
Subcutaneous versus transvenous implantable cardioverter defibrillator in hypertrophic cardiomyopathy: a systematic review and meta-analysis
Highlight box
Key findings
• Subcutaneous implantable cardioverter-defibrillators (S-ICDs) demonstrate a superior profile over transvenous cardioverter-defibrillators (TV-ICDs) concerning device-related complications while offering comparable efficacy and safety.
What is known, and what is new?
• Subcutaneous implanted cardioverter-defibrillators are likely suitable substitutes to TV-ICDs in the general population. Nevertheless, as of the present date, there has been no meta-analysis comparing these two devices specifically in patients with hypertrophic cardiomyopathy (HCM).
• Our study demonstrates that patients with HCM may benefit from S-ICDs, as they exhibit a comparable efficacy profile and safety profile with lower odds of device-related complications than transvenous pacemakers.
What is the implication, and what should change now?
• Subcutaneous implanted cardioverter-defibrillators without pacing indications may be an effective alternative to transvenous ones for HCM patients. Both technique’s long-term safety and effectiveness demand additional research.
Introduction
Background
Hypertrophic cardiomyopathy (HCM), the most common hereditary heart disease, is responsible for left ventricular hypertrophy without additional causes (1), affecting approximately 0.2% of all adults. While some patients remain asymptomatic, others may develop fatigue, exertional dyspnea, lightheadedness, palpitations, syncope, atypical chest discomfort, cardiac arrhythmias, heart failure, and even sudden cardiac death (SCD) due to ventricular diastolic dysfunction (2). The risk of SCD must be stratified for every patient with HCM. Primary prevention implantable cardioverter defibrillator (ICD) therapy is recommended in patients at high risk for SCD (3).
Rationale and knowledge gap
Conventional transvenous ICD (TV-ICD) implant defibrillators lead in the right ventricle, revolutionizing primary and secondary SCD prevention (4). However, TV-ICDs can have device-related complications, which can be categorized into lead-related issues, such as migration, fracture, failure, and infection, and non-lead-related issues, including pocket infection, delayed wound healing, wound discomfort, hematoma, device malfunction, and premature battery depletion (5).
Objective
Since ICDs placed outside the ribs significantly minimize device-related adverse effects, subcutaneous ICDs (S-ICD) were developed as an alternative to transvenous leads (6-8). However, although important records show that S-ICD is effective and reliable in HCM (9,10), data on its use in these patients is still limited. A meta-analysis on the subject has not yet been carried out. Therefore, this study aimed to assess and meta-analyze the effectiveness and safety of subcutaneous and transvenous ICDs in patients with HCM, recognizing that these individuals harbor specific pathophysiological mechanisms, such as cardiac remodeling, which may diverge from the general population. We present this article in accordance with the PRISMA reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-15/rc).
Methods
Search methodology
On December 6th, 2023, PubMed, Scopus, Embase and Cochrane databases were searched for randomized controlled trials and observational studies published since 2004 until December 2023 using the search terms and keywords (“subcutaneous”) AND (“hypertrophic cardiomyopathy”), with no restrictions regarding the language of the studies (Appendix 1). Every recognized study was carefully cross-referenced to ensure no relevant research was overlooked. Two authors (V.M.R.O. and I.C.O.) independently performed data collection and quality evaluation and followed preset criteria. Disagreements were resolved by a third reviewer (A.M.d.S.). The study was registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO/) with the registration number CRD42023417036.
Criteria for eligibility
Studies with the following requirements were included: (I) the implantation methods of S-ICDs and TV-ICD in patients with HCM should be directly compared; (II) a minimum follow-up duration of 6 months was required; and (III) included endpoints of interest. Studies using overlapping patient cohorts were excluded. When there were numerous publications on the same group of patients, we preferred publications that provided the most extensive datasets. Studies that did not include a control group were excluded from the analysis. Our primary endpoints of interest were the incidence rates of appropriate shocks (AS) and inappropriate shocks (IAS), whereas device-related complications were evaluated as secondary endpoints. The prespecified data points extracted from each study included study design, sample size, patient characteristics, intervention details, and outcome measures.
Quality assessment
The Risk of Bias in Non-randomized Studies of Interventions 1 (ROBINS-I) instrument developed by the Cochrane Collaboration was used (11). Based on this instrument, the investigations were placed into one of four risk profiles ranging from insignificant to concerning. Confounding, selection, measurement categorization of interventions, deviations from planned interventions, missing data, measurement of outcomes, and reporting biases were included in these reviews.
Statistical analysis and interpretation
For binary endpoints, treatment effects were pooled using odds ratios (ORs) with 95% confidence intervals (CIs). We adopted the Mantel-Haenszel random-effects model for endpoints demonstrating substantial heterogeneity (I2>25%), while a fixed-effect model was employed for outcomes with low heterogeneity (I2<25%). Based on the recommendations of the Cochrane Handbook, heterogeneity was examined with Cochran’s Q test, I2 statistics, and Tau-square using the DerSimonian and Laird method (12). P values lower than 0.10 and an I2 value greater than 25% were singled out as potential signs of considerable heterogeneity. In cases with significant heterogeneity, we performed a sensitivity analysis by removing one study at a time (the leave-one-out method). All statistical analyses were performed using Review Manager 5.4 (Cochrane Centre, The Cochrane Collaboration, Denmark).
Results
Study selection and baseline characteristics
The first search of the electronic databases yielded 1,114 articles. After eliminating 350 duplicate submissions and 733 studies that did not fulfill the eligibility requirements, 31 publications were selected for a more in-depth analysis. Seven of these publications fulfilled all the inclusion criteria. Figure 1 presents a comprehensive PRISMA flow diagram explaining the search methodology.

A total of 4,347 patients. of whom 3,325 had TV-ICDs, whereas 1,022 had the S-ICD option (23.5%). The average patient age ranged from 39 to 49 years. Seven studies (13-19) were observational, and none used random participants. Notably, only the study conducted by Jankelson et al. (14) used propensity-matched analysis. In contrast, the research carried out by Lambiase et al. (16) used a historical cohort as a control (Table 1).
Table 1
| Study | Study design, country, year | No. of patients (S-ICD/TV-ICD) | Primary prevention, % | Male gender, % | Mean age, years* | LVEF*, % | MLVWT*, mm | Follow-up time* |
|---|---|---|---|---|---|---|---|---|
| Buongiorno et al. (13) | Retrospective observational study, Italy, 2022 | 7/45 | 86.5 | 69.3 | 49±20 | NR | NR | 8±5 years |
| Francia et al. (19) | Retrospective cohort study, Italy, 2023 | 216/211 | S-ICD: 94; TV-ICD: 95 | S-ICD: 76.4; TV-ICD: 48.8 | S-ICD: 43±14; TV-ICD: 50±15 | S-ICD: 60.1±9.4; TV-ICD: 62.2±13.0 | S-ICD: 22.8±5.3; TV-ICD: 23.4±6.7 | S-ICD: 26.5±19.0 months; TV-ICD: 46.9±20.1 months |
| Jankelson et al. (14) | Retrospective cohort study, United States, 2022 | 626/626 | NR | S-ICD: 68.7; TV-ICD: 70.6 | S-ICD: 40.27±15.50; TV-ICD: 40.17±16.33 | NR | NR | S-ICD: 933.47±550.61 days; TV-ICD: 1,600.02±994.16 days |
| Klein et al. (15) | Retrospective cohort study, France, 2017 | 21/156 | NR | NR | NR | NR | NR | 45.6 months† |
| Lambiase et al. (16) | Retrospective cohort study, England, 2016 | 99/2,190 | S-ICD: 87.9; TV-ICD: 83 | S-ICD: 74.7; TV-ICD: 62 | S-ICD: 41.6±15.8; TV-ICD: 42.3 | S-ICD: 65.1±9.9; TV-ICD: NR | S-ICD: 21±7; TV-ICD: NR | S-ICD: 659 days; TV-ICD: 3.7 years |
| Steiger et al. (17) | Prospective cohort study, United States, 2019 | 46/77 | NR | S-ICD: 78; TV-ICD: NR | S-ICD: 39±15; TV-ICD: NR | NR | S-ICD: 19±7; TV-ICD: NR | S-ICD: 2±1.7 years; TV-ICD: 2.8±1.7 years |
| Timmers et al. (18) | Retrospective observational study, Belgium, 2017 | 7/20 | 96.2 | S-ICD: 71.4; TV-ICD: 85 | S-ICD: 42±11; TV-ICD: 43±30 | S-ICD: 70±3; TV-ICD: 67±18 | S-ICD: 20±3; TV-ICD: 21±6 | S-ICD: 386±241 days; TV-ICD: 427±351 days |
†, median; *, mean ± standard deviation. S-ICD, subcutaneous implanted cardioverter-defibrillator; TV-ICD, transvenous implanted cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MLVWT, maximum left ventricular wall thickness; NR, not reported.
This study evaluated results regarding the safety and effectiveness of ICDs in patients with HCM. Seven publications provided evidence of the prevalence of IAS. Among these, five examined the frequency of AS, while five focused on device-related complications.
AS
Across five publications (13,14,16,17,19) there were a total of 4,143 patients who had an AS recorded. Our combined analysis showed no difference between the groups regarding the occurrence of AS (OR 0.49; 95% CI: 0.22–1.08; P=0.08; Figure 2). Notably, there was a clear indication of high heterogeneity across the included studies (I2=75%, P=0.003), as shown in the funnel plot in Figure S1. In addition, cross-validation was performed using the leave-one-out condition. When the publication by Lambiase et al. (16) was withdrawn, the results showed no significant difference in the incidence of AS (OR 1.0; 95% CI: 0.66–1.50; P=0.98), and the heterogeneity was reduced (I2=0%; P=0.66; Figure S2).

IAS
Regarding IAS, findings were presented in seven studies (13-19) that included 4,123 patients. There were no statistically significant differences in the incidence of IAS across the groups (OR 1.03; 95% CI: 0.57–1.84; P=0.93; Figure 3). Notably, heterogeneity across the studies was considerable (I2=65%; P=0.01). During the leave-one-out cross-validation, excluding the data from Steiger et al. (17) resulted in reduced heterogeneity (I2=41%; P=0.15), but the chances of IAS remained the same (OR 0.89; 95% CI: 0.52–1.53; P=0.68) as demonstrated in Figure S2.

Device-related complications
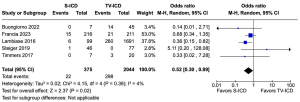
An analysis that pooled data from five studies (13,16-19), including 2,419 patients, found statistically significant differences in the frequency of device-related complications in S-ICD compared to TV-ICD (OR 0.52; 95% CI: 0.30–0.89; P=0.02; Figure 4). No outlying studies were found in this particular setting, and the degree of heterogeneity among the studies was null (I2=4%; P=0.39).
Risk of bias
Two studies were found to have serious overall risk of bias during quality assessment. The total risk of bias for the studies by Steiger et al. (17), Klein et al. (15) and Francia et al. (9) was rated as moderate, whereas that for the study by Lambiase et al. (16) was rated as critical and for Jankelson et al. (14) as low. Figure S3 summarizes the risks associated with biased assessments.
Discussion
Key findings
This review compares the efficacy and safety of S-ICD and TV-ICD devices in 4,347 patients with HCM. The relevant findings from the pooled analysis of the seven studies include: (I) a significantly lower frequency of device-related complications in the S-ICD group than in the TV-ICD group, and (II) ICD shock rates (both appropriate and inappropriate) were similar between the S-ICD and TV-ICD groups.
Explanations of findings and comparison with similar studies
Device-related complications
While lead-related complications were less frequent, the total incidence of device-related complications—encompassing sensing issues, inappropriate antitachycardia pacing (IATP), and malfunctioning devices—was noted. The overall incidence of device-related complications (including sensing issues, IAS, and malfunctioning devices), as the occurrence of non lead-related complications was not significantly different (6). In contrast, our meta-analysis of patients only with HCM revealed a significant difference between S-ICDs and TV-ICDs. Therefore, it is imperative that ongoing clinical trials, including the PRAETORIAN trial (20) and the Avoid Transvenous Leads in Appropriate Subjects trial (21), strive for follow-up periods exceeding 5 years to collect long-term safety and efficacy data and ascertain whether the observed divergence is substantially different in the broader group of patients.
The findings of Lambiase et al. (16), in which they compared the outcomes of patients with S-ICDs with those of a historical TV-ICD group, demonstrated that the annualized infection rates in patients with HCM were equivalent between the groups. This finding is consistent with the study comparing S-ICD and TV-ICD in the general population (6).
IAS
In our study, there was no statistically significant difference in the incidence rates of IAS between S-ICD and TV-ICD devices in patients with HCM. The S-ICD is associated with an increased likelihood of cardiac oversensing as a potential cause of IAS, which was detected by Lin et al. in 23,2% of the patients who underwent deployment of this device (22). Despite the benefits, an increased incidence of inappropriate atrial sensing due to supraventricular tachycardia/atrial fibrillation represents a significant trade-off in using TV-ICDs. Notably, a study has reported that 63% of IAS cases in specific cohorts (23) can be attributed to a high prevalence of atrial tachyarrhythmias in patients with HCM. A study on the Evaluation of Factors Impacting Clinical Outcome and Cost-effectiveness Trials registry revealed that 73% of IAS cases were attributed to cardiac oversensing, primarily caused by low-amplitude signals or T-wave oversensing (20). Another study identified the absence of programming options to correct oversensing as a significant vulnerability for S-ICDs (21).
In contrast, the findings of the Subcutaneous Versus Transvenous Arrhythmia Recognition Testing study indicated that the specificity of the S-ICD was superior to that of the TV-ICD for the detection of supraventricular arrhythmias (7). This underscores the need to employ morphology discrimination algorithms within the conditional shock zone to mitigate IAS occurrences with S-ICDs instead of utilizing interval criteria before applying morphology criteria for TV-ICDs. In our study, 50% used morphological discrimination to enhance S-ICD sensitivity.
Recently, advances have been made to resolve the problem of oversensing in S-ICD devices. These advances include incorporating dual-zone tachycardia detection (24) and using the INSIGHT algorithm and SMART Pass technology, effectively mitigating excessive cardiac oversensing (25).
The PRAETORIAN study (20) revealed that a significant proportion of patients (78%) did not have access to or activate the SMART Pass technology during the initial shock—this lack of utilization results in an increased incidence of IAS. The UNTOUCHED trial, which focused on primary prevention in patients with low ejection fraction, employed innovative selection and programming methods. The study findings revealed the lowest occurrence of IAS in patients with S-ICDs thus far, with a rate of 3.1% at 1 year. This rate is notably lower than that observed in another study on TV-ICDs (26). Rordof et al. (21) conducted a retrospective analysis of device programming and the incidence of IAS in patients who received S-ICDs. Their findings revealed that programming similar to the UNTOUCHED protocol, which involves setting a conditional zone between 200 and 250 beats per minute (bpm) and a shock zone for arrhythmias exceeding 250 bpm, was linked to a reduction in IAS without affecting the rates of appropriate and ineffective shocks. Therefore, further research should be conducted to evaluate the incidence rates of IAS between the most technologically advanced S-ICDs.
AS therapy
The rate of AS therapy was comparable in both groups. Our study demonstrated a significant variation in the rates of AS across both modalities (I2=75%), most likely due to differences in detection algorithms between trials. The PRAETORIAN trial ascribed the greater rate of AS from S-ICDs to the device’s failure to provide antitachycardia pacing (ATP) and double counting of slow ventricular tachycardia if it occurred at a rate lower than the set therapeutic zone. Brouwer et al. (27) recognized the reduced incidence of AS from S-ICDs due to their longer charging time, which permits no sustained ventricular tachycardia to terminate. ATP therapy, a well-established method for treating monomorphic ventricular tachycardia, can be administered via the ICD device as a painless alternative to high-energy shocks (28-30). Significantly, two studies have been conducted to investigate the incidence of ATP, underscoring a notable rate of appropriate therapeutic interventions in patients with TV-ICDs due to ATP episodes. Specifically, Francia et al. (19) reported that 34 out of 49 patients experienced such episodes. In contrast, Jankelson et al. observed that 207 out of 626 patients encountered ATP episodes over a follow-up period of 5 years. However, Jankelson et al. (14) also highlighted that frequent ATP episodes in this cohort did not translate into a reduced incidence of shock delivery compared to patients with S-ICDs. This observation doubts the assumed advantages of empirically administering ATP in patients with HCM.
Future perspectives
The decision to implant an S-ICD must consider the possibility of future ATP use in patients with sustained ventricular tachycardia or pacing needs, which may necessitate upgrading to a cardiac resynchronization therapy defibrillator (5). In one retrospective cohort analysis, the frequency of a downstream pacing requirement among patients without a prior pacing indication was reported to be 34% over a median follow-up of 3.4 years (26). This emphasizes that pacing ability was more critical in the TV-ICD cohort than in the S-ICD cohort.
Nonetheless, recent technological advancements have resulted in the creation of leadless pacemakers controlled by S-ICDs. Although this has been attempted in humans thus far (31), a clinical trial is planned to assess its efficacy in the human population; whether it is promising for future patients who require both pacing and ICD therapy remains to be determined.
Earlier studies found that the average age at ICD implantation in the HCM population was relatively young. With conventional ICDs, these patients are more prone to device- and intravascular lead-associated problems (up to 4%). Recent advances in sensing algorithms and device programming have led to reductions in rates of IAS of up to 68%, according to the pooled. Although, EFFORTLESS/IDE cohorts indicated that S-ICDs were safer than TV-ICDs, their usefulness in HCM therapy remains unclear (32,33).
Limitations
First, the sample size may have needed to be larger to detect significant differences in the incidence of AS and IAS between the two ICD devices, given the lack of studies comparing S-ICD and TV-ICD in patients with HCM. Second, the differences in study design of the six revised publications, which included observational and non-randomized studies, posed challenges in exploring the impact of uncontrolled baseline features on prognosis across both groups. Third, due to the included studies not distinguishing between lead-related and non-lead-related complications, we are unable to separately account for the various types of complication. Finally, including a historical cohort study significantly influenced our meta-analysis’s heterogeneity; not all patients presented data regarding the primary outcomes. Nevertheless, our leave-one-out cross-validation effectively mitigated this problem. Hence, conducting randomized controlled trials to validate further the safety and efficacy of S-ICDs in patients with HCM would bolster our findings.
Conclusions
This meta-analysis, which included 4,347 patients with HCM, indicated a notably lower incidence of device-related complications in patients who received S-ICDs than in those who received TV-ICDs. In addition, S-ICDs exhibited no significant variance in the rates of AS and IAS among patients with HCM.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-15/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-15/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-15/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maron BJ, Desai MY, Nishimura RA, et al. Management of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol 2022;79:390-414. [Crossref] [PubMed]
- Teekakirikul P, Zhu W, Huang HC, et al. Hypertrophic Cardiomyopathy: An Overview of Genetics and Management. Biomolecules 2019;9:878. [Crossref] [PubMed]
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2020;76:3022-55. [Crossref] [PubMed]
- Ali H, Lupo P, Cappato R. The Entirely Subcutaneous Defibrillator - A New Generation and Future Expectations. Arrhythm Electrophysiol Rev 2015;4:116-21. [Crossref] [PubMed]
- Fong KY, Ng CJR, Wang Y, et al. Subcutaneous Versus Transvenous Implantable Defibrillator Therapy: A Systematic Review and Meta-Analysis of Randomized Trials and Propensity Score-Matched Studies. J Am Heart Assoc 2022;11:e024756. [Crossref] [PubMed]
- Araújo CS, Silva CLB, da Silva Menezes Júnior A, et al. Efficacy and Complications of Subcutaneous versus Conventional Cardioverter Defibrillators: A Systematic Review and Meta-analysis. Curr Cardiol Rev 2022;18:e081221198647. [Crossref] [PubMed]
- Boersma L, Barr C, Knops R, et al. Implant and Midterm Outcomes of the Subcutaneous Implantable Cardioverter-Defibrillator Registry: The EFFORTLESS Study. J Am Coll Cardiol 2017;70:830-41. [Crossref] [PubMed]
- Arbelo E, Protonotarios A, Gimeno JR, et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J 2023;44:3503-626. [Crossref] [PubMed]
- Francia P, Olivotto I, Lambiase PD, et al. Implantable cardioverter-defibrillators for hypertrophic cardiomyopathy: The Times They Are a-Changin'. Europace 2022;24:1384-94. [Crossref] [PubMed]
- Maron MS, Steiger N, Burrows A, et al. Evidence That Subcutaneous Implantable Cardioverter-Defibrillators Are Effective and Reliable in Hypertrophic Cardiomyopathy. JACC Clin Electrophysiol 2020;6:1019-21. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Higgins JPT, Thomas J, Chandler J, et al. editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available online: www.training.cochrane.org/handbook
- Buongiorno AL, Blandino A, Bianchi F, et al. 102 ICD implantation in patients with hypertrophic cardiomyopathy (HCM): A long-term single-center experience. Eur Heart J Suppl 2022;24:suac121.590.
- Jankelson L, Garber L, Sherrid M, et al. Subcutaneous versus transvenous implantable defibrillator in patients with hypertrophic cardiomyopathy. Heart Rhythm 2022;19:759-67. [Crossref] [PubMed]
- Klein CC, Pentiah AD, Klug D, et al. Predictors od ICD-related adverse events in patients with hypertrophic cardiomyopathy. Eur J Heart Fail 2017;19:5-601.
- Lambiase PD, Gold MR, Hood M, et al. Evaluation of subcutaneous ICD early performance in hypertrophic cardiomyopathy from the pooled EFFORTLESS and IDE cohorts. Heart Rhythm 2016;13:1066-74. [Crossref] [PubMed]
- Steiger N, Burrows A, Rowin EJ, et al. Abstract 13876: Novel Safe and Effective Role for Subcutaneous-ICDs in Patients With Hypertrophic Cardiomyopathy. Circulation 2019;140:A13876.
- Timmers L, Goethals P, Purnode P, et al. Hypertrophic Cardiomyopathy: A comparison of transvenous and subcutaneous ICDs. Interv Card Electrophysiol 2017;48:1-134.
- Francia P, Ziacchi M, Adduci C, et al. Clinical course of hypertrophic cardiomyopathy patients implanted with a transvenous or subcutaneous defibrillator. Europace 2023;25:euad270. [Crossref] [PubMed]
- Knops RE, Pepplinkhuizen S, Delnoy PPHM, et al. Device-related complications in subcutaneous versus transvenous ICD: a secondary analysis of the PRAETORIAN trial. Eur Heart J 2022;43:4872-83. [Crossref] [PubMed]
- Rordorf R. The ATLAS Randomised Clinical Trial: What do the Superiority Results Mean for Subcutaneous ICD Therapy and Sudden Cardiac Death Prevention as a Whole? Arrhythm Electrophysiol Rev 2022;11:Suppl 1.
- Lin G, Nishimura RA, Gersh BJ, et al. Device complications and inappropriate implantable cardioverter defibrillator shocks in patients with hypertrophic cardiomyopathy. Heart 2009;95:709-14. [Crossref] [PubMed]
- O'Mahony C, Lambiase PD, Quarta G, et al. The long-term survival and the risks and benefits of implantable cardioverter defibrillators in patients with hypertrophic cardiomyopathy. Heart 2012;98:116-25. [Crossref] [PubMed]
- Lloyd MS, Brisben AJ, Reddy VY, et al. Design and rationale of the MODULAR ATP global clinical trial: A novel intercommunicative leadless pacing system and the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm O2 2023;4:448-56. [Crossref] [PubMed]
- Monkhouse C, Wharmby A, Carter Z, et al. Exploiting SMART pass filter deactivation detection to minimize inappropriate subcutaneous implantable cardioverter defibrillator therapies: a real-world single-centre experience and management guide. Europace 2023;25:euad040. [Crossref] [PubMed]
- Zeitler EP, Friedman DJ, Loring Z, et al. Complications involving the subcutaneous implantable cardioverter-defibrillator: Lessons learned from MAUDE. Heart Rhythm 2020;17:447-54. [Crossref] [PubMed]
- Brouwer TF, Yilmaz D, Lindeboom R, et al. Long-Term Clinical Outcomes of Subcutaneous Versus Transvenous Implantable Defibrillator Therapy. J Am Coll Cardiol 2016;68:2047-55. [Crossref] [PubMed]
- Santini M, Lunati M, Defaye P, et al. Prospective multicenter randomized trial of fast ventricular tachycardia termination by prolonged versus conventional anti-tachyarrhythmia burst pacing in implantable cardioverter-defibrillator patients-Atp DeliVery for pAiNless ICD thErapy (ADVANCE-D) Trial results. J Interv Card Electrophysiol 2010;27:127-35. [Crossref] [PubMed]
- Strickberger SA, Canby R, Cooper J, et al. Association of Antitachycardia Pacing or Shocks With Survival in 69,000 Patients With an Implantable Defibrillator. J Cardiovasc Electrophysiol 2017;28:416-22. [Crossref] [PubMed]
- Adduci C, Semprini L, Palano F, Musumeci MB, Volpe M, Autore C, Francia P. Safety and efficacy of anti-tachycardia pacing in patients with hypertrophic cardiomyopathy implanted with an ICD. Pacing Clin Electrophysiol 2019;42:610-6. [Crossref] [PubMed]
- Nieves J, Laslett DB, Basil A, et al. Simultaneous Leadless Pacemaker and Subcutaneous ICD Implantation With Intraoperative Screening: Workflow in Two Patients. JACC Case Rep 2022;4:101535. [Crossref] [PubMed]
- Lambiase PD, Theuns DA, Murgatroyd F, et al. Subcutaneous implantable cardioverter-defibrillators: long-term results of the EFFORTLESS study. Eur Heart J 2022;43:2037-50. [Crossref] [PubMed]
- Weiss R, Knight BP, Gold MR, et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944-53. [Crossref] [PubMed]


