
Dilated phenotype of hypertrophic cardiomyopathy: cardiac magnetic resonance assessment and 9-year follow-up
Although most patients with hypertrophic cardiomyopathy (HCM) have a good prognosis (1), approximately 7% rapidly progress to severe systolic dysfunction (2), which is known as the dilated phase, end-stage, or decompensated HCM (DHCM) (3). Patients with DHCM usually have a poor prognosis and are difficult to identify at an early stage. The dynamic evolution of cardiac abnormalities in patients with DHCM has rarely been reported.
Herein, we describe the case of a 48-year-old man who progressed to DHCM and underwent three cardiac magnetic resonance (CMR) scans in 2010, 2014, and 2017. The patient, with a 4-year history of HCM, presented to our center in 2010 with shortness of breath after heavy activity. The patient had no family history of malignant tumors, heart disease, diabetes, hypertension, hereditary disease, and contagious disease. Electrocardiography revealed T-wave changes, a first-degree atrioventricular block, and occasional atrial and ventricular premature beats. Echocardiography showed left atrial (LA) enlargement, a thickened left ventricular (LV) wall, and varying degrees of septal thickening. Blood tests revealed elevated N-terminal pro-brain natriuretic peptide (NT-proBNP; 2,042.6 pg/mL; normal values, 0–250 pg/mL) and cardiac troponin I (cTnI; 1.244 ng/mL; normal values, 0–0.04 ng/mL). Coronary computed tomography angiography (CCTA) showed no abnormalities during follow-up. The first CMR scan showed a mildly dilated LA chamber, a small LV chamber, and a significantly asymmetrically thickened LV wall, especially the interventricular septum (Figure 1, A1, B1). Over time, the LA and LV chambers enlarged, and the LV wall became progressively thinner (Figure 1, A2, B2, A3, B3). The LV mass also gradually decreased with disease progression from 300 g in 2010 to 242 g in 2017, with the LV ejection fraction (LVEF) also decreasing from 57% in 2010 to 20% in 2017. The initial CMR scan showed late gadolinium enhancement (LGE) in the mid-wall of the interventricular septum and the LV free wall (Figure 1, C1, D1). Subsequent CMR scans revealed more types of LGE patterns, including extensive mid-wall and subendocardial LGE in the interventricular septum and LV free wall, with a focal transmural appearance (Figure 1, C2, D2, C3, D3). The extent of LGE also increased by 16%, 41%, and 49% over time (Figure 2A), indicating aggravated myocardial fibrosis. Global longitudinal strain (GLS) progressively increased (Figure 2B), suggesting a declining LV systolic function. Moreover, LV myocardial strains were all significantly impaired during the follow-up period (Figure 3). Owing to ineffective medical treatment, the patient underwent heart transplantation in 2018. Pathological findings revealed that the biventricular chambers were dilated, and the thickness of the LV wall was 1.4 cm. Microscopic examination revealed extensive fibrotic scar tissue. The pathological diagnosis was HCM. Unfortunately, the patient developed postoperative immune rejection and died in 2019 of multiple organ failure.
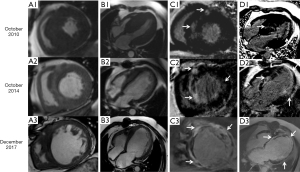

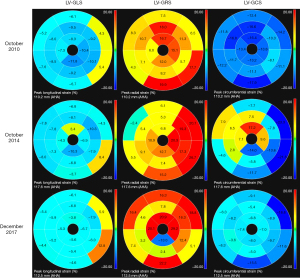
The three CMR examinations in this case dynamically demonstrated the rapid progression of HCM to the decompensated stage in less than a decade, indicating that more attention should be paid to patients with DHCM in clinical practice. The diagnosis of dilated cardiomyopathy (DCM) is straightforward in the presence of LV enlargement and ventricular wall thinning (4). Myocardial infarction (MI) can be easily diagnosed in the presence of subendocardial or transmural LGE (5). However, DHCM should also be considered a diagnostic possibility when these CMR findings are present. Unlike DCM and MI, DHCM is characterized by the thinning of a previously hypertrophied ventricular wall and LGE multiple patterns, including mid-wall, subendocardial, and transmural LGE. Additionally, LGE in DHCM is more widely distributed across all LV segments, whereas LGE in DCM is mainly localized to the interventricular septum (6).
To the best of our knowledge, this study represents the first long-term follow-up report clearly depicting the dynamic evolution of DHCM using serial CMR scans. This report emphasizes the importance of “one-stop” CMR in assessing the morphological, functional, and histological features of the heart, providing important diagnostic and prognostic information for patients with DHCM.
Acknowledgments
We thank all supporting staff for their contributions to this work and we would also like to thank Editage (http://www.editage.cn/) for English language editing.
Funding: This study was supported by
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-160/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-160/coif). S.Z. reports that this study was supported by the National Key R&D Program of China (Nos. 2021YFF0501400 and 2021YFF0501404) and the Key Project of National Natural Science Foundation of China (No. 81930044). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s relatives for the publication of these images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nagueh SF, Phelan D, Abraham T, et al. Recommendations for Multimodality Cardiovascular Imaging of Patients with Hypertrophic Cardiomyopathy: An Update from the American Society of Echocardiography, in Collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2022;35:533-69. [Crossref] [PubMed]
- Marstrand P, Han L, Day SM, et al. Hypertrophic Cardiomyopathy With Left Ventricular Systolic Dysfunction: Insights From the SHaRe Registry. Circulation 2020;141:1371-83. [Crossref] [PubMed]
- Xiao Y, Yang KQ, Jiang Y, et al. Recent progress in end-stage hypertrophic cardiomyopathy. Am J Med Sci 2015;349:448-53. [Crossref] [PubMed]
- Heymans S, Lakdawala NK, Tschöpe C, et al. Dilated cardiomyopathy: causes, mechanisms, and current and future treatment approaches. Lancet 2023;402:998-1011. [Crossref] [PubMed]
- Wang X, Pu J. Recent Advances in Cardiac Magnetic Resonance for Imaging of Acute Myocardial Infarction. Small Methods 2024;8:e2301170. [Crossref] [PubMed]
- Machii M, Satoh H, Shiraki K, et al. Distribution of late gadolinium enhancement in end-stage hypertrophic cardiomyopathy and dilated cardiomyopathy: differential diagnosis and prediction of cardiac outcome. Magn Reson Imaging 2014;32:118-24. [Crossref] [PubMed]


