3) | 94.5 (83.9, 98.6) | |||
| Negative predictive value (95% CI) (%) | 89.2 (84.8, 92.5) | 85.7 (81.0, 89.4) | 94.7 (91.1, 96.6) | 95.3 (91.8, 97.3) | 74.3 (62.6, 83.5) | 67.9 (63.7, 81.2) | 93.0 (85.6, 96.9) | 94.8 (87.8, 98.1) | |||
| Accuracy (95% CI) (%) | 93.8 (91.4, 95.5) | 91.6 (89.0, 93.7) | 95.5 (93.5, 97.0) | 95.5 (93.4, 97.1) | 86.2 (79.0, 90.3) | 88.2 (82.0, 92.5) | 94.1 (89.0, 97.0) | 94.7 (89.8, 97.5) | |||
| P value | 0.12 | 0.82 | 0.53 | 0.75 | |||||||
CCTA, coronary computed tomography angiography; AI, artificial intelligence; CTO, chronic total occlusion; CI, confidence interval.

With ICA as the reference method in locating and detecting CTO lesions, the sensitivity, specificity, PPV, NPV, and accuracy of CCTA-AI in the training dataset were 90.3% (86.3%, 93.3%), 98.1% (95.1%, 99.2%), 98.2% (95.2%, 99.4%), 89.2% (84.8%, 92.5%), 93.8% (91.4%, 95.5%), and 80.0% (70.3%, 87.2%), 96.4% (86.8%, 99.3%), 97.4% (90.2%, 99.5%), 74.3% (62.6%, 83.5%), 86.2% (79.0%, 90.3%) in the testing dataset. No significant difference was found in detecting CTO lesions between AI and manual method (P=0.12 and 0.53). The ROC analysis showed good and moderate accuracy of CCTA-AI in the training dataset and the testing dataset (AUC =0.942 and 0.874) (Figure 4).

In locating and detecting lesions with 50–99% stenosis, the sensitivity, specificity, PPV, NPV, and accuracy of CCTA-AI in the training dataset were 92.7% (88.7%, 95.5%), 97.7% (95.2%, 99.0%), 97.1% (93.8%, 98.7%), 94.7% (91.1%, 96.6%), 95.5% (93.5%, 97.0%), and 87.7% (75.7%, 94.5%), 97.9% (91.9%, 99.6%), 96.1% (85.7%, 99.3%), 93.0% (85.6, 96.9%), 94.1% (89.0%, 97.0%) in the testing dataset. No significant difference was found in detecting lesions with 50–99% stenosis between AI and manual methods (P=0.82 and 0.75). The ROC analysis also showed good accuracy of CCTA-AI in the training dataset and the testing dataset (AUC =0.953 and 0.928) (Figure 4). However, the proposed AI method in differentiating the ICA confirmed subtotal occlusion (STO) (95%≤ stenosis <100%), which is a “functional” total occlusion or a slow contrast penetration through the occluded segment, and CTO lesions (100%) was found to be poor, with the sensitivity, specificity, PPV, NPV and accuracy of CCTA-AI in the training dataset (STO n=117) was 53.0% (43.6%, 62.2%), 79.6% (74.6%, 83.9%), 49.6% (40.6%, 58.6%), 81.7% (76.8%, 85.8%), 72.3% (67.0%, 75.9%), and 45.2% (27.8%, 63.7%), 75.8% (65.7%, 83.7%), 37.8% (22.9%, 55.2%), 80.9% (70.9%, 88.2%), 68.3% (61.8%, 78.2%) in the testing dataset (STO n=31) (Table 3).
Table 3
| Variables | Training dataset | Testing dataset | |||
|---|---|---|---|---|---|
| CCTA-AI | CCTA-manual | CCTA-AI | CCTA-manual | ||
| Number of lesions | 117 | 117 | 31 | 31 | |
| Sensitivity (95% CI) (%) | 53.0 (43.6, 62.2) | 64.1 (54.7, 72.6) | 45.2 (27.8, 63.7) | 61.3 (42.3, 77.6) | |
| Specificity (95% CI) (%) | 79.6 (74.6, 83.9) | 85.8 (81.2, 89.4) | 75.8 (65.7, 83.7) | 84.2 (75.0, 90.6) | |
| Positive predictive value (95% CI) (%) | 49.6 (40.6, 58.6) | 63.0 (53.6, 71.6) | 37.8 (22.9, 55.2) | 55.9 (38.1, 72.4) | |
| Negative predictive value (95% CI) (%) | 81.7 (76.8, 85.8) | 86.3 (81.8, 89.9) | 80.9 (70.9, 88.2) | 87.0 (77.9, 92.8) | |
| Accuracy (95% CI) (%) | 72.3 (67.0, 75.9) | 79.8 (71.5, 85.2) | 68.3 (61.8, 78.2) | 78.6 (68.3, 84.4) | |
| P value | 0.66 | 0.78 | |||
CCTA, coronary computed tomography angiography; AI, artificial intelligence; STO, subtotal occlusion; CI, confidence interval.
Both intra-observer and inter-observer agreements were good (ICC =0.933 for observer A, ICC =0.905 for observer B, and ICC =0.891 for inter-observer agreement, all P<0.05).
Discussion
Key findings
In our study, we proposed a new AI model to facilitate automated segmentation and detection of CTO lesions, which is more efficient than traditional manual image reconstruction and diagnosis (reduces 75% of time to reconstruct and interpret images). With ICA as the reference method, the accuracy of CCTA-AI in the detection of CTO lesions and lesions with 50–99% stenosis is good, and no significant difference is revealed between the AI method and the manual method.
Comparison with similar researches and explanations of findings
DL has been shown to be a promising tool in image segmentation and recognition (25,26). AI applies CNN to achieve vessel extraction, automatically identifying main vessels and branches and generating high-quality reconstructed images, which fulfilled the requirement for clinical routine diagnosis and thus has been widely used in the evaluation of CAD. To detect coronary arterial lesions with stenosis, Kang et al. (27) proposed a structured learning technique, with good sensitivity, specificity, and accuracy achieved. Promising results were demonstrated in the automated detection of obstructive and nonobstructive lesions from CCTA. A multi-center, international study (CLARIFY study) additionally validated the capability of AI-assisted analysis in swiftly and precisely assessing vessel shape and narrowing (14). Some studies have also confirmed that DL algorithms exhibited excellent diagnostic accuracy in coronary atherosclerotic conditions, while also considerably decreasing the duration of post-processing and interpretation of CCTA images (15,19).
However, the reduction in the contrast medium at the occlusion site or the distal vessel is prone to cause vessel segmentation errors, following which, the vessel segments were disconnected, and those before and after occlusion were difficult to be demonstrated in reconstructed images, which brings challenges in the automatic location and detection for CTO lesions. Only a few research papers on AI in the segmentation and reconstruction of CTO lesions were published (19), resulting in decreased time required for postprocessing CTO quantification and demonstrating strong correlation and agreement in the anatomical evaluation of occlusion characteristics. However, no previous reports have investigated the performance of AI in detecting CTO lesions, and additional research is necessary to develop a completely automated algorithm for the segmentation, reconstruction, and identification of CTOs.
To solve the problem of automatically detecting the CTO lesions of the coronary arteries, we developed a new DL algorithm to enable automated extraction of the centerlines of coronary arteries and locate the CTO lesions by completing the missing segments. Based on myocardial segmentation and coronary segmentation results, we labeled each branch of the centerline and identified the missing segments of the vessels, which in turn located and displayed the CTO lesions and increased the ability of CTO lesions detection. Consequently, AI was confirmed to be a simple, reliable, and efficient tool to detect CTO lesions.
With the ICA as the reference standard, we found that the proposed AI model demonstrates commendable diagnostic capabilities not only in detecting CTO lesions but also coronary arteries lesions with 50–99% stenosis. Furthermore, our proposed AI method saved 75% of time in post-processing and interpreting the CCTA images when compared to the traditional manual method. The diagnostic accuracy of the CCTA-AI method in detecting CTO lesions (86.2% in the testing dataset) and lesions with 50–99% was good (94.1% in the testing dataset). The ROC analysis showed moderate accuracy and good accuracy of CCTA-AI in CTO lesions and lesions with 50–99% stenosis (AUC =0.874 and 0.928 in the testing dataset, respectively). No significant difference was found in detecting CTO lesions between AI and manual methods (P<0.05), but in some conditions such as the presence of larger vascular branches in close proximity to the occluded segment (Figure 5) or heavy calcification (peripheral calcification: maximal encircling ≥180° and cross-sectional area ≥50%) may result in AI recognition errors, which needs to be cautious.

Besides, the effectiveness of the proposed AI approach in distinguishing between ICA-confirmed STO and CTO lesions was subpar, and the diagnostic accuracy was reduced to about 70.0%. In anatomical imaging tests, both CTO and STO lesions exhibit a complete interruption of the contrast-enhanced arterial lumen, while functional imaging tests indicate myocardial ischemia. There is still a challenge in noninvasively discriminating CTO from STO lesions (28). Further studies are needed to improve the ability of AI in the automatic segmentation and detection of CTO lesions.
Limitations
Despite the advantages of CCTA-AI in detecting CTO lesions, this study has some limitations. First, the sample size was small as this study was based on a single-center experience. In the following study, we will aim to enroll multicenter data to further test and improve the performance of AI in detecting CTO lesions. Second, we did not differentiate the early and late stages of CTO lesions, which might cause some misidentifications, such as a high density of non-calcified components in occlusions of later stages compared to those in the earlier stages. The presence of microvessels in the late stage of the occlusions exhibits an increase in vascular density, making it easier to detect (29), which was a valid concern. Finally, we excluded patients with stented lesions or after coronary artery bypass grafting surgery. Furthermore, no analysis was carried out in bypass grafts as only the assessment of native vessels was included.
Conclusions
AI can automatically detect CTO lesions based on CCTA images, with good diagnostic accuracy and efficiency. AI is becoming an automatic vascular segmentation and analysis tool with promising possibilities and applications. Our algorithm necessitates additional improvement, and a greater number of external validations are imperative for its clinical implementation.
Acknowledgments
Funding: This study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-407/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-407/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-407/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-407/coif). Z.S. serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2023 to August 2025. X.R. report that she is an employee of Shukun (Beijing) Technology Co. Ltd., China. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Beijing Anzhen Hospital in China (No. 2021164X) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koelbl CO, Nedeljkovic ZS, Jacobs AK. Coronary Chronic Total Occlusion (CTO): A Review. Rev Cardiovasc Med 2018;19:33-9. [Crossref] [PubMed]
- Qin Q, Chen L, Ge L, et al. A comparison of long-term clinical outcomes between percutaneous coronary intervention (PCI) and medical therapy in patients with chronic total occlusion in noninfarct-related artery after PCI of acute myocardial infarction. Clin Cardiol 2022;45:136-44. [Crossref] [PubMed]
- Kim SH, Behnes M, Mashayekhi K, et al. Prognostic Impact of Percutaneous Coronary Intervention of Chronic Total Occlusion in Acute and Periprocedural Myocardial Infarction. J Clin Med 2021;10:258. [Crossref] [PubMed]
- Brilakis ES, Mashayekhi K, Tsuchikane E, et al. Guiding Principles for Chronic Total Occlusion Percutaneous Coronary Intervention. Circulation 2019;140:420-33. [Crossref] [PubMed]
- Sadamatsu K, Okutsu M. Cardiac Computed Tomography for Success in Percutaneous Coronary Intervention for Chronic Total Occlusion. JACC Cardiovasc Imaging 2022;15:172. [Crossref] [PubMed]
- Hong SJ, Kim BK, Cho I, et al. Effect of Coronary CTA on Chronic Total Occlusion Percutaneous Coronary Intervention: A Randomized Trial. JACC Cardiovasc Imaging 2021;14:1993-2004. [Crossref] [PubMed]
- Opolski MP. Cardiac Computed Tomography for Planning Revascularization Procedures. J Thorac Imaging 2018;33:35-54. [Crossref] [PubMed]
- Opolski MP, Achenbach S, Schuhbäck A, et al. Coronary computed tomographic prediction rule for time-efficient guidewire crossing through chronic total occlusion: insights from the CT-RECTOR multicenter registry (Computed Tomography Registry of Chronic Total Occlusion Revascularization). JACC Cardiovasc Interv 2015;8:257-67. [Crossref] [PubMed]
- Tatsugami F, Higaki T, Nakamura Y, et al. Deep learning-based image restoration algorithm for coronary CT angiography. Eur Radiol 2019;29:5322-9. [Crossref] [PubMed]
- Baskaran L, Maliakal G, Al'Aref SJ, et al. Identification and Quantification of Cardiovascular Structures From CCTA: An End-to-End, Rapid, Pixel-Wise, Deep-Learning Method. JACC Cardiovasc Imaging 2020;13:1163-71. [Crossref] [PubMed]
- Jun Guo B, He X, Lei Y, et al. Automated left ventricular myocardium segmentation using 3D deeply supervised attention U-net for coronary computed tomography angiography; CT myocardium segmentation. Med Phys 2020;47:1775-85. [Crossref] [PubMed]
- Zhang N, Yang G, Zhang W, et al. Fully automatic framework for comprehensive coronary artery calcium scores analysis on non-contrast cardiac-gated CT scan: Total and vessel-specific quantifications. Eur J Radiol 2021;134:109420. [Crossref] [PubMed]
- Lin A, Kolossváry M, Motwani M, et al. Artificial Intelligence in Cardiovascular Imaging for Risk Stratification in Coronary Artery Disease. Radiol Cardiothorac Imaging 2021;3:e200512. [Crossref] [PubMed]
- Choi AD, Marques H, Kumar V, et al. CT Evaluation by Artificial Intelligence for Atherosclerosis, Stenosis and Vascular Morphology (CLARIFY): A Multi-center, international study. J Cardiovasc Comput Tomogr 2021;15:470-6. [Crossref] [PubMed]
- Han D, Liu J, Sun Z, et al. Deep learning analysis in coronary computed tomographic angiography imaging for the assessment of patients with coronary artery stenosis. Comput Methods Programs Biomed 2020;196:105651. [Crossref] [PubMed]
- Gohmann RF, Pawelka K, Seitz P, et al. Combined cCTA and TAVR Planning for Ruling Out Significant CAD: Added Value of ML-Based CT-FFR. JACC Cardiovasc Imaging 2022;15:476-86. [Crossref] [PubMed]
- Han D, Kolli KK, Al'Aref SJ, et al. Machine Learning Framework to Identify Individuals at Risk of Rapid Progression of Coronary Atherosclerosis: From the PARADIGM Registry. J Am Heart Assoc 2020;9:e013958. [Crossref] [PubMed]
- Liu L, Ding F, Shen Y, et al. Automatic assessment of collaterals physiology in chronic total occlusions by means of artificial intelligence. Cardiol J 2023;30:685-95. [Crossref] [PubMed]
- Li M, Ling R, Yu L, et al. Deep Learning Segmentation and Reconstruction for CT of Chronic Total Coronary Occlusion. Radiology 2023;306:e221393. [Crossref] [PubMed]
- Shrimpton PC, Hillier MC, Lewis MA, et al. National survey of doses from CT in the UK: 2003. Br J Radiol 2006;79:968-80. [Crossref] [PubMed]
- Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342-58. [Crossref] [PubMed]
- Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135-44. [Crossref] [PubMed]
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012;59:379-87. [Crossref] [PubMed]
- Korb E, Bağcıoğlu M, Garner-Spitzer E, et al. Machine Learning-Empowered FTIR Spectroscopy Serum Analysis Stratifies Healthy, Allergic, and SIT-Treated Mice and Humans. Biomolecules 2020;10:1058. [Crossref] [PubMed]
- Atasever S, Azginoglu N, Terzi DS, et al. A comprehensive survey of deep learning research on medical image analysis with focus on transfer learning. Clin Imaging 2023;94:18-41. [Crossref] [PubMed]
- Hesamian MH, Jia W, He X, et al. Deep Learning Techniques for Medical Image Segmentation: Achievements and Challenges. J Digit Imaging 2019;32:582-96. [Crossref] [PubMed]
- Kang D, Dey D, Slomka PJ, et al. Structured learning algorithm for detection of nonobstructive and obstructive coronary plaque lesions from computed tomography angiography. J Med Imaging (Bellingham) 2015;2:014003. [Crossref] [PubMed]
- Choi JH, Kim EK, Kim SM, et al. Noninvasive Discrimination of Coronary Chronic Total Occlusion and Subtotal Occlusion by Coronary Computed Tomography Angiography. JACC Cardiovasc Interv 2015;8:1143-53. [Crossref] [PubMed]
- Wu Q, Yu M, Li Y, et al. Natural History of Untreated Coronary Total Occlusions Revealed with Follow-Up Semi-Automated Quantitative Coronary CT Angiography: The Morphological Characteristics of Initial CT Predict Occlusion Shortening. Korean J Radiol 2018;19:256-64. [Crossref] [PubMed]


