
Sub-acute stent thrombosis in a bifurcation lesion: the devil is in the details
A 62-year-old male patient, presented with inferior wall ST-segment elevation myocardial infarction (MI) and underwent percutaneous coronary intervention (PCI) to a culprit right coronary artery (RCA)-posterior descending artery (PDA) bifurcation lesion with implantation of 3.0 and 2.5 mm Resolute Onyx (Medtronic Inc., Minneapolis, USA) zotarolimus-eluting stents respectively according to the “T-and-protrusion” (TAP) technique. Post-PCI dual antiplatelet therapy was composed of maintenance doses of aspirin and clopidogrel. Emergency coronary angiography performed four days later for recurrent angina and ST-segment elevation in the inferior leads revealed in-stent occlusion just proximal to the distal RCA bifurcation (Figure 1A). Recanalization was achieved after angioplasty using a 1.5 mm balloon inflated across both ostia of the bifurcation, followed by manual aspiration of a large thrombus from the ostium of the PDA.
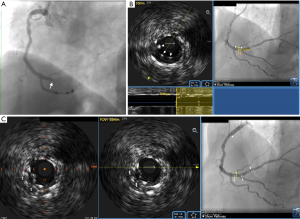
Subsequent intravascular ultrasound (IVUS) imaging (20 MHz Eagle Eye catheter; Philips, San Diego, USA) from the RCA revealed excessive protrusion of the PDA stent inside the distal RCA, resulting in generation of a long neocarina (Figure 1B) and distortion of the RCA stent proximal to the bifurcation, with stent struts protruding into the lumen, being overlapped with each other, and interspersed within the subacute thrombus (Figure 1C). The passage of the IVUS catheter to the PDA was getting obstructed just proximal to the bifurcation thereby precluding imaging. Review of the index PCI showed that the PDA stent was positioned rather deeply protruding inside the RCA thereby favoring the generation of a long neocarina (Figure 2A). Furthermore, an undersized balloon was used back then in the RCA for the final kissing balloon inflation (KBI), thereby explaining the generation of stent distortion. The latter had likely been facilitated by a long, X-shaped pattern of overlap of the kissing balloons (Figure 2B), which has been shown to result in underdilation of the main-vessel stent proximal to the carina. A pro-thrombogenic blood flow disturbance with high shear rate was therefore created by the distorted stent and redundant neocarina resulting in stent thrombosis. During PCI for ST, we applied a “sequential KBI technique” using properly sized balloons positioned laterally to each other with a good angiographic result (Figure 2C). Final IVUS imaging revealed re-expansion of the RCA stent and reconstruction of the neocarina (Figure 2D). Despite not being tested for clopidogrel resistance, the patient was switched from clopidogrel to ticagrelor after angioplasty. The mechanism of stent failure is detailed in the schematic illustration presented in Figure 3.
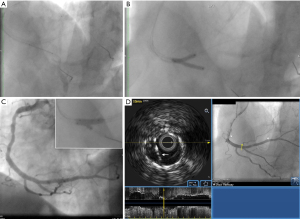

This case highlights the utility of IVUS imaging in revealing the mechanism of stent failure, thereby guiding patient management. We refrained from conversion to a culotte because the PDA stent could be expanded up to an average diameter of 3.3 mm, leading to malapposition and likely napkin ring formation in the RCA. We also refrained from conversion to internal crush, despite that this technique seemed appropriate since it could achieve re-expansion of the RCA stent and elimination of the long neocarina. Because of a long proximal protrusion of around 5 mm of the PDA stent into the RCA, the performance of the internal crush technique would have resulted in a large volume of crushed stent wrapping around one side of the RCA stent and potentially forming an area with multiple layers of struts at the site of the distorted RCA stent. This was deemed unwanted as it could induce inadequate strut apposition which could in turn enhance local thrombogenicity thereby promoting future thrombotic events. Given these circumstances, it was considered prudent to perform a less complex technique especially during the acute phase, such as the “sequential KBI technique”, achieving correction of the most consistent predictor of both stent thrombosis and in-stent restenosis (ISR), that is a suboptimal (≤5.0 mm2) post-stent minimal stent area (MSA). Specifically, the MSA at the site of RCA stent distortion increased from 4.5 to 12.0 mm2 after PCI. The tradeoff of this approach, however, was the preservation of a long neocarina. Revealing a long neocarina by IVUS imaging was instrumental in the success of “sequential KBI”, since it entailed the need to leave a sufficiently long balloon across the PDA ostium during dilatation of the RCA stent. This minimized the risk of PDA occlusion by struts shifting, allowed re-expansion of the excessively protruding PDA stent during a subsequent dilatation and facilitated a final KBI that ensured the reconstruction of a centrally positioned neocarina.
Bifurcation-lesion PCI is inherently complex, partly owing to the heterogeneity of such lesions and is associated with a higher risk of adverse events such as ISR, stent thrombosis, peri-procedural MI and cardiovascular mortality. The inability to accurately determine the extent and distribution of atheroma or to optimally expose the side branch (SB) ostium in certain cases by conventional angiography, the need to have the stent modified in order to match the tapered anatomy of the coronary vessel at the bifurcation site and still be well apposed in all segments and the procedural complexity entailed by the various stenting techniques which frequently require multiple manipulations (i.e., rewiring, advancement of balloons for proximal optimization and KBI, advancement and implantation of a SB stent, use of guide-extension catheters, deep seating of guide catheters), heighten the risk of suboptimal acute results and hence poorer outcomes. Mechanical stent abnormalities such as malapposition, underexpansion and loss of stent integrity (i.e., stent gap, stent fracture, deformation) constitute major contributing factors to bifurcation stent thrombosis whilst the latter two factors are also associated with bifurcation ISR. Compared with angiography, the use of IVUS to guide bifurcation-lesion PCI has been associated with lower rates of mortality, MI and late and very late stent thrombosis. This is achieved via pre-procedural anatomic information afforded by IVUS (i.e., diameters of the main and daughter vessels, atheroma burden, morphology and distribution, stenosis severity and assessment of negative remodeling mainly at the SB ostium, lesion length, stent landing zones and reference segment analysis) allowing selection of the most appropriate stent technique to be executed with use of the most appropriate tools (adequately sized stents and balloons) and also by assessing the result of PCI (i.e., mechanical stent abnormalities, geographic miss, stent edge dissection) allowing actions targeting optimization. Unintended stent deformation (USD) is not infrequent during bifurcation-lesion PCI and may cause mechanical obstruction and stent distortion and fracture or could leave parts of the lesion uncovered potentially resulting in adverse outcomes such as stent thrombosis, ISR, procedure-related MI requiring bailout stenting or target lesion revascularization. Contemporary data have shown that intracoronary imaging greatly facilitates the diagnosis of USD which in a setting of bifurcation-lesion PCI owes to accidental abluminal rewiring or guide catheter (or extension catheter) collision with the proximal stent edge; however, in nearly 50% of the cases, USD may remain undetected. The detection of USD when followed by corrective measures such as postdilatation, bailout stenting or correction by subsequent steps in the applied stent technique ensures a good outcome. Furthermore, rewiring of a SB through an inappropriate stent cell, SB postdilatation without simultaneous or subsequent main-vessel postdilatation or KBI using an undersized main-vessel balloon or balloons having an inappropriate overlapping configuration have also been associated with deformation and potentially malapposition of the main-vessel stent.
In the case presented herein, distortion of the RCA stent proximal to the bifurcation with a PDA was ascribed to KBI using an undersized main-vessel (RCA) balloon and an inappropriate overlapping configuration (x-shaped) of the kissing balloons and resulted in a suboptimal MSA. A “sequential KBI technique” entailing successive postdilatation of the RCA and PDA stents with optimally sized balloons before KBI was applied in order to re-expand the distorted RCA stent. Thus, we selected the balloons to match the diameter of the vessels just distal to the bifurcation as measured by IVUS and inflated them successively at high pressure (20 atm). Subsequently, KBI was performed using the same balloons positioned laterally to each other, which were inflated simultaneously up to 8 atm and deflated also simultaneously to ensure that a central position of the neocarina is achieved. As far as the neocarina is concerned, there have been reported IVUS data documenting the variability of its length (2.7±1.4 mm) obtained in clinical practice, thereby suggesting that the task of completing the “TAP” technique having achieved a short neocarina is difficult. Perhaps, in the case presented herein, knowledge that the Y-shaped distal RCA bifurcation is associated with a longer, oval-shaped PDA ostium, implying the need for wider protrusion of the PDA stent inside the RCA and hence creation of a longer neocarina could have made the operator more vigilant to ensure that appropriate recrossing into the PDA through a distal stent cell and hence adequate scaffolding of the proximal rim of the PDA ostium that could help limiting the amount of stent protrusion and consequently the length of the neocarina has been achieved. Such knowledge could have also prompted the operator to perform a different stent technique (i.e., crush technique) or even accept a suboptimal result in a patent PDA with the aim to optimize the result in a subsequent procedure.
The comprehensive study of this case points to a lack of precision partly owing to inexperience in bifurcation-lesion PCI as the main reason for the suboptimal result during the index PCI that resulted in an adverse patient outcome. This argument is further supported by the fact that the operator made no mention about any possible suspicion of stent distortion or a long neocarina which is otherwise highly mobile and usually difficult to appreciate by angiography. Interrogation with IVUS could have been performed, should the operator had suspected the complications. Lack of suscpicion of the complications is therefore further supported by the fact that the result of index PCI has not been assessed by IVUS. Adequate training and an individualized lesion-specific approach and optimization of the performance of the technique facilitated by a judicious use of intracoronary imaging are key steps to a successful bifurcation-lesion PCI and hence an optimal outcome.
AcknowledgmentsOther Section
I wish to thank Mr. Andreas Procopiou for providing the schematic illustration.
Funding: None.
FootnoteOther Section
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-183/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-183/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of Images in Clinical Medicine was not obtained from the patient or the relatives after all possible attempts were made.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.



